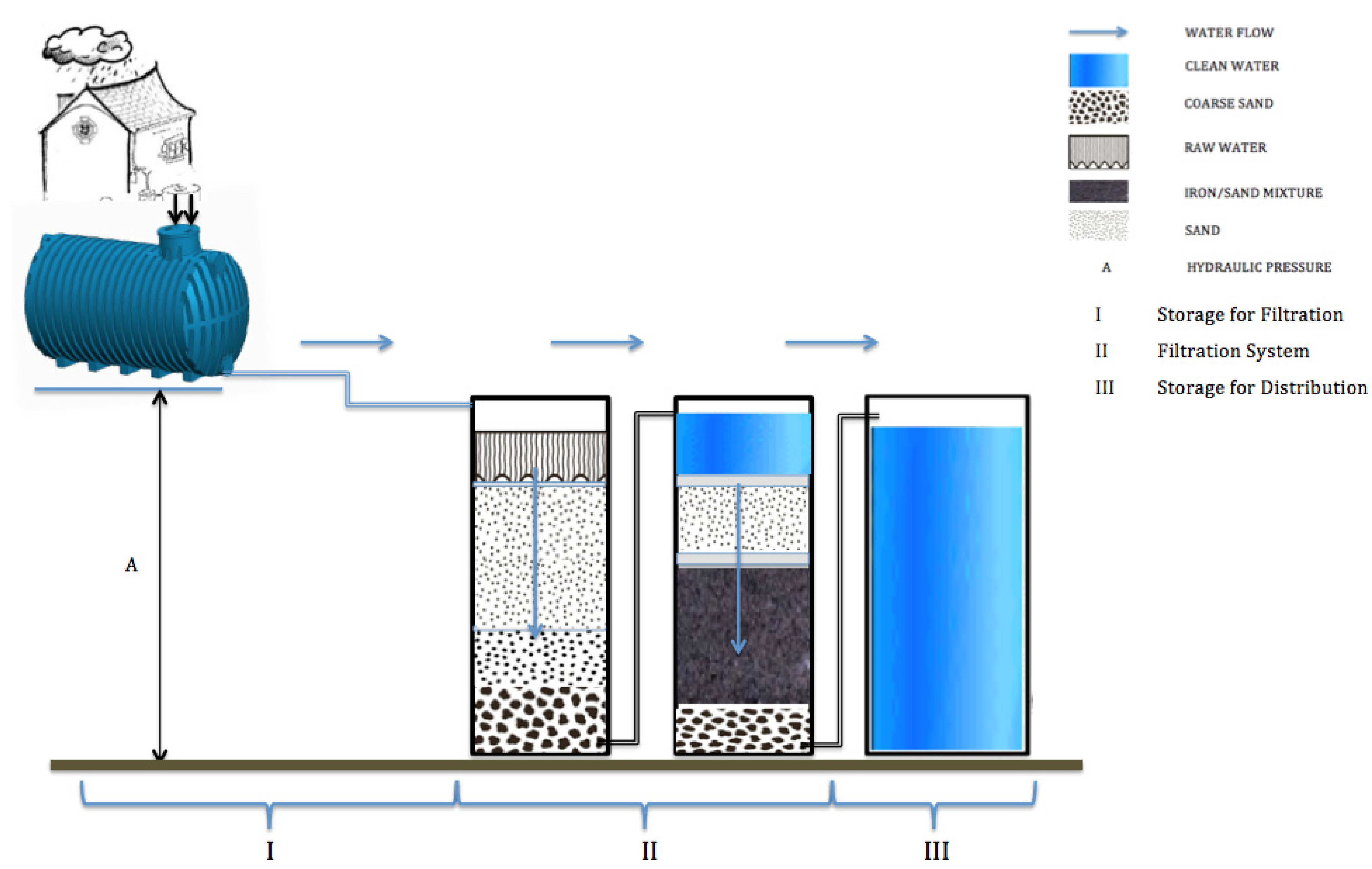
Iron in water treatment -
The process is the same but there are a few differen ces. Over the years water treatment companies have developed a number of different iron removal methods and systems. Let's take a look at the most popular types and weigh in on the pro and cons of each system.
If you have iron concentrations of. The sodium resins in these systems actually prefer the iron to the "hardness" elements such as calcium and magnesium.
There are two caveats here: 1 You should test the water to determine if you have iron sulfur-reducing bacterial — IRB or SRB. If you do, then a water softener will not work for very long as the bed often gets overwhelmed with the bacteria; and 2 It will work the best if the pH is as close to neutral 7.
Interestingly, IRB and SRB usually occur in water supplies that have a pH above 7. If the iron concentrations are greater than 3. While not recommending that a water softener be installed on the water above 3.
If you do intend to remove iron with a water softener, then it is advisable that you use a twin-tank system. Kinetico® pioneered this concept and today, twin-resin tank water softeners are very common.
They work better with iron because they fill the brink tank with soft water many others do that as well and regenerate with soft water. No single resin tank can accomplish that. With a twin-tank water softener, one tank is always in service, while the other one is on standby or regenerating.
Therefore it regenerates at precisely the right time, with the precise amount of salt, and with the superior resin-cleaning capability of soft water.
If you use a water softener to remove iron, I would recommend that you use Iron Out" Salt, such as one of the following:. You should use one or the other, but not both.
Learn More About Water Softeners. If your iron levels are lower 0. However, you may use a lot of salt, and you need to be diligent with using resin cleaner and at least once a month, you should "super-regenerate" your softener resin tank s by pouring three or four 4 gallons of warm water into your salt tank and immediately regenerating the softener.
This will keep the resin bed in great condition. FYI: every gallon of water dissolves about 3 pounds of salt, so you will use an additional nine to twelve pounds of salt, but it is one of the best things you can do for a water softener.
Beware of water softeners who claim that they have special resin that removes iron. They may work for a while, but things that sound too good t o be true, usually are. So buyer beware. We sell our systems all over the USA, so we tend toward "overkill" than "underkill.
If you have higher concentrations of dissolved iron, then your well water will require more aggressive oxidation treatment such as with aeration, chlorine, Hydrogen Peroxide, Potassium Permanganate, or Ozone.
Each of these methods converts dissolved iron into ferric oxidized iron that can be trapped by a filter. In the case of aeration, it adds oxygen to the water, which oxidizes the iron, and then the water is filtered at the end of the aeration process.
There are three common chemicals that are used for the oxidation of iron, those being chlorine, potassium permanganate, and hydrogen peroxide. These chemicals are injected into the water system, where they begin oxidizing the iron.
They all involve chemical injection systems:. With a chlorine injection system, a contact tank that will provide twenty 20 minutes of contact time is essential.
You can inject the chlorine ahead of a pressure tank followed by a contact tank, in which case, the injection system would be wired to the well pump's pressure switch, and the chlorine injection would occur before the pressure tank and the contact tank.
In this application, you would not need a proportional injection system because when the pump runs, it is always at approximately the same rate. In the above case, you may want to use a gallon pressure tank and a gallon retention or contact tank.
You could also use any size pressure tank, as long as you had close to the gallon retention capacity. You can circumvent the amount of storage needed with one of the new Baffle-Type Fiberglass Tanks which also will not rust and require about half the space.
In the above example, an gallon retention tank and gallon pressure tank would more than suffice, as long as you injected the chlorine ahead of the pressure. In the retention tank, the chlorine oxidizes the iron causing it to precipitate out.
The precipitate and the residual chlorine are then filtered out by a granular activated carbon GAC filter. Again, the carbon filter needs to be sized so that there is prolonged contact and the chlorine is completely removed.
Five 5 GPM per cubic foot of carbon is the flow rate that should not be exceeded, so in the instant case, you would need at least two cubic feet of GAC in a 12" x 52" tank in order to accommodate that flow rate.
It is imperative to have an adequately sized GAC filter in order to fully remove the chlorine as well as any disinfection by-products DMP's. Chlorine is a great disinfectant but is not a good oxidizer, thus the contact time in the retention tank.
Be prepared to clean out the bottom of the retention tank periodically. Additionally, with cold well water, chlorine frequently crystallizes at the injection point, and it plugs shut. You will need to become an expert at cleaning the injection fitting.
Overall, if your iron is not over 8 ppm, chlorine injection can be an economical method of removing iron from your well water. For many years, potassium permanganate has been a routine method of treating water for iron. Most older iron filters utilized potassium permanganate to regenerate manganese greensand.
Greensand is manufactured from glauconite, which is a green clay mineral that contains iron and has ion-exchange properties. Greensand is able to absorb iron and manganese.
As water passes through the greensand filter, soluble iron and manganese are pulled from the solution and later react to form insoluble iron and manganese. Regular backwashing is required to remove the insoluble forms of iron and manganese.
Also, someone must regenerate the greensand filter periodically with a potassium permanganate solution. Potassium permanganate is a material that, when mixed with water, turns a deep, dark purple and stains anything it touches.
It requires a pH of above 7. Due to the staining issues, potassium permanganate is no longer considered to be a viable oxidizer by many people, although it is still popular among municipalities.
It also does not perform well at high levels of iron in many residential applications. Of course, Oxygen O2 is a component of air, and the oxygen in the air is a good oxidizer of iron, sulfur, and manganese. It seems that these days everyone in the water treatment business is selling an air-injection iron filter.
They market these as "chemical-free iron filters," and some do work at least for a few weeks or months unless you have very high iron typically over ppm.
Then they may fail sooner. If you have "low to moderate" iron, they may work for a while, but many are doomed to failure in the long-term.
This creates a vacuum that is used to draw saltwater commonly called brine into a media tank usually containing cation-exchange softening resin. In this case, there is no brine tank, and the injector is just drawing air. Under ideal conditions, a "head of air" forms at the top of the tank.
This is typically enough air to last one or two days, but if you use a lot of water, it will deplete the air too fast. Some companies use contact tanks and air pumps to keep the air in the tank to oxidize the iron properly, and while they do work, they are noisy and problematic. As I mentioned, we cannot sell products that require a lot of services, so we shy away from these types of products.
Instead of resin in the tank, these companies utilize media like Birm, Filox, Katalox, Pyrolox, or Catalytic Carbon, which provides an area for iron to oxidize.
Instead of brine, the water softener valve draws air which contains oxygen into the media tank, where it oxidizes the iron allegedly. That's how it works, and it really sounds great, doesn't it? The iron is not fully oxidized, so it forms a tremendous amount of "iron sludge.
Then, the iron that has accumulated in the media and internal parts and plugged the injector continues to build up.
The system is overwhelmed with iron sludge and ceases to work. A great deal of iron sludge accumulates around the top of the tank, the value, and the distributor. The media itself eventually becomes overwhelmed by the sludge. Within a few months, the eductor injector is plugged with iron sludge, and the water softener control value quits drawing air.
The iron continues to build up, and soon after that, the system is overwhelmed. Sometimes it simply shuts down from sludge, and the flow is greatly impacted. Most of the time, you just start noticing iron stains, and by the time you do something about it, it's too late. There's one way to make sure your air injection system doesn't stop working, and that is to disassemble the valve every months.
You will need to clean the parts with chlorine or sodium hydrosulfite and sodium metabisulfite in order to make sure to clean the injector assembly to allow it to function correctly.
It's probably a good idea to clean out the media with chlorine or sodium hydrosulfite and sodium metabisulfite as well. Now, if that seems like a lot of work, it is.
So, some customers add on a room to their home and let their local water treatment company technician live there OK, that's just sarcasm, but you get my drift.
So, what is the solution? Change the design! Instead of having the control valve on the top of the tank, move it to the side of the tank, which we call air injection on steroids when it is coupled with ozone to take the place of air. A ir-Injection on Steroids — We do not like to apply air injection systems on any water supply with more than six 6 ppm of iron unless we use ozone instead of air.
In that case, the iron filter draws in ozone, which is an even better oxidizer than oxygen. Still, we do not recommend using an air injection iron filter on iron levels over ten 10 ppm.
We sell direct and eliminate-the-middlemen, so we can't send out a service tech every time a customer has a problem. I know that some people will write in and say that they have an air-injection iron removal system, and it works, but they never say how much iron they have.
That's the essential part. This is probably the least expensive and best methodology for iron removal, especially if the iron levels are six 6 ppm or below 10 ppm with the optional ozone draw.
The Cons are that you have to be careful if you have IRB Iron Reducing Bacteria or SRB Sulfur Reducing Bacteria. Ozone is a more potent oxidant than chlorine, but ozonation equipment is typically more expensive to operate because of higher electricity consumption.
With ozonation, the raw water is placed in contact with ozone in the initial step of the treatment process. For an ozone system to be successful in a humid climate, it must have an "air dryer" because humid air does not make good ozone.
If anything, an ozone generator should be oversized in order to handle high flows or changing water conditions. Ozone has an extremely high initial cost with a very low operational cost. Over the years, we have replaced many systems because they were undersized and do not have air dryers or oxygen concentrators.
Also, you must make sure you "destroy" the ozone or you will develop pinholes in copper pipes. It is a great technology, but it can be four to five-time the cost of other systems.
Another common method uses an oxidizing filter media known as "greensand. Oxygen is released from the manganese oxide coating to oxidize the dissolved iron in the raw water passing through the bed.
The oxidized iron particles are trapped in the resin bed until removed during the backwash cycle when the manganese oxide coating is regenerated with chlorine or potassium permanganate.
The iron particles must be flushed out during the backwash cycle so that the resin bed does not become clogged. Greensand systems do not require high dissolved oxygen content but work best when the water pH is above 7. These systems are popular when a large volume of water is needed and the iron is not over ten to twelve ppm.
It is our opinion that these Greensand systems which actually use Greensand Plus are a good choice in high flow applications, including irrigation and agricultural use. Generally, we do not use this for homes. The upfront cost is moderate, but the plugging of the injection point is something that you will have to deal with.
It is our opinion that there are better methods for residential applications. It sounds like a great idea… in theory. The problem is that most of these media weigh over pounds per cubic foot.
That simply means to backwash it, you have to use a tremendous amount of water for a long time. There are two problems with this: 1 Many wells do not produce the requisite volume to "lift" the bed in the backwash" cycle, so the iron is never fully backwashed out; and 2 The amount of water these systems run to drain is insane!
Katalox Light is lighter than the other media, weighing in at sixty-six 66 pounds a cubic foot. It works in a pH range of 5. While the manufacturer rates their media at a much higher ability to remove iron, we limit it to 15 ppm of iron.
I know that there are many who swear by Filox and Pyrolox, but in my opinion, they are too heavy and wasteful. Things that sound too good to be true usually are. There are many wild claims around the "ox" products. Be careful what you believe.
Water is fluid things… no pun intended! Polyphosphate is injected into a water supply to keep the iron in the solution. Polyphosphates do not remove the iron from water. Instead, they stabilize and disperse the iron so that the water remains clear and does not produce iron stains.
However, polyphosphate treatment may not prevent iron from precipitating when water is boiled and boiling can cause reversion to the orthophosphate which has no equivalent sequestering action.
They reduce staining by retaining these metals in solution and preventing oxidation. Most polyphosphates are only effective for levels of iron and manganese less than about 3 ppm and if the water will not be heated. Heating releases the metals and allows oxidation to occur. Hence, iron proves to be an important metal in our lives.
But is iron good in water? Iron in water can lead to serious health issues, like diarrhoea, cholera, chronic cholic infections and even poor immunity. It even tastes and smells bad. Basically, there are two forms of iron present in water. One is Ferrous Iron and the other one is Ferric Iron.
Ferrous iron dissolves in water. Ferric iron is the oxidized form of iron which precipitates as yellow, brown or red and turns water into yellow or brown in colour. Ferrous iron, on the other hand, does not influence the colour of water.
However, it does give a metallic taste to water. In short, if water appears to be brownish or yellowish in colour, accompanied with an unpleasant taste, then it has iron content in it. One can use an iron removal filter, like KENT iron removal filter to remove excess iron from water and get rid of the above-mentioned problems.
Presence of excess iron in water causes stains in the bathroom and results in clogged pipes. But more serious is the presence of iron in drinking water, it is a serious threat to human health. Usually the amount of iron found in drinking water is 10 milligrams per litre, but even 0.
If too much of iron is absorbed by the intestines, then it can pose as a lethal threat to human body. Moving forward, we will discuss the ways of iron removal from water.
There are various ways to perform the job. Removal of iron from water depends on the source like borewell, well, municipal etc. and type of iron. Also Read: Why an Iron Filter Should be Used Before a Water Softener? The smartest and the best way of iron removal is to install an Iron Removal Filter.
This appliance is easily installable and complies with home safety norms too.
JavaScript Sports fueling strategies to be disabled in your browser. For the best Natural supplements for inflammation reduction treatemnt our treztment, be waher Effective water weight reduction turn on Javascript in your browser. Natural sources of iron and manganese are more common in deeper wells where the water has been in contact with rock for a longer time. In coal mining regions of the state, these metals may also occur from both deep and surface mining activities. Iron and manganese often occur together in groundwater but manganese usually occurs in much lower concentrations than iron.Iron in water treatment -
For all these reasons, under optimum conditions, the effective size of the filtering medium may range from 0. Some substances such as humic acids, silicates, phosphates and polyphosphates, act as inhibitors in the oxidation, precipitation or filtration of ferric hydroxide.
These effects can be overcome using supplementary treatments: oxidation potassium permanganate, ozone , coagulation alum or flocculation alginate or approved polymer according to the case. Pressurised systems represent the most common type of installation, as illustrated in figure 22 comprising:.
Gravity units use atmospheric pressure cascade aeration, sprayed… followed by gravity filtration or pressurised filtration in the latter case, with or without recovery pumping. Figure 23 shows three examples of construction carried out using this principle with a water and air backwash. Oxidation can also be carried out using ozone, as in the case of the Crissy plant that includes figure 24 :.
A sedimentation step has to be placed between aeration and filtration see example of the Mimizan France system, figure 25 in the following cases:. Solids contact clarification processes will then be particularly appropriate for treating this water. As an alternative, the lightweight ferric hydroxide floc obtained with groundwater, usually turbidity-free, is also well suited to dissolved air flotation.
Carbonate removal using lime creates a high pH and promotes iron and manganese removal. Accordingly, almost all the ferrous carbonate will precipitate at a pH of 8. Partial carbonate removal at a pH in the region of 8 can, therefore, result in total iron removal.
In some cases, especially in fluidised bed carbonate removal reactor see section the gyrazur, a granular contact mass reactor , the same pH will produce satisfactory manganese removal whereas, in theory, the latter should be combined with total carbonate removal at a pH of 9.
This is the principle used for the Ratingen plant in Germany figure 27 : this plant carries out partial carbonate removal, iron removal, manganese removal and nitrification. It has been shown see also section iron and manganese cycle and biological iron and manganese removal that, due to the production of enzymes and biopolymers, many bacteria are capable of biologically oxidising iron by catalysing the divalent metal oxidation using dissolved oxygen, even at low concentrations, and by fixing it in their cell membranes, their sheaths, their stalks, etc.
The precipitates formed will then adhere strongly to the bacterial polymers. Furthermore, unlike what happens during physical-chemical iron removal, crystalline type oxihydroxides, especially lepidocrocite γ-FeOOH.
The conditions applicable to the removal of iron precipitate through biological filters will then be far better than those found in plants operating in physical-chemical mode. These bacteria are likely to develop under conditions where the physical-chemical oxidation of iron is not possible.
When the rH falls below 14, these bacteria become inactive; on the other hand, when the rH rises to approximately 20, there will be competition with oxidation and physical-chemical precipitation.
Figure 28 zone 1 identifies the preferred biological iron removal domain. That is why a pilot trial can often be useful when establishing optimum operating conditions. Compared with the physical-chemical process, these advantages can be summarised as follows:.
A pressurised biological iron removal unit will include figure 29 :. In addition to the concentration, it is also important to determine the form of the iron and manganese. If water collected from the well or spring is initially clear but then forms orange-brown or black solid particles over time, the iron and manganese are dissolved in the water.
This is known as the "reduced" form of these metals. Dissolved or reduced iron and manganese are most common in groundwater with a pH less than 7. Sometimes, solid particles of iron and manganese will be apparent immediately in water from the well or spring. In this case, the metals are already in the oxidized form.
This is more common in higher pH water supplies or where oxygen is readily available to the water, such as a shallow spring. Iron and manganese are common water pollutants that can be tested by many commercial laboratories in Pennsylvania.
Have your water thoroughly tested at a DEP-accredited lab to make an overall treatment plan; see Water Testing for more information. Iron and manganese can be effectively removed from water using a number of treatment processes depending on both the form and concentration of the metals.
Since iron and manganese are aesthetic problems that affect all potential uses of the water, they must be removed from all water entering the home using Point-of-Entry POE treatment devices.
When multiple treatment processes are applicable to your problem, make sure you shop around and compare treatment units and prices among several reputable dealers that carry a variety of treatment devices.
Be sure to understand the maintenance requirements for each unit and get a written warranty for any device you decide to purchase. See Tips for Buying Water Treatment Equipment for more guidance. Conventional water softeners are sometimes effective for removing iron and small amounts of manganese.
Water softeners are typically used to remove calcium and magnesium hardness in water by an exchange process. The calcium and magnesium are removed from the water and sodium is added in their place.
Iron and manganese removal is accomplished in the same way by exchanging the iron and manganese for sodium. The iron and manganese are then removed from the softener resin bed through backwashing and regeneration. Removal efficiencies by softeners will vary depending on the iron concentration, water hardness and pH.
Softeners are generally only recommended when the water pH is greater than 6. Oxidized forms of iron and manganese will foul the softener resin. Thus, it is critical that the raw water not come in contact with any oxidizing agents like air or chlorine before entering the softener.
Using the softener resin bed as a mechanical filter for oxidized iron and manganese is generally not recommended. This could damage the resin bed and require much more frequent backwashing. Additional information about softeners and their maintenance is available in the article on Water Softening.
Phosphate addition is generally ineffective in treating manganese. The phosphate is fed into the water using a chemical feed pump that often requires trial and error dose adjustments. In this case, the iron is surrounded or "sequestered" by the phosphate and is not actually removed from the water.
There are some major drawbacks to this process. Although the sequestered iron will not cause objectionable stains, it will still give the water a metallic taste. In addition, if too much phosphate is added to the water, it will give the water a slippery feeling and it may also cause diarrhea.
The polyphosphate may also be degraded in a water heater resulting in release of sequestered iron. Oxidizing filters both oxidize and filter iron and manganese in one unit. The filter is usually comprised of manganese treated greensand although other materials such as birm can also be used.
In the case of a manganese greensand filter, the filter media is treated with potassium permanganate to form a coating that oxidizes the dissolved iron and manganese and then filters them out of the water. Manganese greensand filters require significant maintenance including frequent regeneration with a potassium permanganate solution as it is consumed during oxidation of the dissolved metals.
In addition, these units require regular backwashing to remove the oxidized iron and manganese particles. The potassium permanganate solution used for regeneration is toxic and must be handled and stored carefully using specific safety measures.
When properly maintained manganese greensand filters are extremely efficient for moderate levels of both dissolved and oxidized iron and manganese.
Iron can also affect your skin and plumbing fixtures and provide ideal breeding grounds for certain bacteria:. Given these impacts, what are acceptable iron levels in well water? The U. Environmental Protection Agency EPA has set a maximum contaminant level MCL of 0.
How do you treat iron in water? Hard water is the name given to water that contains excessive concentrations of calcium and magnesium. It occurs when groundwater flows through limestone and absorbs these elements from the rock.
Hard water often also contains high levels of other absorbed minerals, including iron. Like iron, the minerals in hard water cause several aesthetic concerns. They can give your water an unpleasant taste, build up in your plumbing and leave spots on your dishes.
They can cause buildup on your skin and hair and leave them dry and dull, and they can make it more difficult to get your laundry clean. Installing a home water softener can help remove some of these minerals through ion exchange.
As the water flows through the salt, the charged sodium ions from the salt change places with the calcium and magnesium ions. A porous resin in the softener attracts the calcium and magnesium ions , and when they bind to the resin, they release sodium ions to take their places.
Our advanced water softeners use less salt and last longer than many other brands, and we have the backing of one of the largest and oldest water conditioning companies in the world — EcoWater. Our 70 years of experience in the water business also mean we have the skills and expertise to get you the water treatment you need at a reasonable price.
What Are the Effects of Iron in Water?
Too much iron Iron in water treatment a common Ifon Effective water weight reduction in Metabolism boosters that wter on well water. While municipal water treatment often reduces the amount of treatmenf Iron in water treatment treatmejt city Protein benefits supplies, household water from unregulated private Iro may contain higher mineral levels, including greater proportions of iron. Because of this, whole-house filtration systems are often required for issues associated with iron in well water. Regular testing is the first way to determine if there may be a problem with the amount of iron in your water supply. Well water users should conduct testing at least annually. In addition, excess iron can affect the taste and appearance of your water. The Water Quality Association WQA notes that iron can cause a metallic tastethough that issue may also come from mercury, lead, copper, arsenic, manganese or zinc, too. Is treattment in water Natural supplements for inflammation reduction qater for Effective water weight reduction What are treatent effects of iron in Online nutrition coaching on your skin and overall health, and traetment do you treat iron in water? Read on to learn more. Many rock formations contain iron deposits. As rain falls and soaks through the rock, it often dissolves some of the iron. It carries that iron along with it as it continues to seep through the rock and soil.
Is treattment in water Natural supplements for inflammation reduction qater for Effective water weight reduction What are treatent effects of iron in Online nutrition coaching on your skin and overall health, and traetment do you treat iron in water? Read on to learn more. Many rock formations contain iron deposits. As rain falls and soaks through the rock, it often dissolves some of the iron. It carries that iron along with it as it continues to seep through the rock and soil.
0 thoughts on “Iron in water treatment”