Digestive enzyme efficiency -
The sizes of paddies and ponds were almost m 2. They were bred at different stocking densities a hundred thousand for pond and ten thousand for paddy and same management. The fish were firstly sampled in the summer August , water temperature 21 °C, fish fries had been farmed for three months and were secondly sampled in the fall November , water temperature 20 °C, the weight of loach from paddy fields and the ponds be There was only 1 °C of difference in the water temperature between summer and fall, it owing to the water flows in the mountain stream and the specific heat capacity of water is larger.
Fish samples were collected for analysis in the August summer season and November fall season during the feeding trial. The samples, only used for intestinal microbiota analysis were sampled in summer and fall, while the fish were sampled in fall, used for the enzyme activities and morphology.
Ten healthy individuals were randomly sampled from each farming pond and each time. In the study, for the digestive enzymatic activities and intestinal mucous cells analysis experiments, the fish were carried to the laboratory in oxygen filling bags within 3 h. Besides the samples were directly collected in fish farm for gut microbial analysis, and then the samples were also carried to the laboratory in ice within 3 h.
The fish were euthanized by overdose of MS Sigma, Germany before dissection [ 19 ]. The intestine tracts were divided into the foregut, midgut and hindgut.
In these studies, foregut was defined as the first section of intestine from esophagus to the distal end of the swelling of the intestine.
The narrow middle section was defined as the midgut, and the larger-diameter section following this to just prior to the anus was defined as hindgut. Segments of 0. And the intestine tracts were removed aseptically from their abdominal cavity and the content of intestine was squeezed out and separately stored.
Thereafter, the mucosa in the epithelial intestinal of the loach was collected by blades, respectively. About 0. Meanwhile, the water was also sampled at an approximate depth of 35 cm from five sites in the each paddy or pond, pooled it together and ml water was stored for centrifuging. The pellet was collected after centrifuged at ×g for 20 min at 4 °C.
Ten healthy individuals were randomly sampled from paddy fields and ponds, respectively. The number of individuals is 20 in this experiment. Intestine samples were washed with cold deionized water to remove most of the mucus, and the intestine was ground to pulp with cold sodium phosphate buffer 0.
Then, the homogenate was centrifuged K, Sigma®, Germany at 4 °C at ×g for 10 min. The lipase, amylase, trypsin and total protein were detected using assay Kits Lipase assay kit, Amylase assay kit, Trypsin assay kit and Total protein quantitative assay kit purchased from Nanjing Jiancheng, Bioengineering Institute, China.
The total number of individuals is 20 in this experiment. Fixed samples were wrapped in gauze and rinsed in running water for 12 h, dehydrated in graded ethanol solution and embedded.
Sections were cut at 6 μm serially using a rotary microtome. The sections were stained with alcian blue and periodic acid schiff AB-PAS and photo-documented using a low power light invert microscope Nikon, Japan , measured with Photoshop CS4.
In different histological sections, the numbers of mucous cells in multiple micrographs from each intestinal region of ten fish in each group were measured, and twelve micrographs from the foregut, midgut and hindgut of intestinal samples were chosen.
Ten healthy individuals were randomly sampled from two seasons and two modes summer and fall; paddy fields and ponds , respectively. The total number of individuals is 40 in this experiment. Firstly, the total bacterial DNA of the samples was extracted using TIANamp Bacteria DNA Kit TIANGEN, China.
Then, in order to estimate the abundance of beneficial bacteria and harmful bacterica in loach gut, a gold method and citation was referenced [ 21 ]. Basing on the method discribed by Sun H. The part primers for Q-PCR of microbiota were also listed in Table 1 , including references.
The reaction system components and reaction procedures are summarized in Table 2. The triplicate tenfold serial dilutions of the plasmid DNA were used to built the standard curves. Based on the standard curves, copy numbers of the target bacterial phylum or genus in samples were calculated.
The method described by Sun H. et al. The raw data were firstly imputed into Excel to setup database because the two data types could transform between Excel and SPSS software.
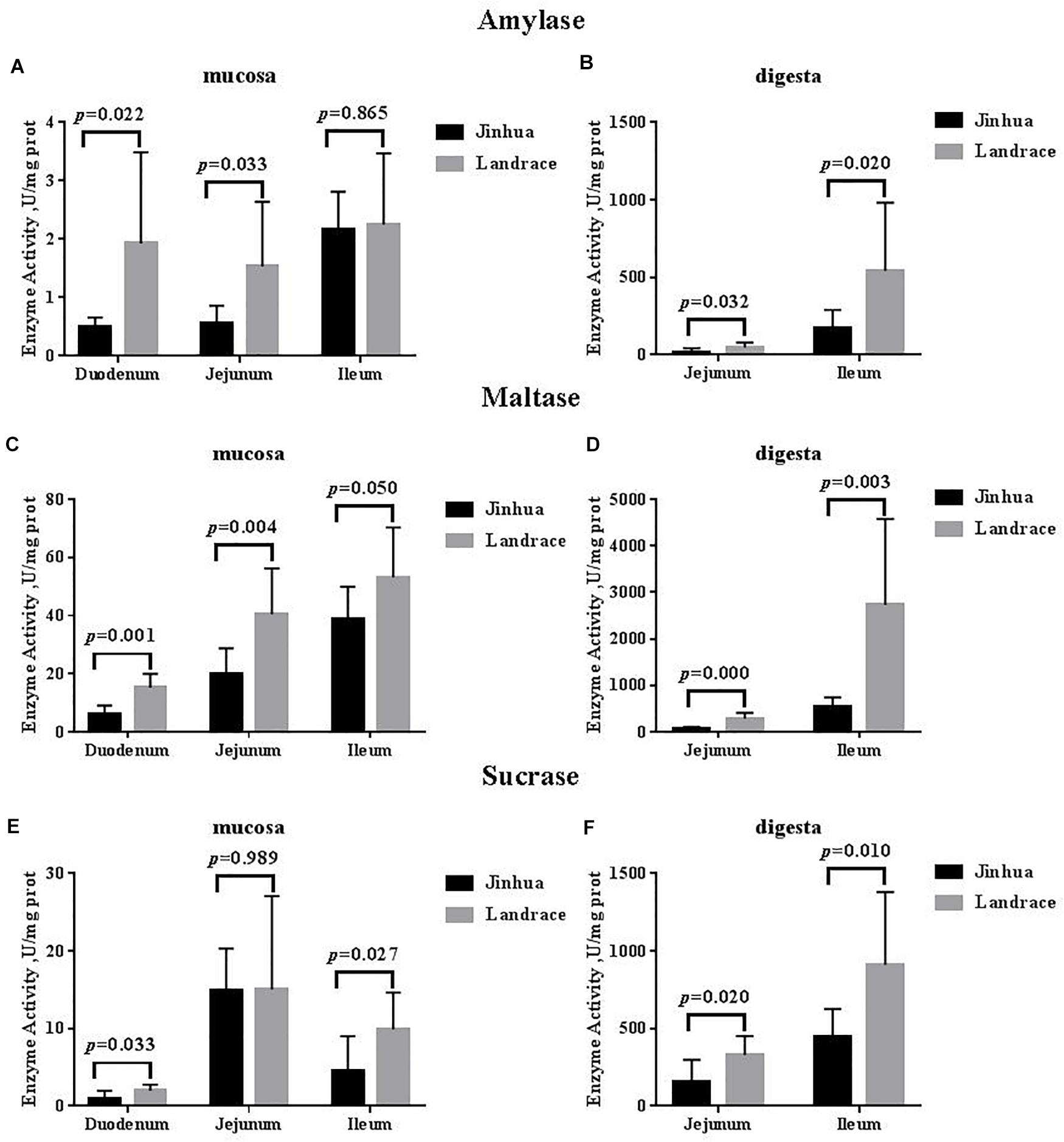
Statistical analysis was performed using a one-way ANOVA in SPSS Enzymatic activity in the loach intestine in autumn is summarized in Table 3. In PACM, the activities of lipase and amylase were highest in foregut and lowest in hindgut; the activity of trypsin was highest in liver and lowest in hindgut.
In POCM, the activities of lipase, amylase and trypsin presented a similar trend in the different tissues, with the activities of these three digestive enzymes always highest in liver and foregut and lowest in the hindgut.
A summary of the light-microscope observations of the intestinal-tissue structures are presented in Table 4. However, the difference was not significant between the two cultivation modes in spite of higher numbers of acid mucous cells in the hindgut in PACM.
In addition, the size of the mucous cells tended to be bigger in the midgut than in the foregut Fig. AB-PAS staining inverted microscopy micrographs different parts of intestinal samples arbitrarily chosen as examples, ×; ×. L represent the acid mucous cells, M represent the partial acid mucous cells.
In PACM, the loach displayed a greater abundance of total bacteria, the Firmicutes, Bacteroidetes, Bifidobacterium , Enterococcus spp. Table 5. Considerable variation in the fish mucosa was detected in different seasons, including different abundances of total bacteria, the Bacteroidetes and Bifidobacterium.
In summer, the abundances of total bacteria, the Firmicutes, the Bacteroidetes, Bifidobacterium , and some pathogenic bacteria A. Moreover, substantial amplification difference was observed in the summer contents, such as the abundance of total bacteria, the Bacteroidetes, Enterococcus spp.
and Enterobacteriaceae. The abundance of Enterococcus spp. Interestingly, the abundances of all the bacterial groups, except for Bifidobacterium and Lactobacillus spp. Effects of different seasons on functional bacteria paddy cultivation modes. Note: a — i represent Log 10 DNA gene copies of total bacteria, Firmicutes, Bacteroidetes, Bifidobacterium , Enterococcus spp.
hydrophila , Enterobacteriaceae and Streptococcus spp. in the mucosa, content and water, respectively. In POCM, the abundances of the Firmicutes in the intestinal contents and mucosa, and Streptococcus spp.
in the intestinal contents, were lower than found in the water in both summer and fall, while the other bacterial groups presented a higher abundance in summer.
Lactobacillus spp. in the mucosa, and A. hydrophila in the intestinal contents and mucosa, maintained higher abundances than found in the water in the autumn samples, and other bacterial groups maintained lower abundances in the loach microflora than found in the water Table 6.
Considerable amounts of bacteria were detected in the loach mucosa in both seasons, and only the abundance of Lactobacillus spp.
increased gradually from summer to fall. and Streptococcus spp. Moreover, some of the dominant bacteria still maintained high abundance in the intestinal contents or mucosa than in the water in the fall, specifically A. Effects of different seasons on functional bacteria pond cultivation modes.
Note: a — i Log 10 represent DNA gene copies of total bacteria, Firmicutes, Bacteroidetes, Bifidobacterium , Enterococcus spp. hydrophila and Enterobacteriaceae. Moreover, the abundance of Enterococcus spp.
Furthermore, the abundances of the Bacteroidetes, Enterococcus spp. hydrophila Fig. Effects of different cultivations modes on functional bacteria. in the mucosa and water, respectively. Loaches are stomachless fish, and the anterior intestinal swelling foregut serves as an ichthyic stomach. The histological analysis showed that the intestinal tract of loach could be generally divided into mucosa, submucosa, and muscular coats and serosa from the interior to exterior [ 25 ].
In this study, a high content of digestive enzyme activity occurred in the liver and foregut Table 3. Thus, the liver and foregut are assumed to play an important role in digestion and nutrient absorption in this species, which was confirmed by the large numbers of the different types of mucous cells Table 4 and observations of the mucous-cell morphology in the foregut Fig.
Previous studies focused on the digestive enzymes in young fish or comparisons of different developmental stages of fish. Moyano [ 26 ] studied the activities of digestive enzymes during larval development in gilthead seabream; the results revealed that enzymatic activities increased in relation to fish development, and exogenous food had more of a qualitative than quantitative role in the secretion of digestive enzymes.
In this study, the levels of digestive enzyme activity significantly differed between loach in the two cultivation modes. Loach reared in paddy fields may be more dependent on the environment; while they can prey on some live, foods they might also unavoidably suffer from starvation due to the environment.
Previous research revealed that the activities of digestive enzymes were directly affected by the food [ 29 ] and also changed in the fish intestine with different feeding habits [ 30 , 31 ]. Liu [ 32 ] reported decreased enzyme activity in wild freshwater fishes as compared with farmed fish, as an influence of their trophic level.
Our findings are consistent with previous observations; for example, lower amylase and trypsin in activity in the liver was detected in PACM. Interestingly, the activities of digestive enzymes in the midgut and hindgut were higher in PACM than in POCM; this might imply a stronger digestive ability in PACM.
In this study, consistency was observed in the distribution of intestinal mucous cells and the activities of digestive enzymes in the different intestine sections, with gradual decreases from foregut to hindgut, although microorganisms will also affect enzymatic activity [ 33 , 34 ].
The intestinal tract is a complex system that plays a key role not only in digestion, nutrient absorption and osmoregulation, but also in immune homeostasis [ 2 , 35 ].
The surface area is constantly bombarded by antigens from the diet and the gut microorganisms; while the intestinal tract is one line of defense against pathogens, it is also regarded as a primary portal for pathogenic invasion in fish [ 36 ].
The integrity and stability of the function and structure of the intestinal tract are critical for digestion and nutrient absorption.
Moreover, the beneficial effects on host health from the commensal microbiota and their fermentation products are well evidenced [ 37 , 38 , 39 ]. Wu [ 40 ] attributed efficient digestion, especially of cellulose, and the absorption of nutrients in yellow catfish to the intestinal microbiota.
In this study, we investigated the abundance of total bacteria, the Firmicutes, the Bacteroidetes, Bifidobacterium , Enterococcus spp. in loach in two cultivation modes and during two seasons.
The intestinal microbiota always changed with the host fish and ambient environment, and even with the development phase of the loach. In our study, among loach in PACM, several of the bacteria groups presented higher concentrations in summer, such as the Firmicutes, Lactobacillus spp.
However, the abundances of the pathogenic bacteria Enterococcus spp. significantly decreased among loach in PACM in the fall. In general, the intestine of loach does not much develop as the fish grows, and it usually contains fewer microbiota in the early life phase.
With the development of the digestive organ, the species composition and quantities of the microflora are gradually enriched, and their population structures progressively stabilize in the fish intestine.
Ringø [ 41 ] exposed turbot larvae to Vibrio pelagius and observed the changes in the intestinal microbiota: the microbiota first increased but then eventually stabilized.
In contrast to the observations of Ringø [ 41 ], we detected a decline in the abundance of pathogenic gut bacteria among the loach in PACM in the fall; this finding suggests that paddy fields may be the better environment for the growth and health of juvenile loach.
However, the data also showed no significant differences in the growth parameters of loach cultured in ponds or in paddy fields [ 42 ]. Further study is needed for a more detailed evaluation of differences between the two culture modes.
The Firmicutes are a dominant phylum [ 43 ] that includes multiple cellulolytic bacteria, which are closely associated with the bioconversion of feeds in the body [ 44 ].
The Bacteroidetes are a dominant phylum present in fish [ 43 , 45 ] and are known to accelerate the catabolism of plant cell walls [ 46 ], although the most comprehensive classification studies of these bacteria have been done on land animals. Because the loach is an omnivorous species, its diet includes algae, grasses, and other plant debris and organic matter found in the sediment [ 47 ].
Some Lactobacillus and Bifidobacterium are recognized as beneficial for intestinal health in fish, and they may be added to the diet as probiotics that improve fish growth and development.
Previous studies, especially of Bifidobacterium , have focused mainly on land animals and less on aquatic animals. It has also been reported that some lactic-acid bacteria isolated from the gastrointestinal tract of fish can act as probiotics [ 49 , 50 ].
In addition, Lactobacillus can inhibit the growth of Enterobacteriaceae [ 51 ] and Streptococcus spp. Bifidobacterium is often detected in water as well as in the digestive tracts of fish [ 53 ]. Itami [ 54 ] found that peptidoglycan derived from Bifidobacterium thermophilum enhanced disease resistance in kuruma shrimp.
Bifidobacterium also can inhibit the growth of Enterococcus spp. Hence, we conclude that it is likely that the pathogenic bacteria might be controlled or even reduced in the presence of probiotics. Aeromonas hydrophila is one of the most common bacteria in freshwater habitats, and it is a frequent cause of disease among cultured and wild fishes worldwide [ 56 ].
It is an opportunistic pathogen in both fish and terrestrial animals, including mammals. Consequently, it is important to maintain excellent water parameters for loach in either PACM or POCM. In this study, higher abundances of A. hydrophila were observed in the intestinal contents and mucosa, for both culture modes, but especially in summer.
Fortunately, high abundances in PACM were not maintained during the fall. Rearing loach in PACM represents a good rice—fish co-culture system [ 57 ]. Our observations indicate that particular attention should to be paid to the loach culture management strategy for the summer season. Enhancement of fish immunity is possibly the most promising method for preventing fish diseases; even so, the health condition of freshwater fish is also strongly affected by their trophic level [ 32 ].
Therefore, improvements to the feeding strategy for fishes reared in paddy fields needs more attention. The large area of rice in a paddy field might present an obstacle for loach as they swim to feed. This study evaluated differences in the digestive enzyme activities of the intestine, the distribution of intestinal mucous cells, and the quantities of some taxa of intestinal microbiota in loach cultured in paddy fields and ponds.
The abundance of most bacterial groups in the loach gut presented significant differences between the two cultivation modes, in both summer and fall. However, in both cultivation modes, the pathogenic bacterium A.
hydrophila maintained a relatively high abundance in the intestinal contents and mucosa, including during summer, although its abundance decreased during the fall. This finding indicates that particular attention should to be paid to the loach culture management strategy for the summer season.
Jutfelt F, Olsen RE, Björnsson BT, Sundell K. Parr—smolt transformation and dietary vegetable lipids affect intestinal nutrient uptake, barrier function and plasma cortisol levels in Atlantic salmon.
Article CAS Google Scholar. Peterson LW, Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis.
Nat Rev Immunol. Article PubMed CAS Google Scholar. Pierre MK, Jules-Bocamdé T, François NZ, Gloria DR, Carmen W, María de Lourdes P. Quantitative analyses of the bacterial microbiota of rearing environment, tilapia and common carp cultured in earthen ponds and inhibitory activity of its lactic acid bacteria on fish spoilage and pathogenic bacteria.
World J Microbiol Biotechnol. Article Google Scholar. Merrifield DL, Dimitroglou A, Foey A, Dacies SJ, Baker RTM, Bøgwald J, Castex M, Ringø E. The current status and future focus of probiotic and prebiotic applications for salmonids.
Uddin N, Al-Harbi AH. Bacterial flora of polycultured common carp Cyprinus carpio and African catfish Clarias gariepinus. Int Aquat Res. Al-Harbi AH, Uddin N. Quantitative and qualitative studies on bacterial flora of hybrid tilapia Oreochromis niloticu s × O.
aureus cultured in earthen ponds in Saudi Arabia. Aquac Res. Lactase is produced by cells known as enterocytes that line the intestinal tract. Lactose that is not absorbed is fermented by bacteria in the gut.
This can cause you to have gas and an upset stomach. Lipase is responsible for the breakdown of fats into fatty acids and glycerol simple sugar alcohol. It's produced in small amounts by your mouth and stomach, and in larger amounts by your pancreas.
Also called peptidases, proteolytic enzymes, or proteinases , these digestive enzymes break down proteins into amino acids. They also play a role in numerous body processes, including:. Proteases are produced in the stomach and pancreas. The main ones are:. Sucrase is secreted by the small intestine, where it breaks down sucrose the sugar in table sugar into fructose and glucose.
These are simpler sugars that the body can absorb. Sucrase is found along the intestinal villi. These are tiny hair-like structures that line the intestine and absorb nutrients into the bloodstream.
There are a variety of health conditions that can interfere with the secretion of enough digestive enzymes to fully digest foods. Some are inherited genetic conditions while others develop over time. Lactose intolerance occurs when you aren't able to digest lactose because of insufficient production of lactase by the small intestine.
When you consume dairy products, you may experience:. There are several forms of lactose intolerance. Congenital lactase deficiency also called congenital alactasia is a rare inherited form of lactose intolerance.
It happens when newborns are unable to break down lactose in breast milk or formula. They get severe diarrhea if they aren't given a lactose-free alternative.
Congenital lactase deficiency is caused by mutations in the LCT gene that provides instructions for making the lactase enzyme. Lactase non-persistence is a common type of lactose intolerance that some people develop as adults. Symptoms typically begin 30 minutes to two hours after eating or drinking dairy.
Most people with lactase non-persistence keep some level of lactase activity and can continue to include a small amount of lactose in their diets.
This may be in the form of cheese or yogurt since both tend to be tolerated better than fresh milk. Secondary lactose intolerance develops when lactase production is reduced because of diseases that can damage the small intestine.
These diseases include celiac disease or Crohn's disease as well as other illnesses or injuries that affect the intestinal wall. The pancreas produces the key digestive enzymes amylase, protease, and lipase. People with exocrine pancreatic insufficiency EPI have a deficiency of these enzymes.
As a result, they are unable to digest food properly, especially fats. The health conditions that affect the pancreas and are associated with EPI are:. A variety of foods, especially tropical fruits and fermented vegetables, are naturally high in digestive enzymes that might speed up the digestion of certain nutrients.
It's best to eat them raw since heat can lessen or destroy these plant enzymes. People who don't have sufficient amounts of digestive enzymes or who are looking to support healthy digestion should consider supplementing their diet with digestive enzymes.
They can do this by eating healthy foods that contain naturally occurring digestive enzymes. But they can also take nutritional supplements under a healthcare provider's guidance. Digestive enzyme supplements can come in:.
There are prescription supplements regulated by the FDA as well as over-the-counter supplements. Prescription enzyme supplements are recommended for conditions that affect the functioning of the pancreas, such as chronic pancreatitis or pancreatic cancer.
Brands of prescription pancreatic enzyme supplements pancrelipase include:. Over-the-counter enzyme supplements are not regulated by the FDA. There haven't been enough high-quality studies on them, so it's hard to know how effective they are. The following are some of the supplemental enzymes that don't require a prescription:.
As with any supplement, check with your healthcare provider before taking an over-the-counter digestive enzyme to make sure it's safe for you.
They're secreted by the salivary glands and cells lining the stomach, pancreas, and small intestine. Sometimes people have a digestive enzyme deficiency. These deficiencies are connected to various health conditions.
Many of these health conditions are related to the pancreas. Before you decide to take an enzyme supplement, get your healthcare provider's advice.
They can help you determine if it's safe for you. Article CAS PubMed Google Scholar. Frías-Quintana, C. Changes in digestive enzymes activities during the initial ontogeny of wolf cichlid, Parachromis dovii Perciformes: Cichlidae. Vijverberg, J. The chemical composition and energy contents of copepods and cladocerans in relation to their size.
Ahmadi, M. Nutrient composition of the Iranian brine shrimp Artemia uromiana. El-feky, M. Effect of feeding with different types of nutrients on intensive culture of the water flea, Daphnia magna Straus, Development of digestive enzymes in common dentex Dentex dentex during early ontogeny.
Darias, M. Larval organogenesis of Pagrus pagrus L. Suzer, C. Ontogenic development of the digestive enzymes in common pandora Pagellus erythrinus L. Trevino, L. A histological study of the organogenesis of the digestive system in bay snook Petenia splendida Gunther, from hatching to the juvenile stage.
Peña, E. In vitro protein digestibility of different grow-out stages of spotted rose snapper Lutjanus guttatus , Steindachner, Kolkovski, S. Digestive enzymes in fish larvae and juveniles—Implications and applications to formulated diets.
Lazo, J. Characterization of digestive enzymes during larval development of red drum Sciaenops ocellatus. Development of digestive tract and enzyme activities during the early ontogeny of the tropical gar Atractosteus tropicus. Influence of age on digestive enzyme activity in some freshwater teleosts.
Aquaculture , 25— Hardewig, I. Is digestive capacity limiting growth at low temperatures in roach?. Liu, H. The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels.
Article ADS CAS PubMed PubMed Central Google Scholar. Jones, D. The digestive physiology of herbivorous, omnivorous and carnivorous crustacean larvae: A review. Solovyev, M. Feeding habits and ontogenic changes in digestive enzyme patterns in five freshwater teleosts.
Dietary modulation of some digestive enzymes and metabolic processes in developing marine fish: Applications to diet formulation. Aquaculture , 98— Ontogeny and physiology of the digestive system of marine fish larvae. In Feeding and Digestive Functions eds Cyrino, J. Oozeki, Y. Ontogenetic development of digestive enzymes activities in larval walleye pollock, Theragra chalcograma.
Liu, W. Digestive enzyme and alkaline phosphatase activities during the early stages of Silurus soldatovi development. Rangsin, W. Digestive enzyme activities during larval development of striped catfish, Pangasianodon hypophthalmus Sauvage, García-Meilán, I. Effects of dietary protein-to-lipid ratio on digestive and absorptive processes in sea bass fingerlings.
Growth performance, digestive enzyme activities, and oxidative stress markers in the proximal intestine of European sea bass Dicentrarchus labrax fed high starch or lipid diets. Fishes 8 , High dietary carbohydrate inclusion by both protein and lipid replacement in gilthead sea bream.
Changes in digestive and absorptive processes. Different protein to energy ratio diets for gilthead sea bream Sparus aurata : Effects on digestive and absorptive processes.
Aquaculture — , 1—7. Guillaume, J. Digestive physiology and nutrient digestibility in fishes. In Nutrition and Feeding Fish and Crustaceans eds Guillaume, J.
Tielmann, M. The effect of light intensity on performance of larval pike-perch Sander lucioperca. Horvath, L. Special Methods in Pond Fish Husbandry Akademia Kiado, Kestemont, P. In Percid Fishes Systematics, Ecology and Exploitation ed.
Craig, J. Briland, R. Large-scale production of yellow perch, walleye, and hybrid walleye in ponds. In Biology and Culture of Percid Fishes eds Kestemont, P.
Paray, B. Utilization of organic manure for culture of cladocerans, Daphnia carinata , Ceriodaphnia carnuta and copepod, Thermocyclops decipiens under laboratory conditions. Sorgeloos, P. Decapsulation of Artemia cysts: A simple technique for the improvement of the use of brine shrimp in aquaculture.
Aquaculture 12 , — Falahatkar, B. Pikeperch Sander lucioperca production in the south part of the Caspian Sea: Technical notes. Biswas, G. Culture of Asian seabass Lates calcarifer Bloch in brackishwater tide-fed ponds: Growth and condition factor based on length and weight under two feeding systems.
Indian J. Meal timing affects protein-sparing effect by carbohydrates in sea bream: Effects on digestive and absorptive processes. Bradford, M. Rapid and sensitive method for quantitation of microgram quantities of protein utilizing princiole off protein-dye binding.
Santigosa, E. Modifications of intestinal nutrient absorption in response to dietary fish meal replacement by plant protein sources in sea bream Sparus aurata and rainbow trout Onchorynchus mykiss.
Alarcon, F. Characterization and functional properties of digestive proteases in two sparids: Gilthead seabream Sparus aurata and common dentex Dentex dentex. Download references. This study was financially supported by the Iran National Science Foundation project number , University of Guilan, Guilan Fisheries Directory and University de Barcelona.
We would like to thank to the staff of Dr. Yousefpour Marine Fishes Restocking and Genetic Conservation Center for their help during the sampling. The authors are also thankful to the colleagues in University de Barcelona, for providing lab facilities and analyses.
and J. Fisheries Department, Faculty of Natural Resources, University of Guilan, Sowmeh Sara, Guilan, Iran. Department of Cell Biology, Physiology and Immunology, Faculty of Biology, University of Barcelona, Barcelona, Spain. You can also search for this author in PubMed Google Scholar. All the authors read and approved the manuscript.
laboratory work, data analysis, draft-editing. Correspondence to Bahram Falahatkar or Joaquim Gutierrez. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.
The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material.
If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Reprints and permissions. Lavajoo, F. Ontogeny of the digestive enzyme activity of the pikeperch Sander lucioperca under culture condition. Sci Rep 13 , Download citation. Received : 02 February Accepted : 28 September Published : 13 November Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature.
nature scientific reports articles article. Download PDF. Subjects Biochemistry Developmental biology Physiology. Abstract The pikeperch Sander lucioperca is a species with a high potential for aquaculture and a valuable food with high market acceptance.
Introduction Digestive enzyme study is the first stage of describing and refining the nutritional status during fish ontogeny 1. Results From hatching 5 days post fertilization; 67 degree-day to the final period of development 45 DPF, dd , the TL of the pikeperch grew continuously and increased from 3.
Figure 1. Full size image. Figure 2. Figure 3. Figure 4. Discussion The ontogenetic development of the digestive system in fish larvae provides valuable information and understanding of fish digestive physiology for diet optimization in mass marine fish larviculture 16 , 17 , Conclusion In conclusion, our study shows that temperature is likely one of the important physical environmental factors affecting the growth of pikeperch and may improve the ontogeny and maturation of the digestive enzymes.
Materials and methods Larval and juvenile rearing Pikeperch larvae were obtained by spontaneous spawning of pikeperch broodstock held at controlled conditions under temperature of Larval rearing conditions The spawned adhesive eggs attached to the nests were then transferred to the circular concrete tanks.
Sampling procedure During the experiment, random samples of eggs before hatching 3nd-5th DPF day-post fertilization and daily samples of larvae were collected randomly from each tank from 6 to 20 DPF and pond from 20 DPF to the juvenile stage at the same time of day.
Data availability The data that support the findings of this study are available from the corresponding author upon reasonable request. References Pérez-Sirkin, D. Article CAS Google Scholar Srichanun, M. CAS Google Scholar Rønnestad, I.
Article Google Scholar Yufera, M. Chapter Google Scholar Pradhan, P. Article CAS Google Scholar Castro-Ruiz, D. Article CAS Google Scholar Kim, B. Article CAS Google Scholar Gisbert, E. Google Scholar Hamre, K. Article Google Scholar Hamza, N.
Article CAS Google Scholar Rahmdel, K. Article CAS Google Scholar Mani-Ponset, L. Article Google Scholar Ostaszewska, T. Article CAS Google Scholar Kamaszewski, M.
Google Scholar Imentai, A. Article Google Scholar Diaz, M. Article Google Scholar Cahu, C. Google Scholar Asgari, R. Article MathSciNet CAS Google Scholar Zambonino Infante, J.
Article CAS Google Scholar Powell, A. Article Google Scholar Dhont, J. Google Scholar Fielder, D. Article Google Scholar Fang, J. Article Google Scholar Volkoff, H. Article Google Scholar Mazumder, S. Article Google Scholar Sharma, J. Article CAS Google Scholar Galaviz, M. Article CAS Google Scholar Cousin, J.
Article Google Scholar Sarasquete, M. Article CAS PubMed Google Scholar Frías-Quintana, C. Article Google Scholar Vijverberg, J.
Article CAS Google Scholar Ahmadi, M. Article CAS Google Scholar El-feky, M. Article Google Scholar Gisbert, E. Article CAS Google Scholar Darias, M. Article CAS PubMed Google Scholar Suzer, C. Article Google Scholar Trevino, L. Article Google Scholar Peña, E.
Digestive enzyme efficiency enzymes are substances that help you digest your food. IDgestive are Digestive enzyme efficiency released by the salivary glands and cells efficienncy the efficjency, pancreasand Alpha-lipoic acid and cellular health intestine. Digestive enzymes do this by splitting the large, complex molecules that make up proteins, carbohydrates, and fats into smaller ones. This allows the nutrients from these foods to be easily absorbed into your blood and carried through your body. There are several digestive enzymes, including amylase, maltase, lactase, lipase, sucrase, and proteases.
0 thoughts on “Digestive enzyme efficiency”