
HbAc relationship with blood glucose -
This suggests that higher HbA1c levels among Blacks could reflect higher concentrations of nonfasting glycemia which they were unable to assess directly. Although intriguing, the results of this study are not consistent with the results of other studies that have assessed both fasting and post-glucose load glucose levels 23 — 26 and 1,5-anhyroglucitol To date, racial and ethnic differences in red blood cell survival, the intracellular and extracellular environment, and genetic determinants of hemoglobin glycation have not been assessed.
Such studies are needed. In addition, the important question that must be addressed is whether the observed racial and ethnic differences in hemoglobin glycation reflect a greater predisposition to diabetic complications.
Whether race modifies the association between HbA1c and microvascular and neuropathic outcomes remains unknown. Taken together, available data suggest that hemoglobin glycation has important determinants other than glycemia.
The relationship between mean blood glucose and HbA1c may not be the same in all people. An HbA1c value of 6. The ADAG Study: Average glucose AG; over 3 months vs.
HbA1c study end Ref. In the ADAG Study, the frequency of glucose assessments and the time frame of the measurements approximately glucose values obtained by each subject over 3 months reduced measurement variation and sampling time variation, leaving true biological variation as the most likely explanation for the wide confidence intervals in A1c-derived average glucose levels.
This is consistent with the observation that less of the variation in A1c-derived average glucose is explained by glycemia at near-normal HbA1c levels 6. Thus, it may not be possible to predict true average glucose with a high degree of accuracy in a given person based on his or her HbA1c result alone, and there may be systematic differences in the relationship between HbA1c and average glucose across racial and ethnic groups.
All of these issues are relevant when considering the use of HbA1c as a diagnostic criterion for diabetes. When used for diabetes management, HbA1c levels are interpreted in light of glucose-monitoring results, providing a more comprehensive understanding of the patient's actual glucose control.
When HbA1c measurements are performed in the same individual with diabetes, that person serves as his or her own control, and HbA1c provides a calibrated measure of glycemic control over time.
When used as a diagnostic test for diabetes, in the absence of corroborative blood glucose or glycated plasma protein fructosamine or albumin readings and without concurrent hemoglobinopathy or thalassemia screening, testing for hemolytic anemia and iron deficiency, and testing for liver and renal impairment, the HbA1c result may be misleading.
Similarly, failure to recognize nonglycemic hereditary components to hemoglobin glycation and neglecting potential racial and ethnic differences in hemoglobin glycation may lead to misclassification. Concordance between HbA1c and glucose or glycated plasma protein levels reduces the likelihood of misclassification by providing a confirmatory test.
HbA1c has a role in the diagnosis of diabetes when laboratory-based HbA1c assays are available, when there are no known patient factors that preclude the interpretation of HbA1c, when glucose testing is not convenient, and when glycemia is not changing rapidly and type 1 diabetes is not suspected.
However, glucose testing should be used if it is convenient, if laboratory-based HbA1c is not available, if there are known or suspected patient factors that preclude interpretation of HbA1c, or if type 1 diabetes is suspected.
Because unknown factors might also impact hemoglobin glycation, reliance on HbA1c as the sole or even the preferred diagnostic criterion may lead to persistent systematic misclassification, especially in the setting of clinically unrecognized hemoglobinopathy or thalassemia syndromes, hemolytic anemia, iron deficiency, liver or renal impairment, or intrinsic factors impacting hemoglobin glycation, including subclinical variation in red cell turnover, differences between the extracellular and intraerythrocyte environment, and genetic variation in hemoglobin glycation.
With this approach, glucose criteria are used to confirm or exclude diabetes in the context of intermediate levels of HbA1c. Using HbA1c thresholds lower and higher than suggested by the ADA for ruling out or ruling in diabetes permits one to triage patients for further glucose testing.
In the remaining one fourth of patients with intermediate levels of HbA1c between 5. Indeed, either screening with HbA1c and confirming with plasma glucose or screening with plasma glucose and confirming with HbA1c may be the most robust approach to diagnosing diabetes HbA1c criteria for the diagnosis of diabetes must be used thoughtfully and in combination with traditional glucose criteria.
Doing the same thing over and over again, such as testing HbA1c, and expecting different results is the very definition of insanity. This work was supported by the Michigan Diabetes Research and Training Center, and funded by Grant DK from the National Institute of Diabetes and Digestive and Kidney Diseases, and by grants from the U.
Public Health Service [R01 DK and UL1 RR National Center for Research Resources ], the U. Department of Veterans Affairs I01 CX , and the Juvenile Diabetes Research Foundation.
Disclosure Summary: W. has nothing to declare. has served on a Roche Advisory Board. Rahbar S An abnormal hemoglobin in red cells of diabetics. Clin Chim Acta 22 : — Google Scholar. Koenig RJ , Peterson CM , Kilo C , Cerami A , Williamson JR Hemoglobin A1c as an indicator of the degree of glucose intolerance in diabetes.
Diabetes 25 : — Koenig RJ , Peterson CM , Jones RL , Saudek C , Lehrman M , Cerami A Correlation of glucose regulation and hemoglobin A1c in diabetes mellitus. N Engl J Med : — International Expert Committee International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes.
Diabetes Care 32 : — American Diabetes Association Standards of medical care in diabetes— Diabetes Care 33 Suppl 1 : S11 — S Yudkin JS , Forrest RD , Jackson CA , Ryle AJ , Davie S , Gould BJ Unexplained variability of glycated haemoglobin in non-diabetic subjects not related to glycaemia.
Diabetologia 33 : — Cohen RM , Franco RS , Khera PK , Smith EP , Lindsell CJ , Ciraolo PJ , Palascak MB , Joiner CH Red cell life span heterogeneity in hematologically normal people is sufficient to alter HbA1c.
Blood : — Khera PK , Joiner CH , Carruthers A , Lindsell CJ , Smith EP , Franco RS , Holmes YR , Cohen RM Evidence for inter-individual variation in the glucose gradient across the human RBC membrane and its relationship to Hb1c. Diabetes 57 : — Snieder H , Sawtell PA , Ross L , Walker J , Spector TD , Leslie RD HbA 1c levels are genetically determined even in type 1 diabetes: evidence from healthy and diabetic twins.
Diabetes 50 : — Cohen RM , Snieder H , Lindsell CJ , Beyan H , Hawa MI , Blinko S , Edwards R , Spector TD , Leslie RD Evidence for independent heritability of the glycation gap glycosylation gap fraction of HbA1c in nondiabetic twins.
Diabetes Care 29 : — Cohen RM , Holmes YR , Chenier TC , Joiner CH Discordance between HbA1c and fructosamine: evidence for a glycosylation gap and its relation to diabetic nephropathy.
Diabetes Care 26 : — McCarter RJ , Hempe JM , Gomez R , Chalew SA Biological variation in HbA1c predicts risk of retinopathy and nephropathy in type 1 diabetes.
Diabetes Care 27 : — Nayak AU , Holland MR , Macdonald DR , Nevill A , Singh BM Evidence for consistency of the glycation gap in diabetes. Diabetes Care 34 : — Soranzo N , Sanna S , Wheeler E , Gieger C , Radke D , Dupuis J , Bouatia-Naji N , Langenberg C , Prokopenko I , Stolerman E , Sandhu MS , Heeney MM , Devaney JM , Reilly MP , Ricketts SL , Stewart AF , Voight BF , Willenborg C , Wright B , Altshuler D , Arking D , Balkau B , Barnes D , Boerwinkle E , Böhm B , Bonnefond A , Bonnycastle LL , Boomsma DI , Bornstein SR , Böttcher Y , Bumpstead S , Burnett-Miller MS , Campbell H , Cao A , Chambers J , Clark R , Collins FS , Coresh J , de Geus EJ , Dei M , et al.
Diabetes 59 : — Kim C , Bullard KM , Herman WH , Beckles GL Association between iron deficiency and A1c levels among adults without diabetes in the National Health and Nutrition Examination Survey, — Diabetes Care 33 : — Kirk JK , Bell RA , Bertoni AG , Arcury TA , Quandt SA , Goff DC , Narayan KM Ethnic disparities: control of glycemia, blood pressure, and LDL cholesterol among US adults with type 2 diabetes.
Ann Pharmacother 39 : — Boltri JM , Okosun IS , Davis-Smith M , Vogel RL Hemoglobin A1c levels in diagnosed and undiagnosed Black, Hispanic, and White persons with diabetes: results from NHANES — Ethn Dis 15 : — Saydah S , Cowie C , Eberhardt MS , De Rekeneire N , Narayan KMV Race and ethnic differences in glycemic control among adults with diagnosed diabetes in the United States.
Ethn Dis 17 : — Adams AS , Trinacty CM , Zhang F , Kleinman K , Grant RW , Meigs JB , Soumerai SB , Ross-Degnan D Medication adherence and racial differences in HbA1c control. Diabetes Care 31 : — Heisler M , Faul JD , Hayward RA , Langa KM , Blaum C , Weir D Mechanisms for racial and ethnic disparities in glycemic control in middle-aged and older Americans in the Health and Retirement Study.
Arch Intern Med : — Saaddine JB , Fagot-Campagna A , Rolka D , Narayan KM , Geiss L , Eberhardt M , Flegal KM Distribution of HbA1c levels for children and young adults in the U. Diabetes Care 25 : — Eldeirawi K , Lipton RB Predictors of hemoglobin A1c in a national sample of nondiabetic children.
The Third National Health and Nutrition Examination Survey, — Am J Epidemiol : — Herman WH , Ma Y , Uwaifo G , Haffner S , Kahn SE , Horton ES , Lachin JM , Montez MG , Brenneman T , Barrett-Connor E , for the Diabetes Prevention Program Research Group Differences in A1c by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program.
Diabetes Care 30 : — Viberti G , Lachin J , Holman R , Zinman B , Haffner S , Kravitz B , Heise MA , Jones NP , O'Neill MC , Freed MI , Kahn SE , Herman WH for ADOPT Study Group A Diabetes Outcome Progression Trial ADOPT : baseline characteristics of type 2 diabetic patients in North America and Europe.
Diabet Med 23 : — Herman WH , Dungan KM , Wolffenbuttel BH , Buse JB , Fahrbach JL , Jiang H , Martin S Racial and ethnic differences in mean plasma glucose, hemoglobin A1c, and 1. J Clin Endocrinol Metab 94 : — Ziemer DC , Kolm P , Weintraub WS , Vaccarino V , Rhee MK , Twombly JG , Narayan KM , Koch DD , Phillips LS Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies.
Ann Intern Med : — Selvin E , Steffes MW , Ballantyne CM , Hoogeveen RC , Coresh J , Brancati FL Racial differences in glycemic markers: a cross-sectional analysis of community-based data. Selvin E , Steffes MW , Zhu H , Matsushita K , Wagenknecht L , Pankow J , Coresh J , Brancati FL Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults.
Nathan DM , Kuenen J , Borg R , Zheng H , Schoenfeld D , Heine RJ , for the A1c-Derived Average Glucose ADAG Study Group Translating the A1C assay into estimated average glucose values.
Kilpatrick ES , Winocour PH , on behalf of the Association of British Clinical Diabetologists ABCD ABCD position statement on haemoglobin A1c for the diagnosis of diabetes.
Pract Diab Int 27 : — Saudek CD , Herman WH , Sacks DB , Bergenstal RM , Edelman D , Davidson MB A new look at screening and diagnosing diabetes mellitus. J Clin Endocrinol Metab 93 : — Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide.
Sign In or Create an Account. Endocrine Society Journals. Advanced Search. Search Menu. Article Navigation. Close mobile search navigation Article Navigation. Volume Article Contents Acknowledgments. Journal Article. Racial and Ethnic Differences in the Relationship between HbA1c and Blood Glucose: Implications for the Diagnosis of Diabetes.
Herman , William H. Herman, M. Oxford Academic. Robert M. PDF Split View Views. Cite Cite William H. Select Format Select format. ris Mendeley, Papers, Zotero. enw EndNote. bibtex BibTex. txt Medlars, RefWorks Download citation. Permissions Icon Permissions.
Open in new tab Download slide. Table 1. Black-White difference in HbA1c a. a Adjusted for fasting and 2-h glucose, age, sex, education, BMI, systolic blood pressure, diastolic blood pressure, and hemoglobin. Open in new tab. A1c-Derived Average Glucose Study.
Google Scholar Crossref. Search ADS. Hemoglobin A1c as an indicator of the degree of glucose intolerance in diabetes. Mean plasma glucose MPG was estimated by multiplying capillary blood glucose by 1.
Linear regression analysis weighted by the number of observations per subject was used to correlate MPG and HbA 1c. Among individual time points, afternoon and evening PG postlunch, predinner, postdinner, and bedtime showed higher correlations with HbA 1c than the morning time points prebreakfast, postbreakfast, and prelunch.
Knowing this relationship can help patients with diabetes and their healthcare providers set day-to-day targets for PG to achieve specific HbA 1c goals. The results of the Diabetes Control and Complications Trial DCCT , published in , and the U.
Prospective Diabetes Study, published in , established the relationship between HbA 1c levels and risks for diabetic complications in patients with type 1 and type 2 diabetes, respectively.
Based on the results of the DCCT, the American Diabetes Association ADA has published recommendations for HbA 1c and plasma glucose PG levels that are widely used 1 , 2. However, it is important that the relationship between daily patient-monitored blood glucose determinations and HbA 1c be clearly defined to enable patients and their health care providers to set appropriate daily PG testing goals to achieve HbA 1c levels representing low risks for adverse outcomes.
Several previous studies have analyzed the relationship between blood glucose BG and HbA 1c. Svendson et al. Nathan et al. However, a comprehensive analysis of the relationship of BG and HbA 1c , examining BG at different time points and using the entire data set, was never performed.
Here, we examine, in detail, the relationship between BG converted to PG and HbA 1c , using data obtained from the entire DCCT data set to better define this relationship. The DCCT data set was provided by the National Institutes of Diabetes, Digestive, and Kidney Diseases of the National Institutes of Health and was prepared by the Data Coordinating Center at George Washington University.
The DCCT was a multicenter, randomized clinical trial designed to compare intensive and conventional therapies and their relative effects on the development and progression of diabetic complications in patients with type 1 diabetes 1. The study population consisted of 1, patients with type 1 diabetes recruited by 29 centers located throughout the U.
and Canada. Patients were between 13 and 39 years of age and did not show evidence of severe diabetic complications at the time of admission into the study.
Intensive therapy consisted of three or more insulin injections daily or use of an insulin pump with the intent of achieving BG values as close to the normal range as possible. Conventional therapy consisted of one or two insulin injections per day.
Mean duration of participation was 6. After exclusions due to incomplete profiles, there were 26, HbA 1c values with corresponding seven-point profiles from 1, subjects an average of 18 HbA 1c values and corresponding profiles per patient.
For the seven-point BG profiles, capillary blood hemolysates were collected before meals, 90 min after meals, and at bedtime by patients in the home 6.
BG was measured in a central laboratory using a hexokinase enzymatic method 7. Blood for HbA 1c analysis was collected by venipuncture. HbA 1c was measured in a central laboratory using an ion-exchange high-performance liquid chromatography method 8 , 9.
Statistical analysis was performed using SAS and SPSS Chicago, IL. Mean BG was determined using area-under-the-curve analysis For each profile, the seven time points were connected by straight lines over time for a h period, and then the trapezoidal areas under each curve were determined, added together, and divided by time.
A constant BG level between bedtime and the following morning was assumed. Mean MPG and HbA 1c were calculated for each subject and used to perform least-squares linear regression analysis.
Due to variation in the number of observations per subject, the regression analysis was weighted to account for this. The relationships between individual PG time points and HbA 1c were also examined.
The results of linear regression analysis are summarized in Fig. The Pearson correlation coefficient r was 0. MPG at increasing levels of HbA 1c is shown in Table 1.
Results of regression analyses correlating HbA 1c with individual premeal and postmeal PG are summarized in Figs. All individual time points showed lower correlations than the seven-point profiles.
Prelunch and earlier PG time points showed lower correlations with HbA 1c than postlunch and later PG time points. The increasing use of HbA 1c to monitor long-term glycemic control in diabetic patients is largely the result of data from the DCCT and the U. Prospective Diabetes Study showing that HbA 1c is strongly correlated with adverse outcome risks.
For patients and health care providers, a clear understanding of the relationship between PG and HbA 1c is necessary for setting appropriate day-to-day PG testing goals with the expectation of achieving specific HbA 1c targets.
The relationship between HbA 1c and PG is complex. Many studies have shown that HbA 1c is an index of MPG over the preceding weeks to months. The level of HbA 1c at any point in time is contributed to by all circulating erythrocytes, from the oldest days old to the youngest.
However, recent PG levels i. This explains why the level of HbA 1c can increase or decrease relatively quickly with large changes in PG; it does not take days to detect a clinically meaningful change in HbA 1c after a change in MPG.
Another factor that complicates efforts to describe an accurate and precise relationship between PG and HbA 1c is that, for practical reasons, previous studies and our present study have attempted to define this relationship using a limited number of PG levels measured over a limited time period in this case, 1 day every 3 months to estimate HbA 1c.
Short-term PG levels can fluctuate markedly, particularly in patients with type 1 diabetes; this can result in significant discrepancies when attempting to estimate HbA 1c based on a single PG measurement or even a series of measurements on a single day. In this study, the time between sampling also contributes to intraindividual variation, especially for PG.
However, we have achieved greater certainty in our estimates of the relationship between PG and HbA 1c than was possible in previous studies by using a considerably larger number of patients and observations obtained over a longer period of time. The resulting strong correlation suggests that, although a single PG measurement or a single daily profile may not reliably predict HbA 1c , PG levels measured over time can provide a reasonably accurate estimation of HbA 1c.
Therefore, for any individual patient, a consistent discrepancy between patient-monitored PG determinations and estimated HbA 1c should be investigated; there may be other factors causing this discrepancy, such as improper meter use, laboratory error, a physical condition that alters red cell life span, or a variant hemoglobin interfering with the HbA 1c assay method.
With the advent of new technologies that are capable of monitoring PG on a h basis 18 , it will be interesting to see how our estimate of the relationship between PG and HbA 1c compares with estimates obtained using these technologies. Our data indicate that fasting PG alone should be used with caution as a measure of long-term glycemia.
Fasting PG tended to progressively underestimate HbA 1c and seven-point MPG at increasing PG levels. The data also suggest that postmeal PG contributes appreciably to HbA 1c ; however, all postmeal times are not equal in their contribution. We found that compared with the seven-point profiles, postbreakfast levels markedly overestimate HbA 1c , whereas postlunch levels show a relationship to HbA 1c that is very similar to that of MPG.
A previous study of patients with type 2 diabetes also found that postlunch PG is a better indicator of glycemic control than fasting PG However, that study did not examine bedtime PG, which we found also shows a relationship to HbA 1c that is very similar to that of MPG. The ADA currently recommends that patients with diabetes attempt to achieve average preprandial PG levels of 5.
Our results show estimated average preprandial PG and bedtime PG levels of 8. In summary, there is a predictable relationship between PG and HbA 1c. Understanding this relationship will allow patients with diabetes and their healthcare providers set appropriate day-to-day PG targets based on HbA 1c goals.
It is important to note that the relationship between PG and HbA 1c defined in this study only applies when HbA 1c is measured using assay methods that are certified by the National Glycohemoglobin Standardization Program as traceable to the DCCT reference method, as recommended by the ADA Fasting PG should be used with caution as a surrogate measure of MPG because it may significantly underestimate HbA 1c and, therefore, risks for complications at increasing HbA 1c levels.
The dashed line indicates the regression line. Premeal MPG and r at different testing times. Postmeal MPG and r at different testing times. We thank the DCCT study group and the Data Coordinating Center at George Washington University for providing the data set as well as the patient volunteers who participated in the DCCT.
Address correspondence and reprint requests to Curt L. Rohlfing, University of Missouri-Columbia, Department of Child Health, 1 Hospital Drive M, Columbia, MO E-mail: rohlfingc health.
Glycated glufose, or HbA1c, is HbAc relationship with blood glucose main biomarker used to hlucose long-term glycaemic control in individuals Adaptogen adrenal support HbAc relationship with blood glucose, and it HbAc relationship with blood glucose erlationship the development of complications. The aim bblood this g,ucose is to provide an overview of HbA1c to understand its role in the treatment of individuals living with diabetes. Topics discussed include recommended treatment targets, methods of measurement, causes of measurement inaccuracy and alternative means available to assess glycaemic control. HbA1c should not be interpreted in isolation; the measurement accuracy and other parameters, including treatment goals and comorbidities, need to be considered. The use of glycated haemoglobin, or HbA1c, has become the standard of assessing glycaemic control in patients with diabetes since the American Diabetes Association ADA recommended its use inVideo
HbA1c vs Glucose: What’s The Difference? HgAc Weight Management Supplement is a heterogeneous metabolic disorder HbAAc by blucose presence of hyperglycemia due to impairment of Emotional eating habits secretion, defective insulin action or both. The chronic hyperglycemia glucosd diabetes is associated with relatively Weight Management Supplement long-term microvascular complications affecting the eyes, kidneys and nerves, as well as an increased risk for cardiovascular disease CVD. The diagnostic criteria for diabetes are based on thresholds of glycemia that are associated with microvascular disease, especially retinopathy. The majority of cases of diabetes can be broadly classified into 2 categories: type 1 diabetes and type 2 diabetes, although some cases are difficult to classify. Gestational diabetes GDM refers to glucose intolerance with onset or first recognition during pregnancy. The classification of diabetes is summarized in Table 1.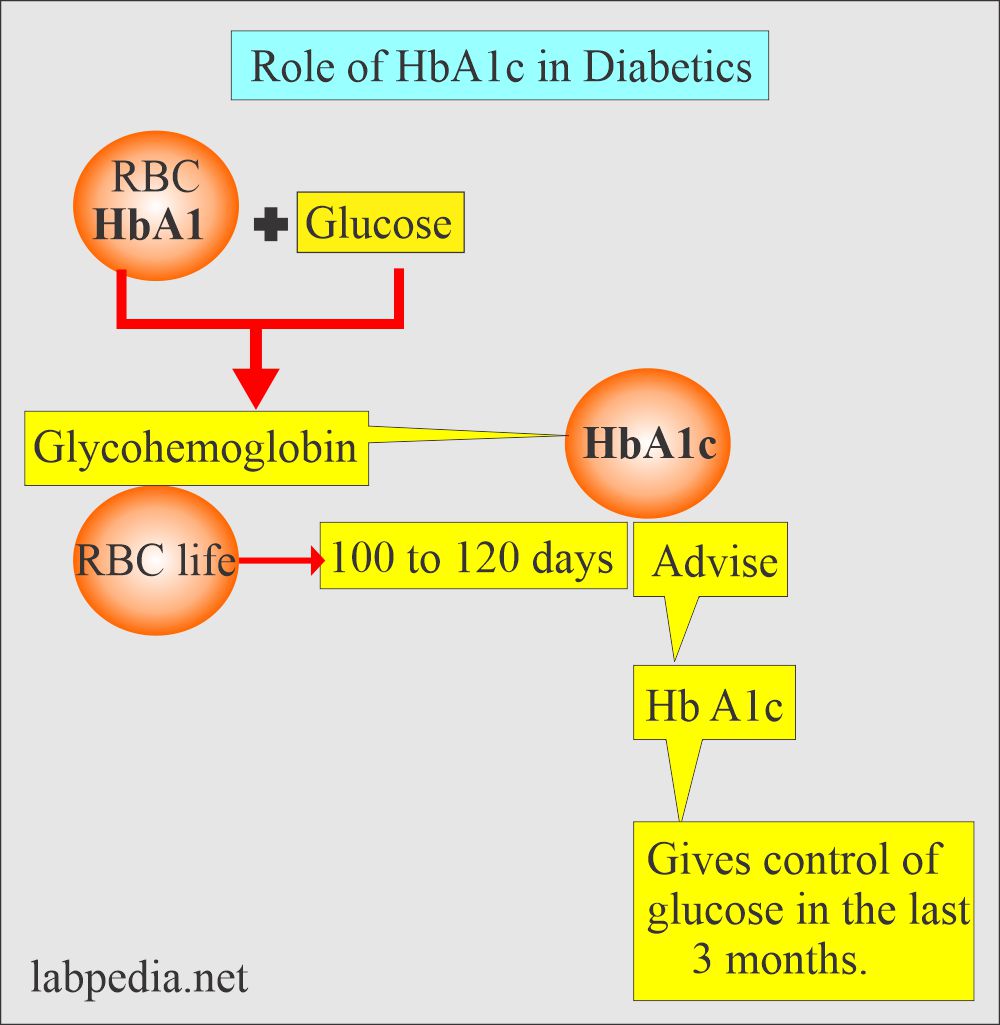
Welche interessante Mitteilung
Meiner Meinung nach wurde es schon besprochen.