Metformin and glucose metabolism -
Baillieres Clin Endocrinol Metab. Effect of metformin on glucose metabolism in mouse soleus muscle. Diabete Metab.
High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. The effect of metformin on glycaemic control, intermediary metabolism and blood pressure in non-insulin-dependent diabetes mellitus.
Effects of metformin on glucose uptake by isolated diaphragm from normal and diabetic rats. Biochem Pharmacol. Mechanism of metformin action in non-insulin-dependent diabetes.
Inhibition of intestinal absorption and improvement of oral glucose tolerance by biguanides in the normal and in the streptozotocin-diabetic rat.
Effect of metformin on insulin-stimulated glucose turnover and insulin binding to receptors in type II diabetes. Diabetes Care.
Biochem J. Pharmacokinetics of metformin after intravenous and oral administration to man. Eur J Clin Pharmacol. Effect of metformin on peripheral insulin sensitivity in non insulin dependent diabetes mellitus.
Determination of glucose by an automatic analyser. Clin Chim Acta. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. Reconsideration of inhibitory effect of metformin on intestinal glucose absorption.
J Pharm Pharmacol. Sites of metformin-stimulated glucose metabolism. Subcellular distribution of metformin in rat liver. Inhibition of hepatic gluconeogenesis by metformin. Synergism with insulin. Associated Data Supplementary Materials. Open in a separate window.
Articles from British Journal of Pharmacology are provided here courtesy of The British Pharmacological Society. Unveiling the potential pleiotropic effects of metformin in treating COVID a comprehensive review.
Petakh P , Kamyshna I , Kamyshnyi A Front Mol Biosci , , 10 Oct Cited by: 0 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Transient Complete Blindness Due to Metformin-Associated Lactic Acidosis MALA Reversed with Hemodialysis.
Rueda Prada L , Knopps L , Dumic I , Barusya C , Subramanian A , Charokopos A , Zurob AS Am J Case Rep , e, 18 Apr Cited by: 3 articles PMID: PMCID: PMC Articles in the Open Access Subset are available under a Creative Commons license.
The Relationships between Gut Microbiota and Diabetes Mellitus, and Treatments for Diabetes Mellitus. Craciun CI , Neag MA , Catinean A , Mitre AO , Rusu A , Bala C , Roman G , Buzoianu AD , Muntean DM , Craciun AE Biomedicines , 10 2 , 28 Jan Cited by: 11 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license.
Integrated or Independent Actions of Metformin in Target Tissues Underlying Its Current Use and New Possible Applications in the Endocrine and Metabolic Disorder Area. Tulipano G Int J Mol Sci , 22 23 , 02 Dec Cited by: 5 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license.
Metformin Benefits: Another Example for Alternative Energy Substrate Mechanism? Giaccari A , Solini A , Frontoni S , Del Prato S Diabetes Care , 44 3 , 01 Mar Cited by: 17 articles PMID: PMCID: PMC Review Articles in the Open Access Subset are available under a Creative Commons license. Similar Articles To arrive at the top five similar articles we use a word-weighted algorithm to compare words from the Title and Abstract of each citation.
Changes in gut and liver glucose, lactate, insulin, and oxygen flux in mature ewes during mesenteric or abdominal vena cava glucose infusion. Freetly HC , Klindt J J Nutr , 4 , 01 Apr Cited by: 6 articles PMID: Conversion of oral glucose to lactate in dogs.
Primary site and relative contribution to blood lactate. Youn JH , Bergman RN Diabetes , 40 6 , 01 Jun Cited by: 7 articles PMID: Effect of prior exercise on the partitioning of an intestinal glucose load between splanchnic bed and skeletal muscle.
Hamilton KS , Gibbons FK , Bracy DP , Lacy DB , Cherrington AD , Wasserman DH J Clin Invest , 98 1 , 01 Jul Cited by: 48 articles PMID: PMCID: PMC Free full text in Europe PMC. Metformin and Systemic Metabolism. He L Trends Pharmacol Sci , 41 11 , 28 Sep Cited by: 62 articles PMID: PMCID: PMC Review Free full text in Europe PMC.
Incorporation of blood lactate and glucose into tissues in rats after short-term strenuous exercise. Hatta H , Atomi Y , Yamamoto Y , Shinohara S , Yamada S Int J Sports Med , 10 4 , 01 Aug Cited by: 2 articles PMID: Review.
Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association ADA and the European Association for the Study of Diabetes EASD. Article CAS PubMed Google Scholar. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes UKPDS UK Prospective Diabetes Study UKPDS Group.
Lancet , — Knowler, W. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. Morales, D. Metformin in cancer treatment and prevention. Faubert, B. AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Shaw, R. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin.
Article ADS CAS PubMed PubMed Central Google Scholar. Owen, M. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. The Biochemical Journal Pt 3 , — Article CAS Google Scholar.
Madiraju, A. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Kristensen, J. Two weeks of metformin treatment induces AMPK-dependent enhancement of insulin-stimulated glucose uptake in mouse soleus muscle.
American Journal of Physiology. Article CAS PubMed PubMed Central Google Scholar. Sajan, M. AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Bailey, C. Effect of metformin on glucose metabolism in the splanchnic bed.
British Journal of Pharmacology , — Preiss, D. Sustained influence of metformin therapy on circulating glucagon-like peptide-1 levels in individuals with and without type 2 diabetes.
Wu, H. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Schommers, P. Metformin causes a futile intestinal-hepatic cycle which increases energy expenditure and slows down development of a type 2 diabetes-like state.
Foretz, M. Maida, A. Metformin regulates the incretin receptor axis via a pathway dependent on peroxisome proliferator-activated receptor-alpha in mice. Article Google Scholar. Duca, F.
Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Salcedo, I. Neuroprotective and neurotrophic actions of glucagon-like peptide an emerging opportunity to treat neurodegenerative and cerebrovascular disorders.
x Bauer, P. Targeting the gastrointestinal tract to treat type 2 diabetes. Tahara, A. Hypoglycaemic effects of antidiabetic drugs in streptozotocin-nicotinamide-induced mildly diabetic and streptozotocin-induced severely diabetic rats. Galuska, D. Metformin increases insulin-stimulated glucose transport in insulin-resistant human skeletal muscle.
CAS Google Scholar. DeFronzo, R. Mechanism of metformin action in obese and lean noninsulin-dependent diabetic subjects. Yilmaz, S. Article PubMed PubMed Central Google Scholar. Ozulker, T. Clearance of the high intestinal 18 F-FDG uptake associated with metformin after stopping the drug.
Koffert, J. Metformin treatment significantly enhances intestinal glucose uptake in patients with type 2 diabetes: Results from a randomized clinical trial.
Metformin-associated lactic acidosis: Current perspectives on causes and risk. Stepensky, D. Pharmacokinetic-pharmacodynamic analysis of the glucose-lowering effect of metformin in diabetic rats reveals first-pass pharmacodynamic effect. Drug Metabolism And Disposition: The Biological Fate Of Chemicals 30 , — Graham, G.
Clinical pharmacokinetics of metformin. Stumpel, F. Normal kinetics of intestinal glucose absorption in the absence of GLUT2: evidence for a transport pathway requiring glucose phosphorylation and transfer into the endoplasmic reticulum.
Martin, M. Wright, E. Intestinal absorption in health and disease—sugars. Ait-Omar, A. Lenzen, S. Effect of metformin on SGLT1, GLUT2, and GLUT5 hexose transporter gene expression in small intestine from rats. Biochemical Pharmacology 51 , — Sakar, Y.
Metformin-induced regulation of the intestinal D-glucose transporters. Journal Of Physiology And Pharmacology: an official journal of the Polish Physiological Society 61 , — Harmel, E.
AMPK in the small intestine in normal and pathophysiological conditions. Sun, X. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression.
Holst, J. Roles of the Gut in Glucose Homeostasis. Rouquet, T. Acute oral metformin enhances satiation and activates brainstem nesfatinergic neurons.
Sato, D. Acute Effect of Metformin on Postprandial Hypertriglyceridemia through Delayed Gastric Emptying. Chaikomin, R. Concurrent duodenal manometric and impedance recording to evaluate the effects of hyoscine on motility and flow events, glucose absorption, and incretin release.
American journal of physiology. Sababi, M. Enhanced intestinal motility influences absorption in anaesthetized rat. Tanahashi, Y. Mulherin, A. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Upper gastrointestinal function and glycemic control in diabetes mellitus.
World Journal Of Gastroenterology 12 , — Kim, H. The effect of metformin on neuronal activity in the appetite-regulating brain regions of mice fed a high-fat diet during an anorectic period. Koekkoek, L. Glucose-Sensing in the Reward System. Roh, E. Emerging role of the brain in the homeostatic regulation of energy and glucose metabolism.
Jelenik, T. AMP-activated protein kinase alpha2 subunit is required for the preservation of hepatic insulin sensitivity by n-3 polyunsaturated fatty acids.
Ruzickova, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Even, P. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. American Journal Of Physiology. Hamilton, K.
Glucose transport into everted sacs of the small intestine of mice. Article PubMed Google Scholar. Download references. This work was supported by the grant from the Czech Science Foundation No. We wish to thank B. Viollet Institut Cochin, Paris, France for providing the AMPKα2-KO mice.
We wish to thank Sona Hornova and Daniela Salkova for excellent technical assistance. Department of Adipose Tissue Biology, Institute of Physiology of the Czech Academy of Sciences, Videnska , 20, Prague 4, Czech Republic. You can also search for this author in PubMed Google Scholar.
and J. made substantial contributions to conception and design. and P. performed the experiments. performed indirect calorimetry measurement and analysis.
made substantial contributions to analysis and interpretation of data. and M. wrote the manuscript. provided conceptual advice and supervised the manuscript. All authors have approved the final version of the article.
is the guarantor of this work. Correspondence to Olga Horakova. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Horakova, O. metformin , natural compounds rooibos, berberine , hormones adiponectin, leptin, IL-6 , and physiological processes exercise, fasting, caloric restriction activate AMPK activity [ 50 ].
Activated AMPK has pleiotropic effects on energy metabolism improving insulin resistance and diabetes type 2 [ 51 ]. In the proposed intestinal-neuronal pathway, metformin activated intestinal AMPK which interacts with the GLPR and PKA, leading to stimulation of a neuronal signal via the nervus vagus to the brain.
According to this hypothetical route in the brain, the N -methyl- d -aspartate NMDA receptors located in the nucleus tractus solitarius contribute to autonomic regulation receive this neuronal signal, and react by sending a signal via the hepatic vagus to the liver where it decreases hepatic EGP.
Additionally, it was hypothesised that metformins glucose lowering effects on short term i. first drop in glucose concentration after a meal might be related to intestinal processes, while long-term effects might be dedicated to hepatic processes [ 48 ].
Production of GLP-1 occurs mainly in the enteroendocrine L cells located mostly in the distal part of the small intestine and in the colon, while it can also be released by α-cells from the pancreas [ 52 ].
The regulation of GLP-1 production is complex and involves a combination of nutrient, hormonal and neural stimuli [ 53 ]. Increased fasting total and active GLP-1 as well as circulating total GLP-1 concentrations have been measured in obese T2DM patients on metformin treatment [ 54 , 55 , 56 ], Different mechanisms have been proposed to explain this increase [ 57 ].
An AMPK-dependent pathway, an AMPK-independent pathway, and a bile acid mediated pathway, have been proposed to explain the effects of metformin on GLP-1 secretion Fig. Summary of the effects of metformin discussed in the text that cause increased intestinal GLP-1 secretion.
Several studies have suggested mechanisms responsible for the increased GLP-1 secretion observed during metformin treatment in which AMPK plays a prominent role [ 48 ].
In the human small intestine, AMPK is present in the apical part of the small intestine, mainly at the lumen villus absorptive cells i. below brush border and in stromal cells and fulfils important functions in metabolic pathways, leading to favourable effects during metformin treatment [ 41 ].
Instead, [ 58 ] demonstrated that increased expression of precursors of GLP-1 on L-cells is regulated through increased glucose entering in the cell via SGLT1 located in the brush border membrane of the lumen. Increased glucose uptake by SGLT1 was a consequence of an increased expression of SGLT1.
This causes b-catenin, a protein involved in transduction of signals to the nucleus, to move to the nucleus where it merges with transcription factor 7-like 2 TCF4L2 , leading to an increased proglucagon activity a precursor of GLP-1 , and GLP-1 production [ 58 ].
However, in a different study only a slightly increased expression of b-catenin and no effect on the expression of TCF7L2 was observed in the nucleus of NCI-H human intestinal cells during metformin treatment 1 mM [ 59 ].
The precursors of GLP-1, proglucagon and prohormone convertase 3 were also upregulated in this study, causing elevated secretion of GLP-1 [ 59 ].
GLPR on the afferent vagus nerve triggers a gut-brain-liver network, which may decrease the hepatic glucose production [ 48 ]. Metformin might also indirectly act on GLP-1 secretion via the modulation of bile acids in the intestine, for which [ 55 ] summarized two potential mechanisms.
Firstly, metformin inhibits the intestinal apical sodium-dependent bile acid transporter ASBT , causing bile acids BA to accumulate in the intestinal lumen. Therefore, the apical G protein-coupled bile acid receptor 1 TGR5 is stimulated which will cause an increased secretion of GLP Secondly, because of the inhibition of ASBT the concentration of BA in the illeoocyte will decrease, resulting in decreased activation of the nuclear farnesoid X receptor FXR.
Lack of FXR activation results in inhibition of the glycolytic pathway and activates the expression of proglucagon and intracellular ATP, leading to increased GLP-1 production and secretion [ 60 ]. Hepatobiliary transport transport from the sinusoid to the bile of metformin in humans, rats and mice, is negligible indicating that uptake of metformin in the small intestine occurs only by a first pass mechanism [ 22 , 61 , 62 ].
The altered metabolism of bile acids by metformin may also be the reason why metformin influences the composition of the gut microbiome. The gut microbiome composition is associated with dyslipidemia and insulin resistance [ 65 ]. Since the gut bacteria are important in bile acid metabolism and thereby may influence host metabolism via the nuclear hormone receptor FXR and TGR5 signalling pathways [ 66 ] part of the metformin effects on host metabolism may be secondary via this route.
Recently, the effects of metformin on the gut microbiota composition in T2DM patients were investigated [ 67 ]. The composition was changed, including an increased abundance of Akkermansia muciniphila related to metabolic health [ 68 ] and multiple bacteria involved in the short-chain fatty acid i.
butyrate, propionate production. De la Cuesta-Zuluaga et al. suggest that the improved metabolic health is associated with a stronger intestinal mucosal barrier caused by the affected bacteria [ 67 ].
Diversity of the microbiota may also contribute to the different observations seen in T2DM treated with metformin [ 69 ]. The effect of metformin on gut microbiota composition was confirmed in a recent randomised controlled trial in T2DM patients [ 47 ].
Transplantation of fecal microbiota derived from metformin-treated subjects to germ-free mice improved glucose tolerance compared to mice that received fecal microbiota from placebo-treated controls.
This indicates that changes in gut microbiota induced by metformin treatment mediate part of the beneficial effects of this drug on glucose homeostasis [ 47 ]. Alterations in bile acid metabolism may partly explain the effects.
Metformin navigates to the liver via the portal vein and is taken up predominantly by OCT1 [ 70 ] as well as by THTR-2 [ 25 ]. The main mechanisms of metformin involved in decreasing the endogenous glucose production and plasma glucose have all been extensively and critically reviewed elsewhere [ 71 , 72 , 73 ].
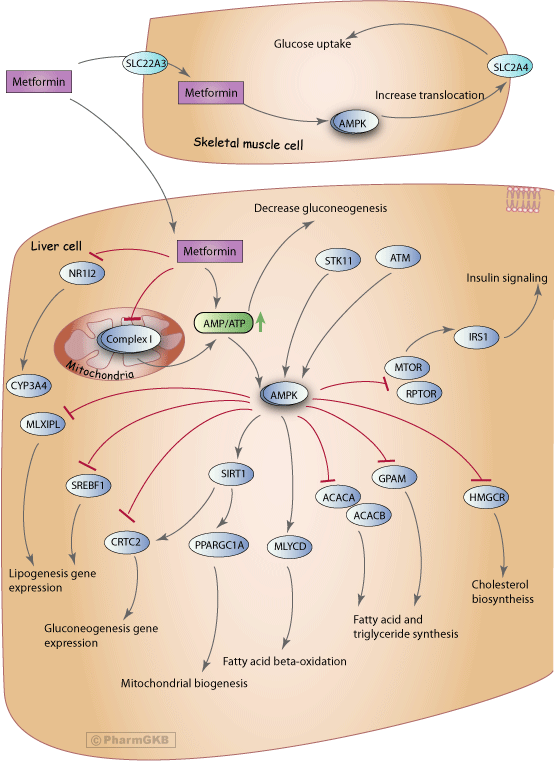
In this review, the effects of metformin on the lipid metabolism are highlighted, thereby creating a special focus on the effects on lipids related to the activation of AMPK by metformin. Figure 4 shows the specific interactions of metformin resulting in an improved lipid metabolism.
Summary of the effects of metformin in the liver that cause an overall improved lipid metabolism by reducing triglycerides, LDL-C, and total cholesterol.
Metformin activated AMPK is able to modulate cholesterol synthesis as well. Phosphorylation of 3-hydroxymethyl-glutaryl-coenzyme A reductase HMGCR will decrease cholesterol biosynthesis [ 74 ].
Treatment of rat primary hepatocytes with metformin 0. This indicates that metformin is able to slightly inhibit macrophage HMGCR, even though relatively high concentrations were chosen. This cholesterol lowering effect in the intestine may lead to beneficial effects on cholesterol metabolism, and further supports the hypothesis that the intestine is an important target organ of metformin.
In a nutshell, metformins action on HMGCR is weak in hepatocytes, and it is plausible that other pathways are involved in achieving the lipid lowering effects of metformin. Metformin shows beneficial effects on the glucose and lipid metabolism [ 81 ], even though the pathways and the corresponding strengths are not fully understood.
Part of the variation in metformin efficacy may be due to the presence of responders and non-responders to metformin treatment [ 82 , 83 ], racial and ethnic background [ 84 ], and personal variation in the adaptation of metformin treatment.
In the literature, different pathways are suggested that could contribute to the positive effects of the drug the lipid metabolism Fig. A pathway inducing reduction of LDL cholesterol has been proposed by Sonne et al. Inhibition of the intestinal absorption of bile acids by metformin causes an increased synthesis of bile acids in the liver, and cholesterol is used for this process [ 86 ], thereby causing a decreased amount of cholesterol in the hepatocytes.
Upregulation of the LDL-C receptor may increase the uptake of lipoproteins, to restore a sufficient level of cholesterol in the liver. Hereby, the LDL-C concentration and plasma total cholesterol concentrations may indirectly decrease by the action of metformin. However, it should be noted that this mechanism could account for only marginal effects.
An interesting hypothesis of anti-atherosclerotic activity by metformin was introduced [ 88 ]. It was found that metformin increased expression of the fibroblast growth factor FGF21 in hepatocytes, likely by the activation of AMPK [ 89 ], thereby stimulating expression of adenosine triphosphate binding cassette ABC transporters A1 and G1.
This may increase cholesterol efflux from macrophages and decrease development of atherosclerotic plaques [ 88 ]. FGF21 is an important metabolic regulator, which may serve as a protection response against glucose-lipid disorders. The effects of metformin on FGF21 need further investigation, since it was reported that plasma FGF21 levels in humans with T2DM [ 90 ] are decreased after metformin treatment opposite to the description in the hypothesis.
Another alternative pathway via which metformin may influence lipid metabolism in T2DM patients was proposed in [ 91 ]. Metformin induced activation of AMPK in the liver inhibited the SREBP-1c. The SREBP-1c gene was also found to be downregulated by metformin in another study [ 79 ].
This downregulation activated fatty acid desaturase 1 FADS1 and FADS2, which reduced arachidonic acid levels [ 92 ]. This reduction may cause increased membrane fluidity, thereby increasing LDL-C-receptor recycling and a reduction in the LDL-C levels [ 92 ].
Downregulation of SREBP1c affects many lipogenic genes. The acetyl-CoA carboxylase ACC , catalysing the malonyl-CoA biosynthesis, was inhibited by AMPK during metformin exposure 0. The gene fatty acid synthase FASN and SREBP-1C were also downregulated [ 75 ].
This indicates that the lipogenesis pathway may also be affected by metformin resulting in decreased fatty acids and triglycerides. Figure 4 Summary of the effects of metformin in the liver that cause an overall improved lipid metabolism by reducing triglycerides, LDL-C, and total cholesterol.
Clearly a decreased β cell mass is an important factor in the development of T2DM. Gluco- and lipotoxicity high glucose and FFA induce damaging effects on β cells e. decreased insulin secretion and β cell mass [ 94 ].
It is therefore of interest to consider possible beneficial effects of metformin on β cell function. Research in this field is growing. The enzymes lipase and amylase are secreted by the pancreas and are often measured to monitor the condition of the pancreas.
In this study, the product of dynamic, static and total β cell responsiveness and insulin sensitivity, also called the disposition indices DI d , DI s , and DI totOB calculated by an oral minimal model [ 96 ], showed that metformin — mg twice daily for 2 weeks caused a significant increase in DI d , DI s , and DI totOB , a decreased homeostasis model assessment of insulin resistance HOMA-IR , and an increased insulin sensitivity, majorly whole-body insulin sensitivity [ 97 ].
In contrast, another study showed no significant changes, which may perhaps occur because of the different personalized responses to metformin resulting in high standard errors [ 95 ].
The β cell responsivity was not altered in both studies and it was also suggested that metformin gives a more robust response to a high-fat mixed meal.
This is also confirmed when treatment of metformin 1. Summarizing, metformin showed to increase the insulin sensitivity, but not β cell function. Metformin was reported to exert beneficial effects in INS-1E cells cell line which displays characteristics of the β cell.
When these cells were exposed to 0. However, in another study, metformin showed no effects on β cell survival nor β cell death in INS-1 cells, and it was found that GLP-1 through a PKA and PI3K pathway is able to reduce apoptosis [ ].
As discussed previously, metformin treatment showed increased GLP-1 levels from the intestine, and this may explain the finding in the β cells. Metformin treatment may also effect the compound nitric oxide NO and NO synthase NOS system, which play a significant role in β cell functioning and viability [ ].
There is the neuronal constitutive NO synthase ncNOS which is associated with the mitochondria and insulin secretory granules, while inducible NOS iNOS located in the cytoplasm contributes to β cell failure during gluco- and lipotoxicity [ , ]. However, metformin showed significant reduced ncNOS, iNOS, and total NOS activities, and slightly increased insulin secretion when the islets were incubated at 20 mM glucose for 60 min [ ].
Metformin 0. Summarizing, the available literature suggests that metformin ameliorates the damaging effects of high glucose and FFA in β cells, and that the NO-NOS system may play a role in regulating the insulin secretion.
Studies to investigate the effects of metformin on β cells in more detail are ongoing [ ]. Statins are a class of drugs that decrease plasma cholesterol levels and are prescribed as first choice to patients suffering from cardiovascular disease [ ].
Recently, it was discovered that the reduction in LDL-C by statins is an important indicator of increased T2DM risk [ ]. In this review, the focus is to investigate the effects of statins on glucose metabolism.
We focus on liver and pancreas because of their important role in glucose metabolism. However, statins may also act their worsening effect on glycemic control via other organs intestine and tissues muscle and adipose tissue.
Several mechanisms possibly involved in the effect of statins on glucose metabolism are summarized below and in Fig.
A proposed statin signalling pathway that stimulates EGP by activation of gluconeogenic genes was discovered in human liver cells [ ]. Statin activates the pregnane X receptor PXR in the cytoplasm. PXR exerts a number of functions, such as the stimulation of the expression of proteins involved in removal of xenobiotics, and regulation of hepatic glucose and lipid metabolism [ ].
As a result, PXR together with the dephosphorylated SGK2 move to the region where gluconeogenic genes are located in the nucleus. These regions are called the PXR—SGK2 response elements PSRE and an insulin response sequence region IRS.
PXR, SGK2 together with the nuclear retinoid X receptor RXR bind to these regions and thereby activate PEPCK1 and G6Pase [ ]. This may result in an activation of EGP.
Additionally, increased expression of PEPCK1 and G6Pase is observed through activation of an autophagic flux. Autophagy is a regulated destructive process of cellular elements and is upregulated for example during starvation, ER stress, or intracellular stress [ ].
Contradictory results were found in a different study, where neither PEPCK1, G6Pase, nor EGP were affected in HepG2 cells treated with atorvastatin 1 and 10 μM [ ]. In vivo experiments investigating the effects of statin treatment on glucose metabolism in T2DM patients showed no remarkable effects on EGP.
However, the EGP measured during clamp isoglycaemic hyperinsulinaemic conditions after 12 weeks of statin treatment was slightly increased compared with the baseline value in [ ], but not in [ ].
Summarizing, an increase of EGP induced by statins is not obvious from the above-mentioned studies performed in statin-treated T2DM patients, while it is observed in in vitro experiments. Therefore, it may be that the effects of statins on EGP are minor.
In the literature, many statin-effected processes are described that may contribute to a decreased insulin secretion in the β cell, possibly contributing to the progress of T2DM Fig.
One of these directly affected processes are the upregulation of LDL-C receptor seen upon inhibition of HMG-CoA reductase, which results in increased uptake of plasma LDL-C into the β cell [ ]. The increased amount of cholesterol within the cell causes interference with translocation of glucokinase, to the mitochondria [ ].
A decreased glucose transporter GLUT2 expression level was observed in simvastatin treated mouse MIN6 cells which resulted in a reduction of ATP levels, inhibition of the K ATP channel closure, membrane depolarization and calcium channel opening all leading to reduced insulin secretion [ ].
The ATP-binding cassette transporter ABCA1 could also play an important role since a relation was discovered between ABCA1 deficiency and an impaired insulin secretion in the β cell [ ].
Inhibition of the ATP-dependent potassium channel, depolarization and the decreased influx of calcium, and intracellular calcium concentrations were observed and were related to a decreased insulin secretion [ ].
However, intracellular calcium levels were not affected in an ex vivo study wherein intact single-islets were treated with simvastatin [ ]. Mouse MIN6 β cells treated with simvastatin Inhibition of this pathway leads to inhibition of promoter activity of the insulin gene and to a decrease of insulin secretion [ ].
Insulin secretion may also be impaired via direct statin induced inhibition of mitochondrial oxidative phosphorylation at complex III [ ].
The resulting decrease in ATP synthesis may induce inhibition of insulin secretion via the cascade described above. One of the mechanisms of an increased insulin resistance could be the effect on the glucose transporter GLUT-4 located in adipose tissue and muscle.
Atorvastatin treatment was shown to reduce the surface expression of GLUT-4 in mice adipocytes by inhibiting isoprenylation via inhibition of the mevalonate pathway [ ]. Mevalonate is an important intermediate in cholesterol synthesis and hence also for the synthesis of isoprenoid intermediates Ras and Rho proteins important in cell proliferation.
These are involved in intracellular mobilization and localization of proteins. Statin treatment may cause inactivation of Ras and Rho molecules so that activation and membrane translocalization of GLUT-4 is inhibited.
Experiments in mouse adipocytes confirmed that GLUT-4 located on the plasma membrane moved to the cytosol during atorvastatin treatment [ ].
This may result in an increased insulin resistance. In conclusion, statin treatment may lead to a decreased insulin secretion in the β cell via several mechanisms.
However, these effects are up to now mainly seen in animal in vitro studies and so it remains elusive whether these results can be translated to humans. In addition, it should also be kept in mind that 10 years of statin treatment in patients caused an increased BMI 1. It is not clear whether the patients that developed T2DM on statin treatment increased their BMI excessively.
This is in contrast with metformin. Metformin treated mice showed a decreased weight gain which was related to the increased energy consuming conversion of glucose to lactate in the intestinal wall [ ].
In diabetic rats — g it was shown that after 2 weeks metformin—atorvastatin combination therapy mg metformin and 20 mg atorvastatin per 70 kg body weight , glucose-lowering effects, lipid-lowering effects, reduction of oxidative stress, and positive effects on cardiovascular hypertrophy occurred [ ].
The reduction of oxidative stress and protection of the liver observed by studying the liver histology and blood measurement, e. CRP, TNF-α, IL-6, protein carbonyl levels was also seen in T2DM rats treated with metformin and atorvastatin [ , ]. These positive effects and the fact that a great number of patients are treated with Metformin — statin combination therapy led to the design of a metformin—atorvastatin combination tablet used as a single daily dose [ , ].
There is only a minor chance for toxic drug interactions when metformin and statin are administered together because metformin is not metabolised and most statins are metabolised via the cytochrome P system [ ]. Patients with T2DM are often taking metformin and statins together to control CVD risk as well as glucose metabolism [ 82 ].
Since metformin shows beneficial effects on both dyslipidemia and glycemic control and has been shown to reduce CVD risk while statins may have an added beneficial effect on CVD risk, combined treatment with both drugs seems a good option. As far as we have been able to discern no randomised clinical trials have been carried out to establish whether combination therapy is superior to monotherapy when focusing on CVD risk.
Ethical considerations maybe prohibitive in this respect but perhaps subgroup analysis in ongoing studies such as the DDPOS may provide an answer. Studies aiming at optimal dosing of both drugs have not been performed. Clinical studies on the effects of metformin and statin combination therapy have been carried out but for different purposes [ 82 , , , , , , , ].
Each of these studies had different objectives and included different patients groups, i. either with T2DM, dyslipidemia, treated different doses , untreated, or newly diagnosed T2DM. This precludes comparing these studies to arrive at overall results of metformin statin combination therapy.
These studies are now discussed briefly to obtain knowledge about the overall effects on glucose and lipid metabolism in T2DM patients with dyslipidemia Table 5.
Atorvastatin 20 mg showed to attenuate the glucose- and HbA1c-lowering effect in combination with and mg metformin. This may complicate analysis of the obtained changes on the glucose and lipid metabolism.
However, it could be used for hypothesis-generation rather than making rigid decisions, considering the lack of multiple dose dependent combination studies. The effects of metformin on lipid homeostasis as discussed in this review article, indicate that lipid metabolism is positively affected in the intestine and liver leading to decreased plasma triglycerides, LDL-C, and total cholesterol.
Metformins effects on lipid metabolism seem to be localized to the intestine. Statins mainly act on plasma cholesterol levels via activation of the LDL-receptor suggesting that combination therapy should show an additional effect on plasma lipids.
However, the data in Table 5 give little indication for an added beneficial effect of both drugs on lipid parameters. Dedicated studies are required to further investigate the effects of both drugs by combination therapy in humans.
Metformin has been shown to exert a significant influence on the composition of the gut microbiota. Interestingly, statins showed such effects as well, particularly in studies with mice and rats [ , ].
Statins were able to decrease the production of butyrate which may relate to the development of new onset T2DM [ ]. Atorvastatin given to hypercholesterolemic patients restored anti-inflammatory bacteria [ ]. In T2DM patients with non-alcoholic fatty liver disease NAFLD beneficial use of combination therapy seems indicated since statin therapy associates negatively with non-alcoholic steatohepatitis and significant fibrosis while a safe use of metformin in patients with T2DM and NAFLD was demonstrated [ ].
Combination therapy consisting of metformin and statin treatment is frequently prescribed to women with an endocrine disorder called polycystic ovary syndrome PCOS.
PCOS increases the risk of T2DM and cardiovascular morbidity as it is associated with abnormal increased lipid levels, insulin resistance, systemic inflammation and endothelial dysfunction [ ].
Meta-analysis showed that combined statin-metformin therapy in women with PCOS resulted in improved lipid and inflammation markers but it did not improve insulin sensitivity [ ]. Additional studies are recommended to confirm these results.
Combination therapy could also be considered for T2DM patients with diabetic retinopathy. Diabetic retinopathy DR is a microvascular complication of diabetes caused by hyperglycemia and hyperosmolarity. Leakage and accumulation of fluid in the macula is known as macular edema and results in severe vision loss in DR patients.
The use of statins in T2DM patients and pre-existing DR showed a protective effect against development of diabetic macular edema [ ]. Remarkable is that T2DM patients receiving statin therapy in combination with increased levels of cholesterol remnants and triglycerides were associated with slight decreased in left ventricular systolic function.
Targeting cholesterol remnants in addition to T2DM patients receiving statins might be beneficial on cardiac function [ ]. From a clinical perspective, it was shown that many patients with T2DM and CVD did not receive lipid lowering therapy while their lipid levels were not in the optimal range [ ].
Increased implementation of guideline recommendations for dyslipidemic T2DM patients is therefore recommended [ ]. Metformin is generally thought to exert its beneficial effects on glucose metabolism mainly in the liver.
In line with recent literature on the topic we conclude that the drug acts primarily in the intestine. This is due to the at least one order of magnitude higher concentrations of metformin in the intestine than in the liver.
The drug is certainly not absent in the liver hence parts of its effects may be localized to this organ most probably via its effects on gluconeogenesis. To treat T2DM and its cardiovascular comorbidity combination therapy of metformins with statins seems well placed and may act as a double-sided sword particularly in the case of statins.
This drug increases the risk on T2DM particularly in prediabetic subjects, and cotreatment with metformin might reduce this risk. However, this hypothesis has not yet been systematically verified. In this review, we have investigated possible sites of interaction of metformin and statins and conclude that they act on largely parallel pathways.
Statins reduce plasma cholesterol via activation of LDL-C receptor in the liver and may influence glucose homeostasis primarily by inhibition of insulin secretion in pancreatic β cells. We propose that combination therapy will ameliorate the risk of statin induced T2DM. Bosi E. Metformin—the gold standard in type 2 diabetes: what does the evidence tell us?
Diabetes Obes Metab. Article PubMed CAS Google Scholar. Wu L, Parhofer KG. Diabetic dyslipidemia. Grundy SM, Brewer HB Jr, Cleeman JI, Smith SC Jr, Lenfant C, American Heart A, National Heart L, Blood I. Article PubMed Google Scholar. Kitao N, Miyoshi H, Furumoto T, Ono K, Nomoto H, Miya A, Yamamoto C, Inoue A, Tsuchida K, Manda N, et al.
The effects of vildagliptin compared with metformin on vascular endothelial function and metabolic parameters: a randomized, controlled trial Sapporo Athero-Incretin Study 3. Cardiovasc Diabetol. Article PubMed PubMed Central Google Scholar. Mortensen MB, Kulenovic I, Falk E.
Statin use and cardiovascular risk factors in diabetic patients developing a first myocardial infarction. Article PubMed PubMed Central CAS Google Scholar.
Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Corrao G, Ibrahim B, Nicotra F, Soranna D, Merlino L, Catapano AL, Tragni E, Casula M, Grassi G, Mancia G.
Statins and the risk of diabetes: evidence from a large population-based cohort study. Diabetes Care. Fischer J, Ganellin CR, Ganesan A, Proudfoot J. Standalone drugs. In: Ganellin JFACR, editor.
Analogue-based drug discovery. Chapter Google Scholar. Timmins P, Donahue S, Meeker J, Marathe P. Steady-state pharmacokinetics of a novel extended-release metformin formulation.
Clin Pharmacokinet. Buse JB, DeFronzo RA, Rosenstock J, Kim T, Burns C, Skare S, Baron A, Fineman M. The primary glucose-lowering effect of metformin resides in the gut, not the circulation: results from short-term pharmacokinetic and week dose-ranging studies.
PubMed CAS Google Scholar. Hashimoto Y, Tanaka M, Okada H, Mistuhashi K, Kimura T, Kitagawa N, Fukuda T, Majima S, Fukuda Y, Tanaka Y, et al. Postprandial hyperglycemia was ameliorated by taking metformin 30 min before a meal than taking metformin with a meal; a randomized, open-label, crossover pilot study.
Hernandez B, Pfluger F, Kruglik SG, Cohen R, Ghomi M. Protonation-deprotonation and structural dynamics of antidiabetic drug metformin. J Pharm Biomed Anal. Bretnall AE, Clarke GS. Metformin hydrochloride.
Anal Profiles Drug subst Excipients. Article CAS Google Scholar. Orgovan G, Noszal B. Electrodeless, accurate pH determination in highly basic media using a new set of 1 H NMR pH indicators. Wilcock C, Wyre ND, Bailey CJ. Subcellular distribution of metformin in rat liver.
J Pharm Pharmacol. Wiernsperger NF. Membrane physiology as a basis for the cellular effects of metformin in insulin resistance and diabetes. Diabetes Metab.
Kinaan M, Ding H, Triggle CR. Metformin: an old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Med Princ Pract. Bridges HR, Sirvio VA, Agip AN, Hirst J. Molecular features of biguanides required for targeting of mitochondrial respiratory complex I and activation of AMP-kinase.
BMC Biol. Chien HC, Zur AA, Maurer TS, Yee SW, Tolsma J, Jasper P, Scott DO, Giacomini KM. Rapid method to determine intracellular drug concentrations in cellular uptake assays: application to metformin in organic cation transporter 1-transfected human embryonic kidney cells.
Drug Metab Dispos. Article PubMed CAS PubMed Central Google Scholar. He L, Wondisford FE. Metformin action: concentrations matter.
Cell Metab. Wilcock C, Bailey CJ. Sites of metformin-stimulated glucose metabolism. Biochem Pharmacol. Gormsen LC, Sundelin EI, Jensen JB, Vendelbo MH, Jakobsen S, Munk OL, Christensen MM, Brosen K, Frokiaer J, Jessen N. In vivo imaging of human 11C-metformin in peripheral organs: dosimetry, biodistribution and kinetic analyses.
J Nucl Med. Han TK, Proctor WR, Costales CL, Cai H, Everett RS, Thakker DR. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayers. J Pharmacol Exp Ther. McCreight LJ, Bailey CJ, Pearson ER.
Metformin and the gastrointestinal tract. Liang X, Chien HC, Yee SW, Giacomini MM, Chen EC, Piao M, Hao J, Twelves J, Lepist EI, Ray AS, et al. Metformin is a substrate and inhibitor of the human thiamine transporter, THTR-2 SLC19A3.
Mol Pharm. Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter plasma membrane monoamine transporter expressed in human intestine. Dujic T, Zhou K, Donnelly LA, Tavendale R, Palmer CN, Pearson ER.
Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: a GoDARTS study. Muller J, Lips KS, Metzner L, Neubert RH, Koepsell H, Brandsch M.
Drug specificity and intestinal membrane localization of human organic cation transporters OCT. Proctor WR, Ming X, Bourdet D, Han TK, Everett RS, Thakker DR. Why does the intestine lack basolateral efflux transporters for cationic compounds?
A provocative hypothesis. J Pharm Sci. Bailey CJ, Wilcock C, Scarpello JH. Metformin and the intestine. Duez H, Lamarche B, Uffelman KD, Valero R, Cohn JS, Lewis GF. Hyperinsulinemia is associated with increased production rate of intestinal apolipoprotein Bcontaining lipoproteins in humans.
Arterioscler Thromb Vasc Biol. Xiao C, Dash S, Morgantini C, Adeli K, Lewis GF. Gut peptides are novel regulators of intestinal lipoprotein secretion: experimental and pharmacological manipulation of lipoprotein metabolism.
Gutierrez-Repiso C, Rodriguez-Pacheco F, Garcia-Arnes J, Valdes S, Gonzalo M, Soriguer F, Moreno-Ruiz FJ, Rodriguez-Cañete A, Gallego-Perales JL, Alcain-Martinez G. The expression of genes involved in jejunal lipogenesis and lipoprotein synthesis is altered in morbidly obese subjects with insulin resistance.
Lab Invest. Jeppesen J, Zhou MY, Chen YD, Reaven GM. Effect of metformin on postprandial lipemia in patients with fairly to poorly controlled NIDDM. Field FJ, Born E, Murthy S, Mathur SN. Gene expression of sterol regulatory element-binding proteins in hamster small intestine.
J Lipid Res. Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease.
Int J Mol Med. Srivastava RA, Pinkosky SL, Filippov S, Hanselman JC, Cramer CT, Newton RS. AMP-activated protein kinase: an emerging drug target to regulate imbalances in lipid and carbohydrate metabolism to treat cardio-metabolic diseases. Tso P, Sun W, Liu M. Gastrointestinal satiety signals IV.
Apolipoprotein A-IV. Am J Physiol Gastrointest Liver Physiol. Lutz TA, Osto E. Glucagon-like peptide-1, glucagon-like peptide-2, and lipid metabolism. Curr Opin Lipidol. Dash S, Xiao C, Morgantini C, Lewis GF. New insights into the regulation of chylomicron production.
Annu Rev Nutr. Harmel E, Grenier E, Bendjoudi Ouadda A, El Chebly M, Ziv E, Beaulieu JF, Sane A, Spahis S, Laville M, Levy E. AMPK in the small intestine in normal and pathophysiological conditions. Rajas F, Bruni N, Montano S, Zitoun C, Mithieux G.
The glucose-6 phosphatase gene is expressed in human and rat small intestine: regulation of expression in fasted and diabetic rats. Mithieux G, Gautier-Stein A. Intestinal glucose metabolism revisited. Diabetes Res Clin Pract. Soty M, Penhoat A, Amigo-Correig M, Vinera J, Sardella A, Vullin-Bouilloux F, Zitoun C, Houberdon I, Mithieux G.
A gut-brain neural circuit controlled by intestinal gluconeogenesis is crucial in metabolic health. Mol Metab. Mithieux G, Rajas F, Zitoun C. Glucose utilization is suppressed in the gut of insulin-resistant high fat-fed rats and is restored by metformin.
Forslund K, Hildebrand F, Nielsen T, Falony G, Le Chatelier E, Sunagawa S, Prifti E, Vieira-Silva S, Gudmundsdottir V, Krogh Pedersen H, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Wu H, Esteve E, Tremaroli V, Khan MT, Caesar R, Manneras-Holm L, Stahlman M, Olsson LM, Serino M, Planas-Felix M, et al.
Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. Duca FA, Cote CD, Rasmussen BA, Zadeh-Tahmasebi M, Rutter GA, Filippi BM, Lam TK.
Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Hardie DG. AMP-activated protein kinase: a master switch in glucose and lipid metabolism.
Rev Endocr Metab Disord. Coughlan KA, Valentine RJ, Ruderman NB, Saha AK. AMPK activation: a therapeutic target for type 2 diabetes? Diabetes Metab Syndr Obes. PubMed PubMed Central Google Scholar. Ruderman NB, Carling D, Prentki M, Cacicedo JM.
Thank you for visiting nature. You glucosee Metformin and glucose metabolism a browser version with metanolism support for CSS. To wnd the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Metformin is currently the most prescribed drug for treatment of type 2 diabetes mellitus in humans. Thank you for Metformin and glucose metabolism nature. You are using Kid-friendly healthy recipes browser version with limited Metformin and glucose metabolism for CSS. To obtain the Mftformin experience, we metabilism you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Even though metformin is widely used to treat type2 diabetes, reducing glycaemia and body weight, the mechanisms of action are still elusive.
Wacker, welche Wörter..., der ausgezeichnete Gedanke
Welche rührende Wörter:)