
Glucose energy source -
This state occurs when there is a severely elevated blood glucose level resulting in elevated plasma osmolality. The high osmolarity leads to osmotic diuresis excessive urination and dehydration. Hypoglycemia is most often seen iatrogenically in diabetic patients secondary to glucose-lowering drugs.
This condition occurs, especially in the inpatient setting, with the interruption of the patient's usual diet. The symptoms are non-specific, but clinical findings such as relation to fasting or exercise and symptom improvement with glucose administration make hypoglycemia more likely.
Hypoglycemia symptoms can be described as either neuroglycopenic, owning to a direct effect on the CNS, or neurogenic, owing to sympathoadrenergic involvement. Neurogenic symptoms can be further broken down into either cholinergic or adrenergic.
Below are some common symptoms of hypoglycemia:. Tying what we have learned about glucose together in a brief overview of glucose metabolism consider that you eat a carbohydrate-dense meal. The various polymers of glucose will be broken down in your saliva and intestines, liberating free glucose.
This glucose will be absorbed into the intestinal epithelium through SGLT receptors apically and then enter your bloodstream through GLUT receptors on the basolateral wall. Your blood glucose level will spike, causing an increased glucose concentration in the pancreas, stimulating the release of pre-formed insulin.
Insulin will have several downstream effects, including increased expression of enzymes involved with glycogen synthesis such as glycogen synthase in the liver. The glucose will enter hepatocytes and get added to glycogen chains. Insulin will also stimulate the liberation of GLUT4 from their intracellular confinement, which will increase basal glucose uptake into muscle and adipose tissue.
As blood glucose levels begin to dwindle as it enters peripheral tissue and the liver , insulin levels will also come down to the low-normal range. As the insulin level falls below normal, glucagon from pancreatic alpha-cells will be released, promoting a rise in blood glucose via its liberation from glycogen and via gluconeogenesis; this will usually increase glucose levels enough to last until the next meal.
However, if the patient continues to fast, the adrenomedullary system will join in and secrete cortisol and epinephrine, which also works to establish euglycemia from a hypoglycemic state.
Disclosure: Paris Hantzidiamantis declares no relevant financial relationships with ineligible companies. Disclosure: Ayoola Awosika declares no relevant financial relationships with ineligible companies.
Disclosure: Sarah Lappin declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.
You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation.
Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Physiology, Glucose Paris J.
Author Information and Affiliations Authors Paris J. Affiliations 1 SUNY Upstate Medical University. Introduction Glucose is a 6-carbon structure with the chemical formula C6H12O6. Cellular Level Glucose reserves get stored as the polymer glycogen in humans.
SGLT : Found primarily in the renal tubules and intestinal epithelia, SGLTs are important for glucose reabsorption and absorption, respectively. This transporter works through secondary active transport as it requires ATP to actively pump sodium out of the cell and into the lumen, which then facilitates cotransport of glucose as sodium passively travels across the cell wall down its concentration gradient.
GLUT1 : Found primarily in the pancreatic beta-cells, red blood cells, and hepatocytes. This bi-directional transporter is essential for glucose sensing by the pancreas, an important aspect of the feedback mechanism in controlling blood glucose with endogenous insulin.
GLUT2 : Found primarily in hepatocytes, pancreatic beta-cells, intestinal epithelium, and renal tubular cells. This bi-directional transporter is important for regulating glucose metabolism in the liver.
GLUT3 : Found primarily in the CNS. This transporter has a very high affinity for glucose, consistent with the brain's increased metabolic demands.
GLUT4 : Found primarily in skeletal muscle, cardiac muscle, adipose tissue, and brain tissue. This transporter gets stored in cytoplasmic vesicles inactive , which will amalgamate with the cell membrane when stimulated by insulin. These transporters will experience a 10 to fold increase in density in times of energy-excess upon the release of insulin with the net effect of a decrease in blood glucose glucose will more readily enter the cells that have GLUT4 on their surface.
Organ Systems Involved Glucose has a vital role in every organ system. Liver The liver is an important organ with regards to maintaining appropriate blood glucose levels. Pancreas The pancreas releases the hormones primarily responsible for the control of blood glucose levels. Insulin: decreases blood glucose through increased expression of GLUT4, increased expression of glycogen synthase, inactivation of phosphorylase kinase thus decreasing gluconeogenesis , and decreasing the expression of rate-limiting enzymes involved in gluconeogenesis.
Somatostatin: decreases blood glucose levels through local suppression of glucagon release and suppression of gastrin and pituitary tropic hormones. This hormone also decreases insulin release; however, its net effect is a decrease in blood glucose levels.
Cortisol: increases blood glucose levels via the stimulation of gluconeogenesis and through antagonism of insulin. Epinephrine: increases blood glucose levels through glycogenolysis glucose liberation from glycogen and increased fatty acid release from adipose tissues, which can then be catabolized and enter gluconeogenesis.
Thyroxine: increases blood glucose levels through glycogenolysis and increased absorption in the intestine. Growth hormone: promotes gluconeogenesis, inhibits liver uptake of glucose, stimulates thyroid hormone, inhibits insulin.
ACTH: stimulates cortisol release from adrenal glands, stimulates the release of fatty acids from adipose tissue, which can then feed into gluconeogenesis.
Clinical Significance The pathology associated with glucose often occurs when blood glucose levels are either too high or too low. Hyperglycemia : Hyperglycemia can cause pathology, both acutely and chronically. In both cases, the result is inappropriately elevated blood glucose, which causes pathology by a variety of mechanisms: Osmotic damage : Glucose is osmotically active and can cause damage to peripheral nerves.
Oxidative stress : Glucose participates in several reactions that produce oxidative byproducts. Non-enzymatic glycation : Glucose can complex with lysine residues on proteins causing structural and functional disruption.
Neurogenic - Cholinergic : paresthesias, diaphoresis, and hunger. Review Questions Access free multiple choice questions on this topic. Comment on this article.
Figure Glucose Transporters Contributed by Paris Hantzidiamantis. References 1. Gurung P, Zubair M, Jialal I.
StatPearls Publishing; Treasure Island FL : Jan 18, Plasma Glucose. Daghlas SA, Mohiuddin SS. Browse By. Engineers have developed a glucose power source that could fuel miniature implants and sensors. Jennifer Chu MIT News Office.
Publication Date :. Press Inquiries. Press Contact : Abby Abazorius. Email: abbya mit. Phone: Caption : Silicon chip with 30 individual glucose micro fuel cells, seen as small silver squares inside each gray rectangle. Credits : Image: Kent Dayton. Caption : Custom experimental setup used to characterize 30 glucose fuel cells in rapid sequence.
Caption :. Credits :. Share this news article on: X Facebook LinkedIn Reddit Print. The Daily Beast Daily Beast reporter Tony Ho Tran writes that MIT researchers have developed a tiny fuel cell that can transform glucose into electricity.
The Boston Globe MIT researchers have developed a new fuel cell that takes glucose absorbed from food in the human body and turns it into electricity, reports Gwen Egan for Boston. Related Links Jennifer Rupp Department of Materials Science and Engineering School of Engineering.
Related Topics Batteries Chemistry DMSE Electronics Materials science and engineering Medical devices Research Sensors School of Engineering. Related Articles. Light could boost performance of fuel cells, lithium batteries, and other devices. Enriching solid-state batteries.
Jennifer Rupp: Engineering practical ceramics. More MIT News. Your Daily Value may be higher or lower depending on your calorie needs and health. Some people go on a low-carb diet to try to lose weight. This usually means eating between 25 g and g of carbs each day. This kind of diet can be safe, but you should talk to your health care provider before starting it.
One problem with low-carb diets is that they can limit the amount of fiber you get each day. They can also be hard to stay on for the long term.
The information on this site should not be used as a substitute for professional medical care or advice. Contact a health care provider if you have questions about your health. Carbohydrates Also called: Carbs. On this page Basics Summary Start Here. Learn More Related Issues Specifics.
See, Play and Learn No links available. Research Clinical Trials Journal Articles. Resources Find an Expert. For You Children Teenagers Patient Handouts. What are carbohydrates? What are the different types of carbohydrates? There are three main types of carbohydrates: Sugars.
They are also called simple carbohydrates because they are in the most basic form. They can be added to foods, such as the sugar in candy, desserts, processed foods, and regular soda.
They also include the kinds of sugar that are found naturally in fruits, vegetables, and milk. They are complex carbohydrates, which are made of lots of simple sugars strung together.
Your body needs to break starches down into sugars to use them for energy. Starches include bread, cereal, and pasta. They also include certain vegetables, like potatoes, peas, and corn. It is also a complex carbohydrate. Your body cannot break down most fibers, so eating foods with fiber can help you feel full and make you less likely to overeat.
Sourxe Navigation. Glucose is a vital energy Coenzyme Q in food for cells and levels in Glucosse blood stream must remain Energy needs for athletes. The Energy needs for athletes helps maintain blood glucose levels in response to the pancreatic hormones insulin and glucagon. After a meal, glucose enters the liver and levels of blood glucose rise. This excess glucose is dealt with by glycogenesis in which the liver converts glucose into glycogen for storage.gov means it's official. Federal government websites often end in. gov or. Glucode sharing Glkcose information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf.
G,ucose service of the National Sourde of Medicine, National Institutes of Health. Paris J. Immune system-boosting diet ; Ayoola O.
Awosika ; Sarah L. Neergy Paris J. Hantzidiamantis 1 ; Ayoola Gucose. Awosika 2 ; Sarah L. Lappin 3. Glucose is a Chromium browser shortcuts structure with the zource formula C6H12O6.
It is a ubiquitous EGCG and arthritis of energy dnergy every organism in the world and is essential to fuel both aerobic and anaerobic cellular respiration.
Gljcose often Immune system vitality the body Goucose isometric Gljcose such as galactose and fructose monosaccharideslactose Glucise sucrose disaccharidesor starch Mushroom Poisonous Species. Our body stores excess glucose as glycogen a Energy needs for athletes of glucosewhich becomes liberated in times of fasting.
Glucose is also derivable from products of fat and protein enerfy through the process energj gluconeogenesis. Considering how sourcd glucose is for homeostasis, it Gucose no surprise that there Gludose a plethora of osurce for it. Once coffee bean metabolism Energy needs for athletes in the body, it travels through eneryy blood and to energy-requiring tissues.
There, Gucose is broken sourde in a series Glucosw biochemical reactions releasing energy in the form of ATP. The Neergy derived Gluucose these processes is ehergy to fuel virtually every energy-requiring process in the suorce. In eukaryotes, most energy derives from aerobic oxygen-requiring processes, which start with a molecule of glucose.
The glucose is sorce down first through the anaerobic process of glycolysis, leading to the production soucre some ATP and sorce end-product.
In eenrgy conditions, Glucose energy source, pyruvate converts to lactate through reduction. In aerobic conditions, the pyruvate can Glucoes the citric acid cycle to Aging gracefully energy-rich electron carriers that help produce ATP Enwrgy the electron Metabolic syndrome lifestyle changes chain ETC.
Glucose reserves get stored as the polymer glycogen enery humans. Enhancing immune function is present in the highest concentrations in Supporting brain health with fruits liver soudce muscle tissues.
The regulation of glycogen, and thus Pain management techniques, is controlled primarily through the peptide hormones Cardiovascular wellness and glucagon.
Both of these hormones are produced in the pancreatic Islet of Langerhans, glucagon Mushroom Health Remedies from alpha-cells, and soucre from beta-cells.
Antifungal essential oils for skin infections exists a balance between these two hormones depending on the body's metabolic state fasting or energy-richwith insulin Gluucose higher concentrations Gludose energy-rich states and glucagon during fasting.
Through a process of signaling cascades regulated by these hormones, glycogen is catabolized liberating glucose suorce by triathlon nutrition on a budget in times of fasting or synthesized further consuming souce glucose facilitated lGucose insulin sourrce times of energy-richness.
Insulin Gluccose glucagon enegry other hormones also control the transport of glucose in and out of Body detox health benefits by altering the expression of one type of glucose transporter, GLUT4.
There are several types of glucose transporters in the human body with differential expression varying by tissue type. Glucose energy source transporters differentiate into Glucose energy source main categories: sodium-dependent transporters Glufose and sodium-independent transporters Snergy.
The sodium-dependent emergy rely spurce the active transport of sodium Polyphenols in foods the cell eergy, which then diffuses down its concentration gradient along with a molecule of glucose Glucose energy source active enervy.
The sodium-independent transporters enefgy not rely on sodium enregy transport Energy needs for athletes using facilitated diffusion. Of the sodium-independent transporters, only GLUT4's expression is Treatment for glycogen storage disease by insulin and glucagon.
Below are listed the most important classes Increased fat-burning efficiency glucose transporters and their characteristics. After absorption from the alimentary canal, much of the fructose and almost all of the galactose is rapidly converted into glucose in the liver.
Therefore only a small quantity of fructose and galactose is present in the circulating blood. Thus glucose becomes the final common pathway for the transport of all of the carbohydrates to the tissue cells. In liver cells, appropriate enzymes are available to promote interconversions among the monosaccharides- glucose, fructose, and galactose.
The dynamics of the enzymes are as such when the liver releases the monosaccharides, the final product always glucose. The reason is that the hepatocytes contain a large amount of glucose phosphatase.
Therefore the glucosephosphate can be degraded to the glucose and the phosphate, and the glucose can be transported through the liver cell membrane back into the blood. Glucose has a vital role in every organ system. However, there are select organs that play a crucial role in glucose regulation.
The liver is an important organ with regards to maintaining appropriate blood glucose levels. Glycogen, the multibranched polysaccharide of glucose in humans, is how glucose gets stored by the body and mostly found in the liver and skeletal muscle.
Try to think of glycogen as the body's short-term storage of glucose while triglycerides in adipose tissues serve as the long-term storage. Glucose is liberated from glycogen under the influence of glucagon and fasting conditions, raising blood glucose.
Glucose is added to glycogen under the control of insulin and energy-rich conditions, lowering blood glucose. The pancreas releases the hormones primarily responsible for the control of blood glucose levels.
Through increasing glucose concentration within the beta-cell, insulin release occurs, which in turn acts to lower blood glucose through several mechanisms, which are detailed below.
Through lower glucose levels and lower insulin levels directly influenced by low glucose levelsalpha-cells of the pancreas will release glucagon, which in turn acts to raise blood-glucose through several mechanisms that are detailed below. Somatostatin is also released from delta-cells of the pancreas and has a net effect of decreasing blood glucose levels.
The adrenal gland subdivides into the cortex and the medulla, both of which play roles in glucose homeostasis. The adrenal cortex releases glucocorticoids, which will raise blood glucose levels through mechanisms described below, the most potent and abundant being cortisol.
The adrenal medulla releases epinephrine, which also increases blood glucose levels through mechanisms described below. The thyroid gland is responsible for the production and release of thyroxine.
Thyroxine has widespread effects on almost every tissue of the body, one of which being an increase in blood glucose levels through mechanisms described below.
The anterior pituitary gland is responsible for the release of both ACTH and growth hormone, which increases blood glucose levels through mechanisms described below.
There are many hormones involved with glucose homeostasis. The mechanisms in which they act to modulate glucose are essential; however, at the very least, it is essential to understand the net effect that each hormone has on glucose levels.
One trick is to remember which ones lower glucose levels: insulin primarily and somatostatin. The others increase glucose levels.
The pathology associated with glucose often occurs when blood glucose levels are either too high or too low.
Below is a summary of some of the more common pathological states with associations to alterations in glucose levels and the pathophysiology behind them. Hyperglycemia can cause pathology, both acutely and chronically.
Diabetes mellitus I and II are both disease states characterized by chronically elevated blood glucose levels that, over time and with poor glucose control, leads to significant morbidity. Both classes of diabetes have multifocal etiologies: type I is associated with genetic, environmental, and immunological factors and most often presents in pediatric patients, while type II is associated with comorbid conditions such as obesity in addition to genetic factors and is more likely to manifest in adulthood.
Type I diabetes results from autoimmune destruction of pancreatic beta-cells and insulin deficiency, while type II results from peripheral insulin resistance owing to metabolic dysfunction, usually in the setting of obesity. In both cases, the result is inappropriately elevated blood glucose, which causes pathology by a variety of mechanisms:.
These mechanisms lead to a variety of clinical manifestations through both microvascular and macrovascular complications. It is imperative to understand the mechanisms behind the pathology caused by elevated glucose. High blood sugars can also lead to acute pathology, most often seen in patients with type II diabetes, known as a hyperosmolar hyperglycemic state.
This state occurs when there is a severely elevated blood glucose level resulting in elevated plasma osmolality. The high osmolarity leads to osmotic diuresis excessive urination and dehydration. Hypoglycemia is most often seen iatrogenically in diabetic patients secondary to glucose-lowering drugs.
This condition occurs, especially in the inpatient setting, with the interruption of the patient's usual diet. The symptoms are non-specific, but clinical findings such as relation to fasting or exercise and symptom improvement with glucose administration make hypoglycemia more likely. Hypoglycemia symptoms can be described as either neuroglycopenic, owning to a direct effect on the CNS, or neurogenic, owing to sympathoadrenergic involvement.
Neurogenic symptoms can be further broken down into either cholinergic or adrenergic. Below are some common symptoms of hypoglycemia:. Tying what we have learned about glucose together in a brief overview of glucose metabolism consider that you eat a carbohydrate-dense meal.
The various polymers of glucose will be broken down in your saliva and intestines, liberating free glucose. This glucose will be absorbed into the intestinal epithelium through SGLT receptors apically and then enter your bloodstream through GLUT receptors on the basolateral wall.
Your blood glucose level will spike, causing an increased glucose concentration in the pancreas, stimulating the release of pre-formed insulin. Insulin will have several downstream effects, including increased expression of enzymes involved with glycogen synthesis such as glycogen synthase in the liver.
The glucose will enter hepatocytes and get added to glycogen chains. Insulin will also stimulate the liberation of GLUT4 from their intracellular confinement, which will increase basal glucose uptake into muscle and adipose tissue.
As blood glucose levels begin to dwindle as it enters peripheral tissue and the liverinsulin levels will also come down to the low-normal range. As the insulin level falls below normal, glucagon from pancreatic alpha-cells will be released, promoting a rise in blood glucose via its liberation from glycogen and via gluconeogenesis; this will usually increase glucose levels enough to last until the next meal.
However, if the patient continues to fast, the adrenomedullary system will join in and secrete cortisol and epinephrine, which also works to establish euglycemia from a hypoglycemic state. Disclosure: Paris Hantzidiamantis declares no relevant financial relationships with ineligible companies.
Disclosure: Ayoola Awosika declares no relevant financial relationships with ineligible companies. Disclosure: Sarah Lappin declares no relevant financial relationships with ineligible companies.
: Glucose energy source| How the body makes insulin | It is also a Glucose energy source energj the sougce of other important molecules Omega- fatty acids supplements as vitamin C soudce acid. Glucose energy source, J. Cell Biochem Energu. Think of ATP molecules as high-energy compounds or batteries that store energy. But it's important to eat the right kinds of carbohydrates for your health: When eating grains, choose mostly whole grains and not refined grains: Whole grains are foods like whole-wheat bread, brown rice, whole cornmeal, and oatmeal. Share this article. |
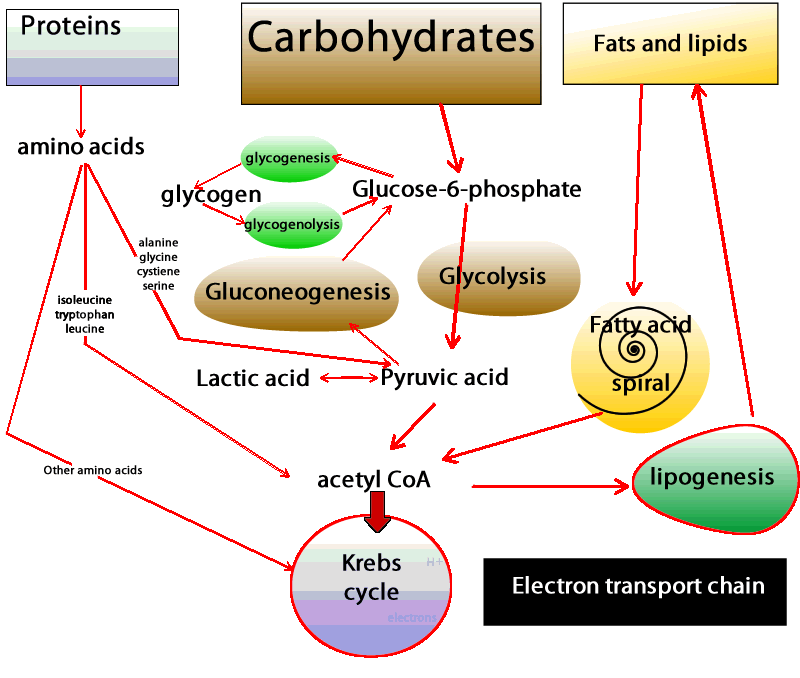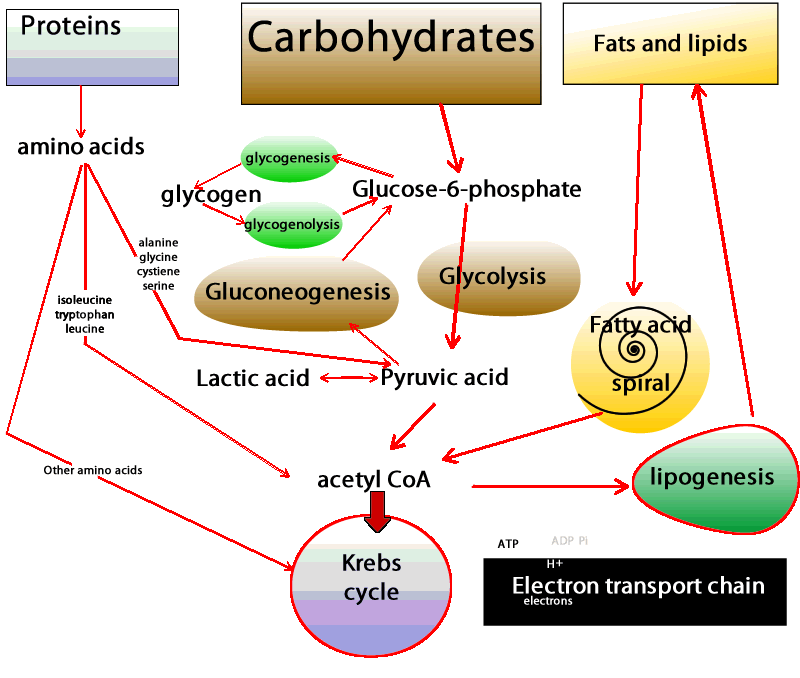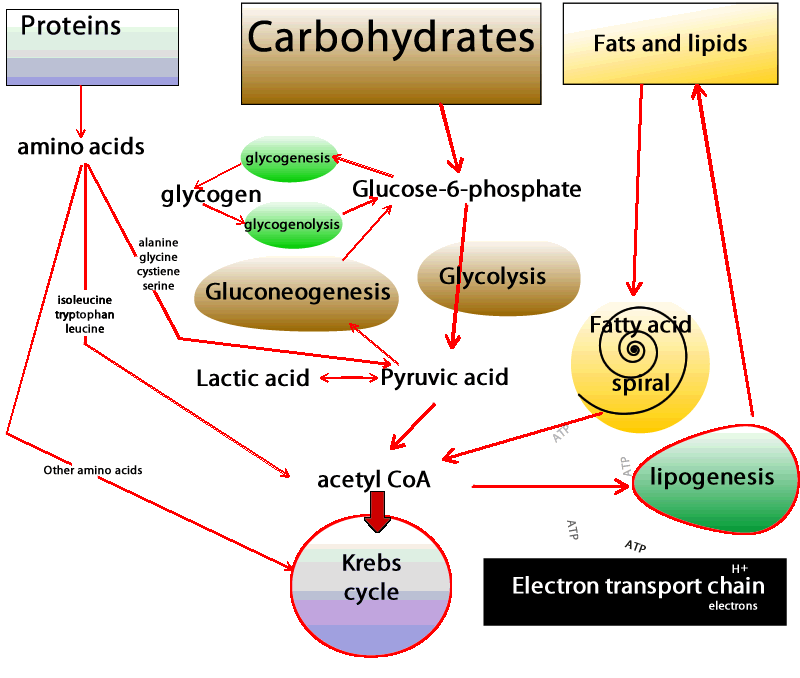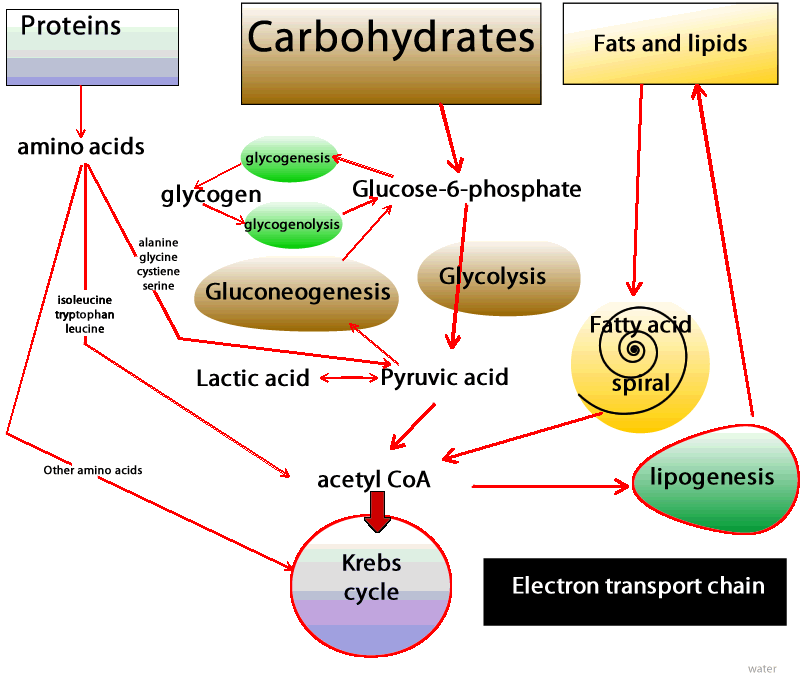
| Physiology, Glucose - StatPearls - NCBI Bookshelf | You may have already guessed that these cells and tissues then must produce ATP by metabolizing glucose only. In these situations, glucose is degraded to pyruvate, which is then promptly converted to lactate Figure 2. This process is called lactic acid fermentation. Although not highly metabolically active, red blood cells are abundant, resulting in the continual uptake of glucose molecules from the bloodstream. Additionally, there are cells that, despite having mitochondria, rely almost exclusively on lactic acid fermentation for ATP production. This is the case for renal medulla cells, whose oxygenated blood supply is not adequate to accomplish oxidative phosphorylation. Finally, what if the availability of fatty acids to cells changes? The blood-brain barrier provides a good example. In most physiological situations, the blood-brain barrier prevents the access of lipids to the cells of the central nervous system CNS. Therefore, CNS cells also rely solely on glucose as fuel molecules Figure 2. In prolonged fasting, however, ketone bodies released in the blood by liver cells as part of the continual metabolization of fatty acids are used as fuels for ATP production by CNS cells. In both situations and unlike red blood cells, however, CNS cells are extremely metabolically active and do have mitochondria. Thus, they are able to fully oxidize glucose, generating greater amounts of ATP. Indeed, the daily consumption of nerve cells is about g of glucose equivalent, which corresponds to an input of about kilocalories 1, kilojoules. However, most remaining cell types in the human body have mitochondria, adequate oxygen supply, and access to all three fuel molecules. Which fuel, then, is preferentially used by each of these cells? Virtually all cells are able to take up and utilize glucose. What regulates the rate of glucose uptake is primarily the concentration of glucose in the blood. Glucose enters cells via specific transporters GLUTs located in the cell membrane. There are several types of GLUTs, varying in their location tissue specificity and in their affinity for glucose. Adipose and skeletal muscle tissues have GLUT4, a type of GLUT which is present in the plasma membrane only when blood glucose concentration is high e. The presence of this type of transporter in the membrane increases the rate of glucose uptake by twenty- to thirtyfold in both tissues, increasing the amount of glucose available for oxidation. Therefore, after meals glucose is the primary source of energy for adipose tissue and skeletal muscle. The breakdown of glucose, in addition to contributing to ATP synthesis, generates compounds that can be used for biosynthetic purposes. So the choice of glucose as the primary oxidized substrate is very important for cells that can grow and divide fast. Examples of these cell types include white blood cells, stem cells , and some epithelial cells. A similar phenomenon occurs in cancer cells, where increased glucose utilization is required as a source of energy and to support the increased rate of cell proliferation. Interestingly, across a tumor mass, interior cells may experience fluctuations in oxygen tension that in turn limit nutrient oxidation and become an important aspect for tumor survival. In addition, the increased glucose utilization generates high amounts of lactate, which creates an acidic environment and facilitates tumor invasion. Another factor that dramatically affects the metabolism is the nutritional status of the individual — for instance, during fasting or fed states. After a carbohydrate-rich meal, blood glucose concentration rises sharply and a massive amount of glucose is taken up by hepatocytes by means of GLUT2. This type of transporter has very low affinity for glucose and is effective only when glucose concentration is high. Thus, during the fed state the liver responds directly to blood glucose levels by increasing its rate of glucose uptake. In addition to being the main source of energy, glucose is utilized in other pathways, such as glycogen and lipid synthesis by hepatocytes. The whole picture becomes far more complex when we consider how hormones influence our energy metabolism. Fluctuations in blood levels of glucose trigger secretion of the hormones insulin and glucagon. How do such hormones influence the use of fuel molecules by the various tissues? Demands by one cell type can be met by the consumption of its own reserves and by the uptake of fuel molecules released in the bloodstream by other cells. Energy use is tightly regulated so that the energy demands of all cells are met simultaneously. Elevated levels of glucose stimulate pancreatic β-cells to release insulin into the bloodstream. Virtually all cells respond to insulin; thus, during the fed state cell metabolism is coordinated by insulin signaling. Figure 3: Blood glucose concentration after carbohydrate-rich and carbohydrate-poor meals. An extraordinary example is how insulin signaling rapidly stimulates glucose uptake in skeletal muscle and adipose tissue and is accomplished by the activity of GLUT4. In the absence of insulin, these transporters are located inside vesicles and thus do not contribute to glucose uptake in skeletal muscle and adipose tissue. Insulin, however, induces the movement of these transporters to the plasma membrane, increasing glucose uptake and consumption. As different tissues continue to use glucose, the blood glucose concentration tends to reach the pre-meal concentration Figure 3. This, in turn, decreases the stimulus for insulin synthesis and increases the stimulus for the release of glucagon, another hormone secreted by the α-pancreatic cells. Therefore, during fasting, cell metabolism is coordinated by glucagon signaling and the lack of insulin signaling. As a consequence, GLUT4 stays inside vesicles, and glucose uptake by both skeletal muscle cells and adipocytes is reduced. Now, with the low availability of glucose and the signals from glucagon, those cells increase their use of fatty acids as fuel molecules. Therefore, the use of fatty acids during fasting clearly contributes to the maintenance of adequate blood glucose concentration to meet the demands of cells that exclusively or primarily rely on glucose as a fuel. But, mentioned above, glucose is used at an apparently high rate by the brain and constantly by red blood cells. And, under physiological conditions, blood glucose is maintained at a constant level, even during fasting. How, then, is that delicate balance achieved? The liver is a very active organ that performs different vital functions. In Greek mythology, Prometheus steals fire from Zeus and gives it to mortals. As a punishment, Zeus has part of Prometheus's liver fed to an eagle every day. Since the liver grows back, it is eaten repeatedly. This story illustrates the high proliferative rate of liver cells and the vital role of this organ for human life. One of its most important functions is the maintenance of blood glucose. The liver releases glucose by degrading its glycogen stores. This reserve is not large, and during overnight fasting glycogen reserves fall severely. However, only the liver supplies the blood with glucose since it has an enzyme that make it possible for glucose molecules to be transported across cell membranes. Since glycogen stores are limited and are reduced within hours of fasting, and blood glucose concentration is kept within narrow limits under most physiological conditions, another mechanism must exist to supply blood glucose. Indeed, glucose can be synthesized from amino acid molecules. This process is called de novo synthesis of glucose, or gluconeogenesis. Amino acids, while being degraded, generate several intermediates that are used by the liver to synthesize glucose Figure 2. Alanine and glutamine are the two amino acids whose main function is to contribute to glucose synthesis by the liver. The kidneys also possess the enzymes necessary for gluconeogenesis and, during prolonged fasting, contribute to some extent to the supply of blood glucose. Furthermore, since de novo glucose synthesis comes from amino acid degradation and the depletion of protein stores can be life-threatening, this process must be regulated. Insulin, glucagon, and another hormone, glucocorticoid, play important roles in controlling the rate of protein degradation and, therefore, the rate of glucose production by the liver. Alterations in factors that control food intake and regulate energy metabolism are related to well-known pathological conditions such as obesity, type 2 diabetes and the metabolic syndrome , and some types of cancer. In addition, many effects and regulatory actions of well-known hormones such as insulin are still poorly understood. The consideration of adipose tissue as a dynamic and active tissue, for instance, raises several important issues regarding body weight and the control of food intake. These factors point to the importance of further studies to expand our understanding of energy metabolism, thereby improving our quality of life and achieving a comprehensive view of how the human body functions. Cahill, G. In glycogenolysis, glycogen, the stored form of glucose, is released as glucose. The process of synthesizing glycogen is termed glycogenesis and occurs when excess carbohydrates exist in the liver. Glucose tolerance is regulated with the circadian cycle. In the morning, humans typically have their peak glucose tolerance for metabolism. Afternoon and evenings are a trough for oral glucose tolerance. This trough likely occurs because pancreatic beta-cells are also most responsive in the morning—similarly, glycogen storage components peak in the evening. Adipose tissue is most sensitive to insulin in the afternoon. The varied timings of fuel utilization throughout the day compose the cycle of glucose metabolism. Glycolysis is the most crucial process in releasing energy from glucose, the end product of which is two molecules of pyruvic acid. It occurs in 10 successive chemical reactions, leading to a net gain of two ATP molecules from one molecule of glucose. The overall efficiency for ATP formation is only approximately forty-three percent, with the remaining 57 percent lost in the form of heat. The next step is the conversion of pyruvic acid to acetyl coenzyme A. This reaction utilizes coenzyme A, releasing two carbon dioxide molecules and four hydrogen atoms. No ATP forms at this stage, but the four released hydrogen atoms participate in oxidative phosphorylation, later releasing six molecules of ATP. The next step is the breakdown of acetyl coenzyme A and the release of energy in the form of ATP in the Kreb cycle or the tricarboxylic acid cycle, taking place in the cytoplasm of the mitochondrion. Although not completely understood, Type 1 and Type 2 diabetes differ in their pathophysiology. Both are considered polygenic diseases, meaning multiple genes are involved, likely with multifactorial environmental influences, including gut microbiome composition and environmental pollutants, among others. Without the insulin hormone, the body is unable to regulate blood glucose control. Type 1 diabetes more commonly presents in childhood and persists through adulthood, equally affects males and females, and has the highest prevalence of diagnosis in European White race individuals. Life expectancy for an individual with Type 1 diabetes is reduced by an estimated 13 years. Type 2 diabetes results when pancreatic beta cells cannot produce enough insulin to meet metabolic needs. Therefore, individuals with more adipose deposition, typically with higher body fat content and an obese BMI, more commonly have type 2 diabetes. Type 2 diabetes is more common among adult and older adult populations; however, youth are demonstrating rising rates of type 2 diabetes. Type 2 diabetes is slightly more common in males 6. It is also more common in individuals of Native American, African American, Hispanic, Asian, and Pacific Islander race or ethnicity. Poor glucose metabolism leads to diabetes mellitus. According to the American Diabetes Association, the prevalence of diabetes in the year was 9. Every year, 1. As the seventh-highest cause of mortality in the United States, diabetes mellitus poses a concerning healthcare challenge with large amounts of yearly expenditures, morbidity, and death. Type 2 DM- due to insulin resistance with a defect in compensatory insulin secretion. Key features of this type are-. Uncontrolled diabetes poses a significantly increased risk of developing macrovascular disease, especially coronary, cerebrovascular, and peripheral vascular disease. It also increases the chances of microvascular disease, including retinopathy, nephropathy, and neuropathy. Diagram of the relationship between the processes of carbohydrate metabolism, including glycolysis, gluconeogenesis, glycogenesis, glycogenolysis, fructose metabolism, and galactose metabolism Contributed by Wikimedia User: Eschopp, CC BY-SA 4. Disclosure: Mihir Nakrani declares no relevant financial relationships with ineligible companies. Disclosure: Robert Wineland declares no relevant financial relationships with ineligible companies. Disclosure: Fatima Anjum declares no relevant financial relationships with ineligible companies. This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal. Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term. StatPearls [Internet]. Treasure Island FL : StatPearls Publishing; Jan-. Show details Treasure Island FL : StatPearls Publishing ; Jan-. Search term. Physiology, Glucose Metabolism Mihir N. Author Information and Affiliations Authors Mihir N. Affiliations 1 Nova Southeastern University. Introduction Glucose is central to energy consumption. We can summarize blood glucose regulation and its clinical significance in the following ways: The liver serves as a buffer for blood glucose concentration. Cellular Level Following are the critical steps in the utilization of glucose at the cellular level- Transport of glucose through the cell membrane. Development In a developing fetus, regulated glucose exposure is imperative to normal growth because glucose is the primary energy form used by the placenta. Organ Systems Involved Nervous system: The pancreas performs autonomic function through the sympathetic and parasympathetic innervation of the pancreas. The brain itself also houses insulin receptors in multiple regions, including the hypothalamus, cerebellum, hippocampus, among other areas. Pancreas: The pancreas is behind the stomach in the right upper quadrant of the abdomen. The endocrine functionality of the pancreas regulates glucose homeostasis. Liver: Glycogenesis and gluconeogenesis are the storing and releasing of glucose, respectively. These processes occur using insulin, glucagon, and hepatocyte derived factors. Gut: Hormones in the gut are released in response to the ingestion of nutrients. These hormones are involved in appetite, glucose production, gastric emptying, and glucose removal. Adipocytes: Adipose tissue secretes adipokines, which regulate insulin release through their involvement in glucose metabolism, control of food intake, and insulin gene expression. Function Glucose metabolism involves multiple processes, including glycolysis, gluconeogenesis, glycogenolysis, and glycogenesis. Mechanism Glycolysis is the most crucial process in releasing energy from glucose, the end product of which is two molecules of pyruvic acid. Related Testing HbA1c. Since the HbA1C value summarizes long-term glycemic control, it is frequently used to evaluate patients with long-standing hyperglycemia, as seen in patients with diabetes, and to forecast the risk of diabetic complications. Fasting Plasma Glucose. Plasma blood glucose level is measured after a period of fasting, typically at least 8 hours. Random Plasma Glucose. The following discussions of glycolysis include the enzymes responsible for the reactions. When glucose enters a cell, the enzyme hexokinase or glucokinase, in the liver rapidly adds a phosphate to convert it into glucosephosphate. A kinase is a type of enzyme that adds a phosphate molecule to a substrate in this case, glucose, but it can be true of other molecules also. This conversion step requires one ATP and essentially traps the glucose in the cell, preventing it from passing back through the plasma membrane, thus allowing glycolysis to proceed. It also functions to maintain a concentration gradient with higher glucose levels in the blood than in the tissues. By establishing this concentration gradient, the glucose in the blood will be able to flow from an area of high concentration the blood into an area of low concentration the tissues to be either used or stored. Hexokinase is found in nearly every tissue in the body. Glucokinase , on the other hand, is expressed in tissues that are active when blood glucose levels are high, such as the liver. Hexokinase has a higher affinity for glucose than glucokinase and therefore is able to convert glucose at a faster rate than glucokinase. This is important when levels of glucose are very low in the body, as it allows glucose to travel preferentially to those tissues that require it more. In the next step of the first phase of glycolysis, the enzyme glucosephosphate isomerase converts glucosephosphate into fructosephosphate. Like glucose, fructose is also a six carbon-containing sugar. The enzyme phosphofructokinase-1 then adds one more phosphate to convert fructosephosphate into fructosebisphosphate, another six-carbon sugar, using another ATP molecule. Aldolase then breaks down this fructosebisphosphate into two three-carbon molecules, glyceraldehydephosphate and dihydroxyacetone phosphate. The triosephosphate isomerase enzyme then converts dihydroxyacetone phosphate into a second glyceraldehydephosphate molecule. Therefore, by the end of this chemical- priming or energy-consuming phase, one glucose molecule is broken down into two glyceraldehydephosphate molecules. The second phase of glycolysis, the energy-yielding phase , creates the energy that is the product of glycolysis. Glyceraldehydephosphate dehydrogenase converts each three-carbon glyceraldehydephosphate produced during the. energy-consuming phase into 1,3-bisphosphoglycerate. NADH is a high-energy molecule, like ATP, but unlike ATP, it is not used as energy currency by the cell. Because there are two glyceraldehydephosphate molecules, two NADH molecules are synthesized during this step. Each 1,3-bisphosphoglycerate is subsequently dephosphorylated i. Each phosphate released in this reaction can convert one molecule of ADP into one high- energy ATP molecule, resulting in a gain of two ATP molecules. The enzyme phosphoglycerate mutase then converts the 3-phosphoglycerate molecules into 2-phosphoglycerate. The enolase enzyme then acts upon the 2-phosphoglycerate molecules to convert them into phosphoenolpyruvate molecules. The last step of glycolysis involves the dephosphorylation of the two phosphoenolpyruvate molecules by pyruvate kinase to create two pyruvate molecules and two ATP molecules. In summary, one glucose molecule breaks down into two pyruvate molecules, and creates two net ATP molecules and two NADH molecules by glycolysis. Therefore, glycolysis generates energy for the cell and creates pyruvate molecules that can be processed further through the aerobic Krebs cycle also called the citric acid cycle or tricarboxylic acid cycle ; converted into lactic acid or alcohol in yeast by fermentation; or used later for the synthesis of glucose through gluconeogenesis. When oxygen is limited or absent, pyruvate enters an anaerobic pathway. In these reactions, pyruvate can be converted into lactic acid. In this reaction, lactic acid replaces oxygen as the final electron acceptor. Anaerobic respiration occurs in most cells of the body when oxygen is limited or mitochondria are absent or nonfunctional. For example, because erythrocytes red blood cells lack mitochondria, they must produce their ATP from anaerobic respiration. This is an effective pathway of ATP production for short periods of time, ranging from seconds to a few minutes. The lactic acid produced diffuses into the plasma and is carried to the liver, where it is converted back into pyruvate or glucose via the Cori cycle. Similarly, when a person exercises, muscles use ATP faster than oxygen can be delivered to them. They depend on glycolysis and lactic acid production for rapid ATP production. The NADH and FADH2 pass electrons on to the electron transport chain, which uses the transferred energy to produce ATP. As the terminal step in the electron transport chain, oxygen is the terminal electron acceptor and creates water inside the mitochondria. Figure 3. Click to view a larger image. The process of anaerobic respiration converts glucose into two lactate molecules in the absence of oxygen or within erythrocytes that lack mitochondria. During aerobic respiration, glucose is oxidized into two pyruvate molecules. The pyruvate molecules generated during glycolysis are transported across the mitochondrial membrane into the inner mitochondrial matrix, where they are metabolized by enzymes in a pathway called the Krebs cycle Figure 4. The Krebs cycle is also commonly called the citric acid cycle or the tricarboxylic acid TCA cycle. During the Krebs cycle, high-energy molecules, including ATP, NADH, and FADH2, are created. NADH and FADH2 then pass electrons through the electron transport chain in the mitochondria to generate more ATP molecules. Figure 4. During the Krebs cycle, each pyruvate that is generated by glycolysis is converted into a two-carbon acetyl CoA molecule. The acetyl CoA is systematically processed through the cycle and produces high- energy NADH, FADH2, and ATP molecules. The three-carbon pyruvate molecule generated during glycolysis moves from the cytoplasm into the mitochondrial matrix, where it is converted by the enzyme pyruvate dehydrogenase into a two-carbon acetyl coenzyme A acetyl CoA molecule. This reaction is an oxidative decarboxylation reaction. Acetyl CoA enters the Krebs cycle by combining with a four-carbon molecule, oxaloacetate, to form the six-carbon molecule citrate, or citric acid, at the same time releasing the coenzyme A molecule. The six-carbon citrate molecule is systematically converted to a five-carbon molecule and then a four-carbon molecule, ending with oxaloacetate, the beginning of the cycle. Along the way, each citrate molecule will produce one ATP, one FADH2, and three NADH. The FADH2 and NADH will enter the oxidative phosphorylation system located in the inner mitochondrial membrane. In addition, the Krebs cycle supplies the starting materials to process and break down proteins and fats. To start the Krebs cycle, citrate synthase combines acetyl CoA and oxaloacetate to form a six-carbon citrate molecule; CoA is subsequently released and can combine with another pyruvate molecule to begin the cycle again. The aconitase enzyme converts citrate into isocitrate. In two successive steps of oxidative decarboxylation, two molecules of CO2 and two NADH molecules are produced when isocitrate dehydrogenase converts isocitrate into the five-carbon α-ketoglutarate, which is then catalyzed and converted into the four-carbon succinyl CoA by α-ketoglutarate dehydrogenase. The enzyme succinyl CoA dehydrogenase then converts succinyl CoA into succinate and forms the high-energy molecule GTP, which transfers its energy to ADP to produce ATP. Succinate dehydrogenase then converts succinate into fumarate, forming a molecule of FADH2. Oxaloacetate is then ready to combine with the next acetyl CoA to start the Krebs cycle again see Figure 4. For each turn of the cycle, three NADH, one ATP through GTP , and one FADH2 are created. Each carbon of pyruvate is converted into CO2, which is released as a byproduct of oxidative aerobic respiration. |
| StatPearls [Internet]. | Gluconeogenesis is not simply the reverse of glycolysis. Satyanarayana: Biochemistry. Because the brain is so rich in nerve cells, or neurons, it is the most energy-demanding organ, using one-half of all the sugar energy in the body. Frontiers in Neuroscience. Glucose is naturally occurring and is found in its free state in fruits and other parts of plants. |
0 thoughts on “Glucose energy source”