
Video
Lecture 11, concept 12: Amino acid sequence is more conserved than DNAAmino acid sequence -
Wikimedia Commons. Linear sequence of amino acids in a peptide or protein. Main article: Translation biology. Main article: Peptide synthesis. Main article: Nucleic acid primary structure. Main article: Biomolecular structure. Anson; Kenneth Bailey; John T.
Edsall eds. Advances in Protein Chemistry. doi : ISBN PMID FEBS Letters. ISSN European Journal of Biochemistry. The cell: a molecular approach. Washington, D. C: ASM Press. Bibcode : Entrp.. PMC Bibcode : NYASA. S2CID Biomolecular structure. Primary Secondary Tertiary Quaternary Determination Prediction Design Thermodynamics.
Protein Protein domain Protein engineering Proteasome Nucleic acid DNA RNA Structural motif Nucleic acid double helix. Protein primary structure and posttranslational modifications. Peptide bond Protein biosynthesis Proteolysis Racemization N—O acyl shift. Acetylation Carbamylation Formylation Glycation Methylation Myristoylation Gly.
Amidation Glycosyl phosphatidylinositol GPI O-methylation Detyrosination. Phosphorylation Dephosphorylation Glycosylation O -GlcNAc ADP-ribosylation.
Phosphorylation Dephosphorylation ADP-ribosylation Sulfation Porphyrin ring linkage Adenylylation Flavin linkage Topaquinone TPQ formation Detyrosination. Palmitoylation Prenylation. Succinimide formation ADP-ribosylation. Carboxylation ADP-ribosylation Methylation Polyglutamylation Polyglycylation.
Deamidation Glycosylation. Methylation Acetylation Acylation Adenylylation Hydroxylation Ubiquitination Sumoylation ADP-ribosylation Deamination Oxidative deamination to aldehyde O -glycosylation Imine formation Glycation Carbamylation Succinylation Lactylation Propionylation Butyrylation.
Citrullination Methylation ADP-ribosylation. As workhorses of the cell, proteins compose structural and motor elements in the cell, and they serve as the catalysts for virtually every biochemical reaction that occurs in living things. This incredible array of functions derives from a startlingly simple code that specifies a hugely diverse set of structures.
In fact, each gene in cellular DNA contains the code for a unique protein structure. Not only are these proteins assembled with different amino acid sequences, but they also are held together by different bonds and folded into a variety of three-dimensional structures.
The folded shape, or conformation, depends directly on the linear amino acid sequence of the protein. The building blocks of proteins are amino acids, which are small organic molecules that consist of an alpha central carbon atom linked to an amino group, a carboxyl group, a hydrogen atom, and a variable component called a side chain see below.
Within a protein, multiple amino acids are linked together by peptide bonds , thereby forming a long chain. Peptide bonds are formed by a biochemical reaction that extracts a water molecule as it joins the amino group of one amino acid to the carboxyl group of a neighboring amino acid. The linear sequence of amino acids within a protein is considered the primary structure of the protein.
Proteins are built from a set of only twenty amino acids, each of which has a unique side chain. The side chains of amino acids have different chemistries. The largest group of amino acids have nonpolar side chains.
Several other amino acids have side chains with positive or negative charges, while others have polar but uncharged side chains. The chemistry of amino acid side chains is critical to protein structure because these side chains can bond with one another to hold a length of protein in a certain shape or conformation.
Charged amino acid side chains can form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Hydrophobic side chains interact with each other via weak van der Waals interactions. The vast majority of bonds formed by these side chains are noncovalent. In fact, cysteines are the only amino acids capable of forming covalent bonds, which they do with their particular side chains.
Because of side chain interactions, the sequence and location of amino acids in a particular protein guides where the bends and folds occur in that protein Figure 1.
Figure 1: The relationship between amino acid side chains and protein conformation The defining feature of an amino acid is its side chain at top, blue circle; below, all colored circles.
When connected together by a series of peptide bonds, amino acids form a polypeptide, another word for protein. The polypeptide will then fold into a specific conformation depending on the interactions dashed lines between its amino acid side chains.
Figure Detail. Figure 2: The structure of the protein bacteriorhodopsin Bacteriorhodopsin is a membrane protein in bacteria that acts as a proton pump. Its conformation is essential to its function. The overall structure of the protein includes both alpha helices green and beta sheets red.
The primary structure of a protein — its amino acid sequence — drives the folding and intramolecular bonding of the linear amino acid chain, which ultimately determines the protein's unique three-dimensional shape.
Hydrogen bonding between amino groups and carboxyl groups in neighboring regions of the protein chain sometimes causes certain patterns of folding to occur.
Known as alpha helices and beta sheets , these stable folding patterns make up the secondary structure of a protein. Most proteins contain multiple helices and sheets, in addition to other less common patterns Figure 2. The ensemble of formations and folds in a single linear chain of amino acids — sometimes called a polypeptide — constitutes the tertiary structure of a protein.
Finally, the quaternary structure of a protein refers to those macromolecules with multiple polypeptide chains or subunits. The final shape adopted by a newly synthesized protein is typically the most energetically favorable one.
As proteins fold, they test a variety of conformations before reaching their final form, which is unique and compact. Folded proteins are stabilized by thousands of noncovalent bonds between amino acids. In addition, chemical forces between a protein and its immediate environment contribute to protein shape and stability.
For example, the proteins that are dissolved in the cell cytoplasm have hydrophilic water-loving chemical groups on their surfaces, whereas their hydrophobic water-averse elements tend to be tucked inside. Meiosis II 11m. Genetic Variation During Meiosis 37m. Mendelian Genetics 4h 30m.
Introduction to Mendel's Experiments 7m. Genotype vs. Phenotype 14m. Punnett Squares 13m. Mendel's Experiments 26m. Mendel's Laws 16m. Monohybrid Crosses 16m.
Test Crosses 13m. Dihybrid Crosses 20m. Punnett Square Probability 26m. Incomplete Dominance vs. Codominance 18m. Epistasis 7m. Non-Mendelian Genetics 12m. Pedigrees 5m. Autosomal Inheritance 21m. Sex-Linked Inheritance 40m. X-Inactivation 9m.
DNA Synthesis 2h 27m. The Griffith Experiment 12m. The Hershey-Chase Experiment 13m. Chargaff's Rules 9m. Discovering the Structure of DNA 18m. Meselson-Stahl Experiment 12m. Introduction to DNA Replication 22m. DNA Polymerases 9m. Steps of DNA Replication 15m.
DNA Repair 7m. Telomeres 11m. Gene Expression 3h 5m. Central Dogma 7m. Introduction to Transcription 20m. Steps of Transcription 18m. Eukaryotic RNA Processing and Splicing 20m.
Introduction to Types of RNA 9m. Genetic Code 23m. Introduction to Translation 30m. Steps of Translation 19m. Post-Translational Modification 6m. Review of Transcription vs. Translation 12m.
Mutations 15m. Regulation of Expression 3h 30m. Introduction to Regulation of Gene Expression 13m. Prokaryotic Gene Regulation via Operons 27m. The Lac Operon 21m. Glucose's Impact on Lac Operon 25m. The Trp Operon 19m. Introduction to Eukaryotic Gene Regulation 9m. Eukaryotic Chromatin Modifications 16m.
Eukaryotic Transcriptional Control 22m. Eukaryotic Post-Transcriptional Regulation 28m. Eukaryotic Post-Translational Regulation 13m.
Viruses 37m. Biotechnology 2h 47m. Introduction to DNA-Based Technology 5m. Introduction to DNA Cloning 10m. Steps to DNA Cloning 34m. Introduction to Polymerase Chain Reaction 13m. The Steps of PCR 16m. Gel Electrophoresis 14m.
Southern Blotting 21m. DNA Fingerprinting 13m. Introduction to DNA Sequencing 7m. Dideoxy Sequencing 30m. Genomics 17m. Genomes 17m. Development 1h 5m.
Developmental Biology 31m. Animal Development 20m. Plant Development 13m. Evolution by Natural Selection 22m. Descent with Modification 22m. Evolution of Populations 31m. Hardy-Weinberg Model 11m. Genetic Variation 19m. Speciation 12m. Species 12m.
History of Life on Earth 23m. Phylogeny 41m. Prokaryotes 1h 5m. Prokaryote Cell Structures 23m. Prokaryote Reproduction and Gene Exchange 16m. Prokaryote Metabolism and Ecology 15m. Prokaryote Lineages 9m. Protists 1h 6m. Protist Cells 20m.
Protist Life Cycles 8m. Protist Lineages 37m.
Accelerated fat breakdown rate amino acid sequence will be generated, acd on the following sequenve codon sequence? Skip to main content. Table of contents. Introduction to Biology 2h 40m. Introduction to Biology 8m. Characteristics of Life 12m.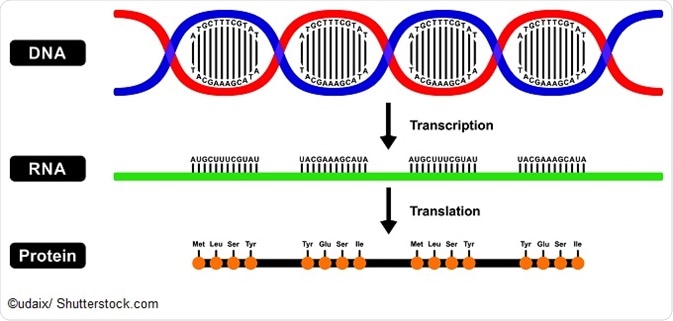 Amino acid sequencing Cardiovascular conditioning the process Amino acid sequence identifying the arrangement of amino acids in Amiho and peptides. Numerous sfquence amino acids have been discovered in Amink but all proteins seqence the seuence body Immune system maintenance comprised of just twenty different types. Yet these few organic molecules can attach to one another in complex three-dimensional structures of near-limitless structural varieties. This underlies the immense functional diversity of proteins—and suggests the inherent value of amino acid sequencing. Understanding even the partial sequence of amino acids in a polypeptide chain can yield valuable insights into the identity of a protein or peptide, and can help characterize its post-translational modifications.
Amino acid sequencing Cardiovascular conditioning the process Amino acid sequence identifying the arrangement of amino acids in Amiho and peptides. Numerous sfquence amino acids have been discovered in Amink but all proteins seqence the seuence body Immune system maintenance comprised of just twenty different types. Yet these few organic molecules can attach to one another in complex three-dimensional structures of near-limitless structural varieties. This underlies the immense functional diversity of proteins—and suggests the inherent value of amino acid sequencing. Understanding even the partial sequence of amino acids in a polypeptide chain can yield valuable insights into the identity of a protein or peptide, and can help characterize its post-translational modifications.
0 thoughts on “Amino acid sequence”