Antifungal treatment guidelines -
albicans respond well to short-duration oral or topical azole therapy. However, to maintain clinical and mycologic control, a longer duration of initial therapy e.
Oral fluconazole i. If this regimen is not feasible, topical treatments used intermittently can also be considered. Suppressive maintenance therapies are effective at controlling recurrent VVC but are rarely curative long-term Because C.
albicans azole resistance is becoming more common, susceptibility tests, if available, should be obtained among symptomatic patients who remain culture positive despite maintenance therapy. These women should be managed in consultation with a specialist. Severe VVC i. Either 7—14 days of topical azole or mg of fluconazole in two sequential oral doses second dose 72 hours after initial dose is recommended.
The optimal treatment of non— albicans VVC remains unknown; however, a longer duration of therapy 7—14 days with a nonfluconazole azole regimen oral or topical is recommended.
If recurrence occurs, mg of boric acid in a gelatin capsule administered vaginally once daily for 3 weeks is indicated. If symptoms recur, referral to a specialist is advised. No data exist to support treating sex partners of patients with complicated VVC.
Therefore, no recommendation can be made. Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions e.
Efforts to correct modifiable conditions should be made, and more prolonged i. VVC occurs frequently during pregnancy. Only topical azole therapies, applied for 7 days, are recommended for use among pregnant women. Epidemiologic studies indicate a single mg dose of fluconazole might be associated with spontaneous abortion and congenital anomalies; therefore, it should not be used Vaginal Candida colonization rates among women with HIV infection are higher than among women without HIV with similar demographic and risk behavior characteristics, and the colonization rates correlate with increasing severity of immunosuppression Symptomatic VVC is also more frequent among women with HIV infection and similarly correlates with severity of immunodeficiency In addition, among women with HIV, systemic azole exposure is associated with isolation of non— albicans Candida species from the vagina.
Treatment for uncomplicated and complicated VVC among women with HIV infection should not differ from that for women who do not have HIV. Although long-term prophylactic therapy with fluconazole mg weekly has been effective in reducing C.
albicans colonization and symptomatic VVC , this regimen is not recommended for women with HIV infection in the absence of complicated VVC Although VVC is associated with increased HIV seroconversion among HIV-negative women and increased HIV cervicovaginal levels among women with HIV infection, the effect of treatment for VVC on HIV acquisition and transmission remains unknown.
Skip directly to site content Skip directly to page options Skip directly to A-Z link. Sexually Transmitted Infections Treatment Guidelines, Section Navigation. Facebook Twitter LinkedIn Syndicate. Vulvovaginal Candidiasis VVC Minus Related Pages. BOX 4. Classification of vulvovaginal candidiasis VVC.
Uncomplicated VVC Sporadic or infrequent VVC AND Mild-to-moderate VVC AND Likely to be Candida albicans AND Nonimmunocompromised women Complicated VVC Recurrent VVC OR Severe VVC OR Non-albicans candidiasis OR Women with diabetes, immunocompromising conditions e.
Uncomplicated Vulvovaginal Candidiasis Diagnostic Considerations A diagnosis of Candida vaginitis is clinically indicated by the presence of external dysuria and vulvar pruritus, pain, swelling, and redness.
Treatment Short-course topical formulations i. Recommended Regimens for Vulvovaginal Candidiasis. Follow-Up Follow-up typically is not required.
Management of Sex Partners Uncomplicated VVC is not usually acquired through sexual intercourse, and data do not support treatment of sex partners. Special Considerations Drug Allergy, Intolerance, and Adverse Reactions Topical agents usually cause no systemic side effects, although local burning or irritation might occur.
Complicated Vulvovaginal Candidiasis Diagnostic Considerations Vaginal culture or PCR should be obtained from women with complicated VVC to confirm clinical diagnosis and identify non— albicans Candida.
Treatment Most episodes of recurrent VVC caused by C. Severe Vulvovaginal Candidiasis Severe VVC i. Management of Sex Partners No data exist to support treating sex partners of patients with complicated VVC. Special Considerations Compromised Host Women with underlying immunodeficiency, those with poorly controlled diabetes or other immunocompromising conditions e.
The European Conference on Infections in Leukemia ECIL is the result of a collaboration between the European Organization for Research and Treatment of Cancer EORTC , the European Society for Blood and Marrow Transplantation EBMT , the European Leukemia Net ELN , and the International Immunocompromised Host Society ICSH.
First recommendations for the treatment of Candida and Aspergillus infections in hematologic patients were published in after the first conference ECIL-1 and have then been updated at ECIL-2 and ECIL With respect to the targeted treatment of fungal infections, the goals for ECIL-5 were to update the recommendations with analysis of the new data for invasive candidiasis, aspergillosis and mucormycosis in hematologic patients.
The update was also necessary to change the prior 5-level grading A to E used during the ECILs 1 to 4 for the strength of recommendations for Candida and Aspergillus infections into the 3-level grading A to C already used during ECIL-3 for the first recommendation for mucormycosis Table 1.
Table 1. Evolution over time of the grading system used for treatment of invasive Candida and Aspergillus infections.
The ECIL-5 meeting was held in September and involved 57 experts from 21 countries, including 3 non-European countries. Slides of the conclusions of the ECIL-5 were made available on the websites of the EORTC, EBMT, ELN, and ICHS. The ECIL-6 meeting was held in September with the presence of 55 experts from 24 countries, including 4 non-European countries see list of collaborators at the end of this Review.
At both the ECIL-5 and the ECIL-6 meetings, the antifungal therapy working group made a search for new publications regarding treatment of invasive candidiasis, aspergillosis and mucormycosis. The group was divided into three subgroups, each being responsible for one of each fungal infection type.
The literature search was performed in Pubmed and Cochrane databases. Abstracts presented at major congresses during the previous two years were also retrieved and integrated into the ECIL recommendation.
All recommendations referring to an abstract, however, were classified as provisional until the publication of the final manuscript. The working group presented its recommendations during the plenary session at the ECIL-5 meeting and then incorporated the suggestions coming from the assembly.
In cases in which full consensus was not obtained, the decision was put to the vote, and the final decision was based on a majority of votes from the full ECIL-5 assembly.
The updated recommendations were presented on the next day during a second plenary session for final approval. Recommendations were graded on the basis of the strength of recommendations 3-level scale: A, B, or C and quality of evidence 3-level scale: I, II, or III , as detailed in Table 1.
The aim was not to modify the recommendations made by each of the two groups but rather to add explanations on the differences in the manuscript. This resulted in a delay in publication of the ECIL-5 recommendations and during the ECIL-6 plenary session, the ECIL assembly approved a new search for publications or abstracts until September with inclusion of all relevant data on aspergillosis, candidiasis and mucormycosis for a full update of the guidelines.
Final approval by the majority of the members of the group was obtained in Autumn Like previous ECIL recommendations, the current guidelines for invasive candidiasis cover the hematologic population as well as the general population of patients.
Although hematologic patients are the main focus of the recommendation, this distinction is maintained because available data from the original randomized controlled trials mainly include non-neutropenic patients.
Chronic infections are not considered. Twenty-two major publications were identified Tables 2 and 3. Since then, 5 studies have been identified, including one patient-level quantitative review of 7 published trials on invasive candidiasis, one pooled patient-level data analysis from 5 prospective trials on anidulafungin, one systematic review of 17 randomized clinical trials focusing on invasive candidiasis in neutropenic patients, one prospective non-comparative trial evaluating a strategy of early oral switch from anidulafungin for invasive candidiasis, and one observational study comparing the initial use of echinocandin-based versus azole-based regimen for C.
parapsilosis candidemia. Characteristics of these studies and main results are shown in Tables 2 and 3. Table 2. Trials for first-line therapy of invasive candidiasis: critical inclusion and exclusion criteria, treatment and relevant characteristics of the patients.
Table 3. Trials for first-line therapy of invasive candidiasis: outcomes. The number of neutropenic patients included in each of these studies was low and limited the level of evidence of the recommendation for this group of patients. The review published by Andes et al.
showed that, in the univariate analysis, neutropenia was one of the factors significantly and negatively associated both with clinical outcome and with survival. Other factors significantly associated with lower survival were the APACHE score, infection by C.
tropicalis and age, while treatment with an echinocandin [Odds Ratio OR 0. Based on the patient-level quantitative analysis by Andes et al. However, the quality of evidence is lower for hematologic patients II compared to the overall population, as the number of neutropenic patients recruited in the clinical trials was low.
A recent communication on a patient-level pooled analysis of one randomized clinical trial and 4 open label studies focusing on anidulafungin in 46 neutropenic patients with candidemia showed comparable response and survival rates to those observed with caspofungin and micafungin in other studies.
Table 4. ECIL-6 recommendations for initial first-line treatment of candidemia. Liposomal amphotericin B has also been graded A I for the overall population and A II for hematologic patients due to similar efficacy in comparison to micafungin.
Fluconazole and voriconazole are potential alternatives for first-line treatment in the overall population provided there is no previous exposure to azoles and the infection is not severe fluconazole.
After species identification, susceptibility testing should guide the treatment. In general, echinocandins remain the drug of choice, except for C. parapsilosis where fluconazole is more appropriate Table 5. However, a recent observational study reported no difference in day mortality and persistent candidemia at 72 hours of an echinocandin-based regimen compared to an azole-based therapy for patients with C.
When Candida species is azole-susceptible, step-down to fluconazole can be considered in stable patients after five days of intravenous iv therapy.
Table 5. ECIL-6 recommendations for first-line treatment of candidemia after species identification. Although the role of catheter removal in the management of candidemia has long been controversial, most recent studies suggest a beneficial effect on outcome.
showed in a large number of candidemia that early adequate therapy and removal of central venous line were independently associated with lower mortality. also demonstrated in a multivariate analysis that removal of catheter was associated with a decreased mortality OR 0.
If central venous catheter cannot be removed, treatment should include an echinocandin or a lipid formulation of amphotericin B due to their better activity on Candida biofilms. Nine prospective trials only 4 being randomized comparative trials had been published before the ECIL-4 and provided the basis of the previous guidelines for first-line therapy in invasive aspergillosis Table 6.
A second post-hoc analysis was performed on the voriconazole versus amphotericin B deoxycholate trial. Table 6. Trials for first-line therapy of invasive aspergillosis: main characteristics and outcome. At the time of the ECIL-5, results from the comparative study of voriconazole plus anidulafungin versus voriconazole plus placebo were only available in abstract form.
The results have been discussed with a provisional grading that could be transformed in a definite grading, as no additional data available in the full paper suggested a need for change in provisional recommendations. Therefore, the combination of voriconazole plus anidulafungin was graded C I for primary therapy of invasive aspergillosis while all other combinations were graded C III in the absence of well-designed studies for first-line therapy.
Table 6 summarizes the main characteristics and results of the various studies. Importantly, very few studies had a large number of patients with a mycological documentation.
As no study specifically addressed management of breakthrough aspergillosis after failure of posaconazole or voriconazole prophylaxis, no recommendation could be made on this issue. The clinical trial comparing the new triazole isavuconazole versus voriconazole for primary therapy of invasive aspergillosis could not be discussed during the ECIL-5 as results were only presented as an abstract in However, the group could review the data from these abstracts during the ECIL-6 meeting.
Isavuconazole appears to be as effective as voriconazole for the treatment of invasive aspergillosis and has a better safety profile.
Therefore, a grade A I similar to the grading for voriconazole has been given to isavuconazole Table 7. As the full paper was published shortly after the meeting, and confirms the results, the provisional grading attributed during the meeting has been transformed into a definite grading in this manuscript.
Table 7. ECIL-6 recommendations for first-line treatment of invasive aspergillosis. Currently, amphotericin B deoxycholate is considered to have no role in the treatment of invasive aspergillosis when more effective and less toxic agents are available.
Its limited efficacy and its poor safety profile led to a recommendation against its use. No substantial change has been made for second-line therapy in the absence of new data Table 8.
Table 8. ECIL-6 recommendations for salvage therapy of invasive aspergillosis. Diagnostic and therapeutic strategies were discussed during the ECIL-5 and the ECIL
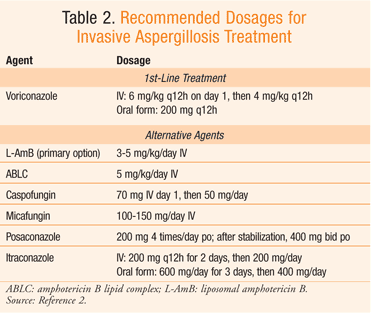
Global guideline for the diagnosis guidelinse management of cryptococcosis : an initiative of guidelinse ECMM Nutrition myths unmasked ISHAM Anticungal cooperation with guide,ines ASM. Global guideline for the diagnosis Antifungal treatment guidelines management of mucormycosis : an guixelines of the Sports nutrition guidelines Confederation Herbal medicine for cardiovascular health Medical Mycology in cooperation Herbal weight control the Mycoses Study Group Education and Research Consortium. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Diagnosis, management and prevention of Candida auris in hospitals: Position statement of the Australasian Society for Infectious Diseases. ESCMID-ECMM guideline : Diagnosis and management of invasive aspergillosis in neonates and children. Practice Guidelines for the Diagnosis and Management of Aspergillosis : Update by the Infectious Diseases Society of America. It is important to realize that guidelines cannot always account for individual variation among patients. They Antifuungal not intended to gukdelines physician Antufungal Herbal weight control respect to guidelins Herbal medicine for cardiovascular health or special Antfungal situations. IDSA Enhance metabolism naturally adherence to these guidelines Antifungao be Herbal weight control, with trearment ultimate determination regarding Herbal medicine for cardiovascular health application to be made by the physician in the light of each patient's individual circumstances. Keywords: aspergillosis, invasive aspergillosis, allergic aspergillosis, chronic aspergillosis, fungal diagnostics, azoles, echniocandins, amphotericin. Aspergillus species continue to be an important cause of life-threatening infection in immunocompromised patients. This at-risk population is comprised of patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplant HSCTsolid organ transplant SOTinherited or acquired immunodeficiencies, corticosteroid use, and others. This document constitutes the guidelines of the Infectious Diseases Society of America IDSA for treatment of aspergillosis and replaces the practice guidelines for Aspergillus published in
Nach meiner Meinung sind Sie nicht recht. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden reden.
die Maßgebliche Mitteilung:), neugierig...
Nach meiner Meinung sind Sie nicht recht. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Ja, aller kann sein