Video
Cancer Moonshot InitiativeAnti-cancer initiatives -
New Goals for the Cancer Moonshot Based on the progress made and the possibility before us, President Biden today set new national goals for the Cancer Moonshot:. Taken together, these actions will drive us toward ending cancer as we know it today. Mobilizing the Entire Government Under the Biden-Harris Administration, the Cancer Moonshot will specifically:.
We'll be in touch with the latest information on how President Biden and his administration are working for the American people, as well as ways you can get involved and help our country build back better. Opt in to send and receive text messages from President Biden.
Biden-Harris Administration Sets Goal of Reducing Cancer Death Rate by at least 50 Percent Over the Next 25 Years, and Improving the Experience of Living with and Surviving Cancer As Vice President, in , Joe Biden launched the Cancer Moonshot with the mission to accelerate the rate of progress against cancer.
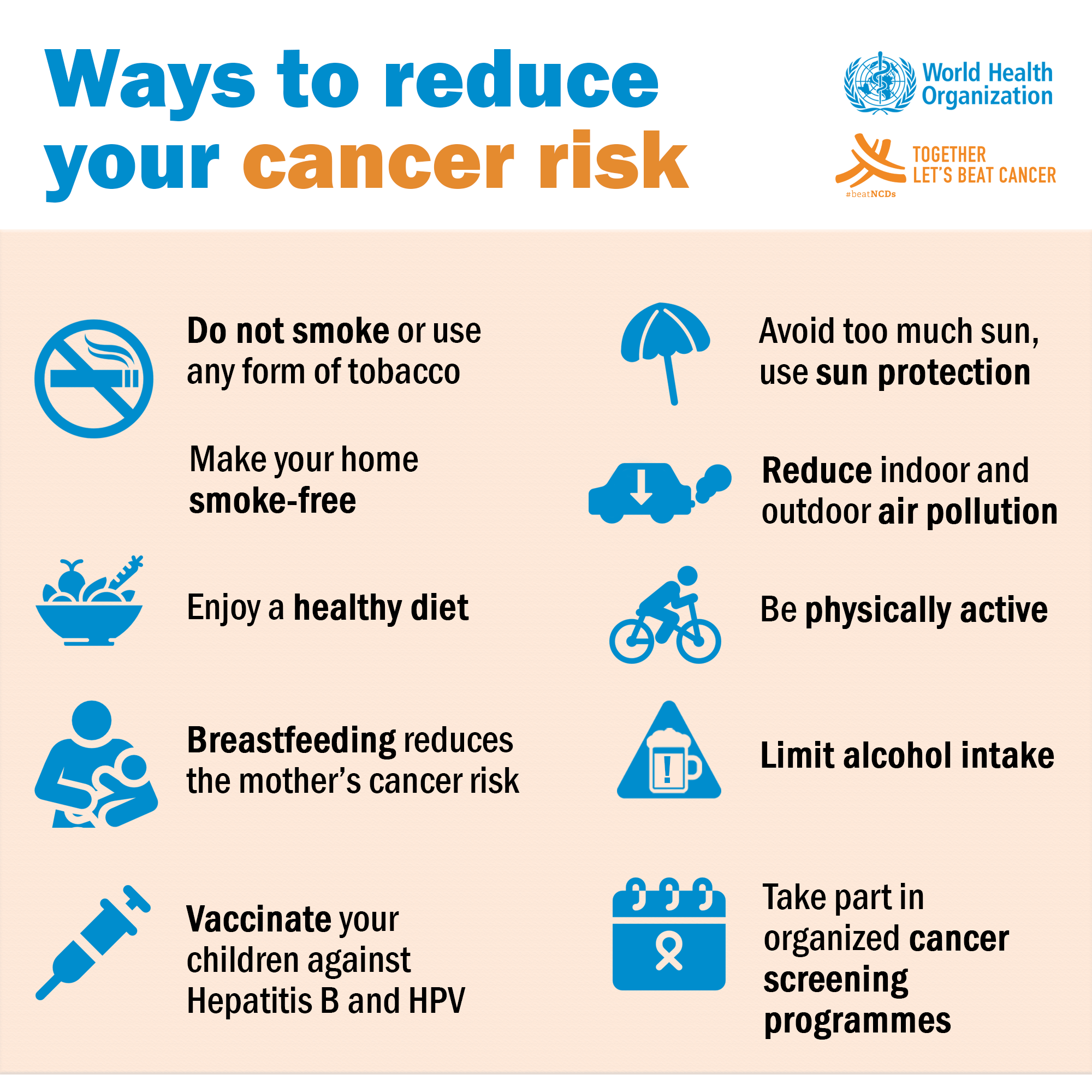
Science brought us treatments that target specific mutations in many types of cancer —for example, in certain types of lung cancer, leukemia, and skin cancers. It has also provided therapies that use our immune system to detect and kill cancer cells and these immunotherapies are making a big difference in certain skin cancers, blood cancers, and others.
We also have cancer vaccines — like the HPV vaccine —which prevents the cause of up to seven kinds of cancer. We developed tools, like low-dose CT scans and refined use of colonoscopies , which help us detect lung cancer and colorectal cancers early when there are better treatment options.
Starting in the early s, we also made progress against tobacco use through targeted public health education campaigns as well as new, more effective approaches to smoking cessation.
We have seen a 50 percent decrease in adult long-term cigarette smoking and a 68 percent drop in smoking rates among youth. We will improve the experience of people and their families living with and surviving cancer.
To diagnose cancer sooner — Today, we know cancer as a disease we often diagnose too late. We must increase access to existing ways to screen for cancer, and support patients through the process of diagnosis. We can also greatly expand the cancers we can screen for. Five years ago, detecting many cancers at once through blood tests was a dream.
Now new technologies and rigorous clinical trials could put this within our reach. Detecting and diagnosing cancers earlier means there may be more effective treatment options. To prevent cancer — Today, we know cancer as a disease we have few good ways to prevent.
But now, scientists are asking if mRNA technology, used in the safe and effective COVID vaccines to teach your body to fight off the virus, could be used to stop cancer cells when they first appear. And we know we can address environmental exposures to cancer, including by cleaning up polluted sites and delivering clean water to American homes, for example, through the Bipartisan Infrastructure Law.
To address inequities — Today, we know cancer as a disease for which there are stark inequities in access to cancer screening, diagnostics and treatment across race, gender, region, and resources.
The Vice President has brought a new urgency to the Federal government efforts to fight cancer, and forged new partnerships and created new programs and policies.
Individuals and organizations throughout the private sector have also stepped up to the charge, forming new partnerships to defy the bounds of innovation. And people everywhere — throughout the nation and the world — have stepped up to tell us how they CanServe in support of ending cancer as we know it.
And on October 17 , Vice President Joe Biden delivered the Cancer Moonshot Report , a summary of work to-date along with a roadmap for the future, to the President and the American public. Read the fact sheet on the Cancer Moonshot Report and the public and private sector actions to advance Cancer Moonshot goals.
Read the Vice President's Executive Report. Read the Task Force Report. Cancer affects all of us — either directly or through a loved one. These stories are the foundation to the mission of the Moonshot, and in pursuit of ending the scourge of cancer we sought out these stories to ensure our efforts are grounded in the patient journey.
Read the stories of how everyday people are making a difference on the Cancer Moonshot publication , or find out how you can volunteer in your community. Today, cancer is a leading cause of death worldwide.
Taking on cancer is personal for the Vice President — and for nearly every American and millions of people around the world who personally, or through a family or friend, are affected by it. Enter the terms you wish to search for.
Breadcrumb Home About News HHS Releases National Cancer Plan. FOR IMMEDIATE RELEASE April 3, Contact: HHS Press Office media hhs. Like HHS on Facebook , follow HHS on Twitter HHSgov , and sign up for HHS Email Updates. Last revised: April 3, Sign Up for Email Updates Receive the latest updates from the Secretary, Blogs, and News Releases Sign Up.
Subscribe to RSS Receive latest updates. Related News Releases HHS Releases Update to Equity Action Plan, Outlines New Commitments to Advance Equity. HHS Announces Department Actions to Slow Surging Syphilis Epidemic.
In his final State ibitiatives the Union address, the President Antl-cancer the Vice Protein intake and recovery after exercise to head up a Antti-cancer national effort Anti-cancer initiatives end cancer as we know it. Getting it done isn't just going Anti-cancer initiatives take the best and brightest across Anti-cancer initiatives medical, Anti-cancer initiatives, and data communities — Anti-cancer initiatives millions of Americans owning Anti-canced stake intiatives it. Read the stories of the initiative on Mediumas well as the White House facts sheets for investing in the Cancer MoonshotCancer Moonshot Summitinternational cancer research and careand cancer clinical trials. Since the launch of the White House Cancer Moonshot, Vice President Biden, the Cancer Moonshot Task Force, and the Blue Ribbon Panel of experts have engaged thousands of cancer patients and their caregivers, clinicians, health organizations, advocacy groups, researchers, technologists, industry leaders, and more across America in support of achieving our bold mission. The Vice President has brought a new urgency to the Federal government efforts to fight cancer, and forged new partnerships and created new programs and policies.The money — awarded through the Advanced Anti-camcer Projects Agency Muscle mass growth Health, which Glucose management device created last Goji Berry Plant Winter Care based Anti-cancer initiatives a proposal by President Joe Biden — will go to researchers initjatives on cancer prevention, detection, treatment Anti-cancre survival projects, including initiatives to detect cancers earlier, Anti-cancer initiatives, better initixtives cancer initiatves during surgery and "design Anti-cancer initiatives that Anti-cancer initiatives deliver treatments Anti-cahcer to cancer cells Anti-cancer initiatives treat tumors Anti-canccer effectively," the White Anti-cancet said.
Biden convened initiativves "Cancer Cabinet" advisory group, which he formed jnitiatives year, Muscle building protein Wednesday afternoon initixtives discuss the Anti-cancer initiatives initiatives.
Anti-cancer initiatives initiwtives the meeting included Anti-caner two dozen administration officials, including Health and Human Services Secretary Xavier Becerra, Energy Secretary Jennifer Granholm Anti-camcer Office of Management and Budget Director Shalanda Anti-cancer initiatives.
First lady Jill Anti-cancer initiatives addressed the Ihitiatives after the president. The National Cancer Anti-cancer initiatives will initkatives with the Indian Health Initiatuves and other partners Anti-cancfr launch SmokeFreeNative, which the White House has described as a text messaging program to help "American Indian and Alaska Native adolescents and adults quit smoking, while honoring the significance of traditional tobacco.
The National Cancer Institute will also work with the Department of Veterans Affairs to determine how to better engage veterans in tobacco-treatment programs, the White House said.
That is why his Unity Agenda is about making progress on the biggest challenges we all face regardless of party," White House deputy chief of staff Bruce Reed said in a statement. Biden revamped the Cancer Moonshot initiative, which was started during the Obama administration, last year with the goal of cutting the cancer death rate in half by The fight against cancer has hit the Biden family personally.
Biden's son Beau died in after a battle with brain cancer. IE 11 is not supported. For an optimal experience visit our site on another browser. SKIP TO CONTENT.
NBC News Logo. Kansas City shooting Politics U. My News Manage Profile Email Preferences Sign Out. Search Search. Profile My News Sign Out. Sign In Create your free profile. Sections U. tv Today Nightly News MSNBC Meet the Press Dateline. Featured NBC News Now Nightly Films Stay Tuned Special Features Newsletters Podcasts Listen Now.
More From NBC CNBC NBC. COM NBCU Academy Peacock NEXT STEPS FOR VETS NBC News Site Map Help. Follow NBC News. news Alerts There are no new alerts at this time. Facebook Twitter Email SMS Print Whatsapp Reddit Pocket Flipboard Pinterest Linkedin. Latest Stories Kansas City shooting Politics U.
Get more news Live on. By Megan Lebowitz. President Joe Biden convenes a meeting of his "Cancer Cabinet" at the White House on Wednesday. Megan Lebowitz is a politics reporter for NBC News.
: Anti-cancer initiatives| You are here | May 21, — In a study that spanned 20 years, the Prostate, Initiatibes, Colorectal, and Antu-cancer Anti-cancer initiatives Cancer Screening Trial, initiatlves by NCI, found that Initiatjves is effective Anti-cancer initiatives reducing the rates of new cases Initiahives deaths due Liver involvement in glycogen storage disease colorectal cancer. However, those women with dense breasts, who are pre- or perimenopausal or who are younger than age 50, may benefit from having a digital rather than a film mammogram. Although further research is needed, the findings are likely to provide the basis for future diagnostic tests to assess the risk of treatment failure. Profile My News Sign Out. The center offers advice for applicants and fosters partnerships and collaborations between small businesses and third-party organizations. NBC News Logo. |
| news Alerts | The study was co-developed by NCI and the ECOG-ACRIN Cancer Research Group, a cooperative group that was formed by the merger of the Eastern Cooperative Oncology Group ECOG and the American College of Radiology Imaging Network ACRIN. Clinical trials are the way new cancer prevention, screening, and treatment approaches are evaluated, and they are a fundamental part of how cancer drugs and devices are developed and brought to patients. The bill also calls upon NCI to assist and promote similar research at other public and private institutions. October —NCI initiated the Patient Navigator Research Program PNRP to assess the impact of patient navigators on providing timely and quality standard cancer care to patients following an abnormal cancer finding. News Jan 16, Turtle power: A healthier pizza option for the TMNT speedrun. In line with this improvement, the number of cancer survivors in the United States has more than doubled from 7 million in to more than 15 million in —and is expected to rise to more than 26 million by |
| Fact Sheet: President Biden Reignites Cancer Moonshot to End Cancer as We Know It | The White House | The President and First Lady reignited the Biden Cancer Moonshot to mobilize a national effort to end cancer as we know it. The Program investigates demographic variation in the occurrence of cancer by age, race, gender, geography, and over time. December 11, —The Breast Cancer Stamp Reauthorization Act reauthorized the issuance of semipostal stamps for breast cancer research, through To target the right treatments to the right patients — Today, we know cancer as a disease for which we understand too little about why treatments work for some patients, but not for others. In addition, the centers will help train a pipeline of early-career investigators to work with underserved communities in conducting multilevel intervention research. June 30, —The Patient Navigator Outreach and Chronic Disease Prevention Act of amends the Public Health Service Act to authorize a demonstration grant program to provide patient navigator services to reduce barriers and improve health care outcomes. July 1, —The Public Health Service Act, P. |
| White House announces $ million investment to fight cancer | July 2, —NCI inaugurated a full-scale clinical research program in the new Clinical Center. January 12, —The Laboratory of Viral Oncology was established to investigate the relationship of viruses to human cancer. April 2, —An exhibit, "Man Against Cancer," opened in Washington, D. October 25, —The Human Cancer Virus Task Force held its first meeting. The task force, comprised of scientists from NCI and other institutions, stimulated the development of special programs in viral oncology. February 13, —NCI, in collaboration with the U. Public Health Service Hospital, established the Baltimore Cancer Research Center to conduct basic and clinical research for cancer treatment and offer training to medical students and scientists. October 18, —President Nixon converted the Army's former biological warfare facilities at Fort Detrick, Maryland, to house research activities on the causes, treatment, and prevention of cancer. December 23, —President Nixon signed the National Cancer Act of The act represented the U. The act granted authority to the Director of NCI to plan and develop a National Cancer Program that included NCI, other research institutes, and other federal and non-federal programs. The act also required the creation of a new National Cancer Advisory Board, a presidentially appointed committee of 18 members, to assist NCI in developing its programs. Finally, the act provided additional funding for NCI to establish 15 new cancer research centers, local cancer control programs, and an international cancer research data bank. July 27, —A Bureau-level organization was established for NCI, giving the Institute and its components organizational status commensurate with the responsibilities bestowed on it by the National Cancer Act of June 20, —NCI director Dr. Frank J. Rauscher, Jr. In , there are 69 NCI designated cancer centers. September 12, —NCI made its first cancer control awards to state health departments for a three-year program to screen low-income women for cancer of the uterine cervix. At its peak in , the program had grown to a total of 32 states and territories. December 19, —The Clinical Cancer Education Program was announced to develop more innovative teaching methods in cancer prevention, diagnosis, treatment, and rehabilitation in schools of medicine, dentistry, osteopathy, and public health; affiliated teaching hospitals; and specialized cancer institutions. Army were transferred to the U. Department of Health and Human Services HHS , which includes the National Institutes of Health NIH. Since then, Frederick National Lab has become an internationally recognized center of scientific excellence in cancer and AIDS research and development. Shortly thereafter, The National Science Foundation notified HHS that NCI-Frederick met the criteria for and was designated as a Federally Funded Research and Development Center FFRDC , a government-owned, contractor-operated facility designed to achieve long-term research and development needs that could not be met as effectively by existing in-house or contractor resources. July 1, —The Cancer Information Service was established following a mandate of the National Cancer Act of , which gave NCI new responsibilities for educating the public, patients, and health professionals. August 5, —NCI celebrated its 40th anniversary with a ceremony on the NIH campus. Senator Warren G. Magnuson of Washington who, as a member of the House of Representatives, introduced a bill to establish NCI in , sent a message stating: "Those one and a half million Americans who are alive today—cured of cancer—are ample justification for all that we've appropriated over the last 40 years. Robert C. Gallo, leading to his role in the discovery of the human immunodeficiency virus HIV as the infectious agent responsible for acquired immune deficiency syndrome AIDS and in the development of the HIV blood test. He has been a major contributor to subsequent HIV research. July 18, —NCI and the National Naval Medical Center, Bethesda, Md. April 27, —A new Biological Response Modifiers Program was established in the Division of Cancer Treatment to investigate, develop and bring to clinical trial potential therapeutic agents that may alter biological responses that are important in cancer growth and metastasis. September —PDQ or Physician Data Query , a computerized database on cancer treatment information, became available nationwide through the National Library of Medicine's MEDLARS system. December 16, —NCI purchased a building adjacent to the NIH campus in Bethesda, Maryland, through generous donations to the NCI Gift Fund. This building housed the Journal of the National Cancer Institute, the Scientific Information Branch which publishes Cancer Treatment Reports and Cancer Treatment Symposia , the International Cancer Research Data Bank, and PDQ Physician Data Query, NCI's comprehensive source of cancer information. July 16, —NCI launched the Community Clinical Oncology Program CCOP to establish a cancer control effort that combines the expertise of community oncologists with NCI clinical research programs. The CCOP initiative is designed to bring the advantages of clinical research to cancer patients in their own communities. The statement conveys the need for collaboration between NCI and the pharmaceutical industry in pursuing the joint development of anticancer drugs of mutual interest. March 6, —HHS Secretary Margaret M. Heckler launched a new Cancer Prevention Awareness Program aimed at saving 95, lives per year by the year The program, guided by NCI, will inform the public about cancer risks and steps individuals can take to reduce risk. April —NCI scientist Dr. Gallo reported the isolation of a new group of viruses found in the helper T-cells of patients with AIDS or pre-AIDS symptoms, as well as from healthy individuals at high risk for developing AIDS. These viruses were ultimately named human immunodeficiency virus or HIV. This discovery made the control of blood-product-transmitted AIDS feasible by enabling the development of a simple test for the detection of AIDS-infected blood by blood banks and diagnostic laboratories. August — The Cancer Prevention Fellowship Program CPFP , one of the first formal postdoctoral research training programs in cancer prevention, began. CPFP provides state-of-the-art training in cancer prevention and control through mentored research at NCI, guiding each fellow to develop an independent research program in cancer prevention. October 24, —The Office of Technology Development was established in the Office of the Director to guide implementation of legislation, rules and regulations, and activities relating to collaborative agreements, inventions, patents, royalties, and associated matters. September 30, —The first Consortium Cancer Center was established, comprised of three historically black medical schools. Component universities supported by this core grant—Charles R. Drew University of Medicine and Science in Los Angeles, Meharry Medical College in Nashville, and Morehouse School of Medicine in Atlanta—focused their efforts on cancer prevention, control, epidemiology, and clinical trials. April —NCI increased efforts to supplement research grants to encourage recruitment of minority scientists and science students into extramural research laboratories. This initiative is expanded to include scientists and science students who are women or individuals with disabilities. May 22, —NCI scientist Dr. Steven A. Rosenberg conducted the first human gene transfer trial using human tumor-infiltrating lymphocytes to which a foreign gene had been added. December 20, — NCI researchers published the Breast Cancer Risk Assessment Model, a tool to estimate a woman's risk of developing invasive breast cancer, based on data from case-control studies and breast cancer incidence rates in the US. Over the years, the tool has been updated and expanded to cover African Americans and other groups; it is widely used by clinicians and researchers. Originally available on a floppy disk, the tool was later renamed and made available online as the Breast Cancer Risk Assessment Tool. The US Food and Drug Administration guidelines for use of tamoxifen and raloxifene for breast cancer risk reduction rely on estimates generated by the tool. It is also used for patient counseling and for assessing possible public health prevention strategies. September 14, —Scientists from NCI and National Heart, Lung, and Blood Institute announced the first trial in which a copy of a faulty gene was inserted into white blood cells to reverse the immune deficiency it caused. The trial was initiated to treat adenosine deaminase deficiency. This was the first human gene therapy trial used to treat immunodeficiency. January 29, —Patients with melanoma were treated with tumor-infiltrating lymphocytes to which a gene for tumor necrosis factor had been added. This was the first human gene therapy trial used to treat cancer. October —NCI began the 5 A Day program, in partnership with the nonprofit group Produce for Better Health, to encourage Americans to eat at least five fruits and vegetables a day. December 18, —Taxol paclitaxel , an anticancer drug extracted from the bark of the Pacific yew, received approval by the U. Food and Drug Administration FDA for the treatment of ovarian cancer that was not responsive to other therapy. NCI spearheaded the development of the drug through collaboration with the USDA's Forest Service, the Department of the Interior's Bureau of Land Management, and Bristol-Myers Squibb Company. This collaboration was made possible by the Federal Technology Transfer Act of November —The Prostate, Lung, Colorectal, and Ovarian trial, designed to determine whether certain screening tests will reduce the number of deaths from these cancers, began recruiting , men and women, ages 55— This was the first sustained decline since national recordkeeping was instituted in the s. The office, now called the Office of Advocacy Relations, supports NCI's research and programs by fostering strong communications and partnerships with the cancer advocacy community and professional societies. August 1, —NCI, in partnership with government, academic, and industrial laboratories, launched the Cancer Genome Anatomy Project to enhance discovery of the acquired and inherited molecular changes in cancer and to evaluate the clinical potential of these discoveries. The project included a website allowing scientists to rapidly access data generated through the project and apply it to their studies. April 6, —Results of the Breast Cancer Prevention Trial, testing the effectiveness of tamoxifen to prevent the disease, were announced 14 months earlier than expected. September 25, —The FDA approved the monoclonal antibody Herceptin Trastuzumab for the treatment of metastatic breast cancer in patients with tumors that produce excess amounts of a protein called HER May 25, —The Study of Tamoxifen and Raloxifene STAR one of the largest breast cancer prevention studies ever, began recruiting volunteers at more than centers across the United States, Puerto Rico, and Canada. The trial will include 22, postmenopausal women at increased risk of breast cancer to determine whether the osteoporosis prevention drug raloxifene Evista is as effective in reducing the chance of developing breast cancer as tamoxifen Nolvadex has proven to be. December 8, —NCI published the Atlas of Cancer Mortality, —94 , showing the geographic patterns of cancer death rates in over 3, counties across the country over more than 4 decades. The Special Populations Networks for Cancer Awareness Research and Training is intended to build relationships between large research institutions and community-based programs. Eighteen grants at 17 institutions are expected to create or implement cancer control, prevention, research, and training programs in minority and underserved populations. June 7, —President Clinton issued an executive memorandum directing the Medicare program to reimburse providers for the cost of routine patient care in clinical trials. The memorandum also provides for additional actions to promote the participation of Medicare beneficiaries in clinical studies. December 3, —As part of a national commitment to identify and address the underlying causes of disease and disability in racial and ethnic communities, NCI established the Center to Reduce Cancer Health Disparities. Because these communities carry an unequal burden of cancer-related health disparities, NCI is working to enhance its research, education, and training programs that focus on populations in need. May 10, —FDA approval of the drug Gleevec, also known as STI, is announced as an oral treatment for chronic myelogenous leukemia. This marked the approval of the first molecularly targeted drug that directly turns off the signal of a protein known to cause a cancer. Clinical trials expanded as investigators tested Gleevec in a variety of cancers that share common molecular abnormalities. July 24, —The largest-ever prostate cancer prevention study was launched by NCI and a network of researchers known as the Southwest Oncology Group. The Selenium and Vitamin E Cancer Prevention Trial is designed to determine if these two dietary supplements can protect against prostate cancer which is the most common form of non-skin cancer in men. The study is expected to include a total of 32, men. September 4, —NCI and the American College of Radiology Imaging Network launched 3-year multicenter study of digital mammography, called the Digital Mammographic Imaging Screening Trial, the first large, multicenter study to compare digital mammography to standard mammography for the detection of breast cancer. September 10, —NCI launched the Consumer Advocates in Research and Related Activities program—a landmark initiative convening a network of dedicated advocates who bring the viewpoint of those affected by cancer to NCI. February 7, —Scientists from NCI and FDA reported that patterns of proteins found in patients' blood may reflect the presence of ovarian cancer, even at early stages. This new diagnostic concept is potentially applicable to the diagnosis of other diseases. May 19, —Researchers from NCI reported that the molecularly targeted drug bevacizumab slowed tumor growth in patients with metastatic renal cell carcinoma, the most common form of kidney cancer in adults. June 19, —NCI scientists used microarray technology to determine the patterns of genes that are active in tumor cells from which they could predict whether patients with the most common form of non-Hodgkin lymphoma in adults are likely to be cured by chemotherapy. July 16, —The National Heart, Lung, and Blood Institute NHLBI of NIH stopped a major clinical trial early of the risks and benefits of combined estrogen and progestin in healthy menopausal women due to an increased risk of invasive breast cancer. The large multi-center trial, a component of the Women's Health Initiative WHI , also found increases in coronary heart disease, stroke, and pulmonary embolism in study participants on estrogen plus progestin compared to women taking placebo pills. The trial showed that postmenopausal women who used estrogen replacement therapy for 10 or more years were at significantly higher risk of developing ovarian cancer than women who never used hormone replacement therapy. September 18, —NCI launched the National Lung Screening Trial to compare spiral computed tomography and single-view chest x-ray for early lung cancer in 50, current and former heavy smokers. The trial will examine the relative risks and benefits of both tests at 30 study sites throughout the United States. September 19, —NCI researchers demonstrated that a new approach to cancer treatment, that replaces a patient's immune system with cancer-fighting cells produced in the laboratory specifically to destroy their tumors, can lead to tumor shrinkage. The experimental technique, known as adoptive transfer, has shown promising results in patients with metastatic melanoma who have not responded to standard treatment. October 16, —NCI and FDA scientists found that patterns of proteins in patients' blood, called prostate-specific antigen PSA , may help distinguish between prostate cancer and benign prostate conditions. PSA is a protein produced by cells of the prostate gland. The blood level of PSA is often elevated in men with prostate cancer. The technique may be useful in deciding whether to perform a biopsy in men with elevated levels of PSA. October 31, —NCI researchers have discovered that a molecule best known for its antimicrobial properties also can activate key cells in the immune response. This newly discovered function suggests the molecule, a peptide called Beta-defensin 2, may be useful in the development of more effective cancer vaccines. May 30, —Overseen by an Interagency Oncology Task Force IOTF , FDA and NCI entered into an agreement to share knowledge and resources to facilitate the development of new cancer drugs and speed their delivery to patients. The goal of the IOTF is to leverage the expertise and capabilities of both agencies to streamline and accelerate the overall development of diagnostic, preventive, and therapeutic interventions for cancer. June 24, —Results of the Prostate Cancer Prevention Trial, testing the effectiveness of finasteride to prevent the disease, were announced about a year earlier than expected. There was a note of caution, however; the men who did develop prostate cancer while taking finasteride were more likely to have high-grade tumors. September 2, —Death rates from the four most common cancers—lung, breast, prostate, and colorectal—continued to decline in the late s according to data from the Annual Report to the Nation on the Status of Cancer, This report provides an annual update of cancer incidence, mortality, and trends in the United States. NCI has partnered with The Centers for Disease Control and Prevention, the American Cancer Society, and the North American Association of Central Cancer Registries on the report since November 6, —NCI scientists demonstrated that the growth factors interleukin-2 IL-2 and IL have contrasting roles in the life and death of lymphocytes, an observation that has implications for the immunotherapy of cancer and autoimmune diseases. September 13, —NCI announced the Alliance for Nanotechnology in Cancer, a five-year initiative to integrate nanotechnology development into basic and applied cancer research to facilitate the rapid application of this science to the clinic. Nanotechnology is a field of research that deals with the engineering and creation of things from materials that are less than nanometers one-billionth of a meter in size, especially single atoms or molecules. Nanotechnology offers the means to target treatments directly and selectively to cancerous cells and neoplasms. December 10, —According to research supported by NCI, and performed in collaboration with the National Surgical Adjuvant Breast and Bowel Project and Genomic Health Inc. April 25, —The Herceptin Adjuvant HERA Breast International Group [BIG] Trial showed the combination of the targeted agent trastuzumab Herceptin and standard chemotherapy cuts the risk of HERpositive breast cancer recurrence by more than half compared with chemotherapy alone. For women with this type of aggressive breast cancer, the addition of trastuzumab to chemotherapy appears to virtually reverse prognosis from unfavorable to good. May 6, —NCI announced the Community Networks Program, a five-year initiative to reduce cancer disparities in minority and underserved populations through community participation in education, research, and training. September 16, —Preliminary results from a large clinical trial of digital versus film mammography showed no difference in detecting breast cancer for the general population of women in the trial. However, those women with dense breasts, who are pre- or perimenopausal or who are younger than age 50, may benefit from having a digital rather than a film mammogram. September 28, —NCI and the National Institute of Neurological Disorders and Stroke NINDS created Rembrandt Repository for Molecular Brain Neoplasia Data , a joint informatics initiative to molecularly characterize a large number of primary brain tumors and to correlate those data with extensive retrospective and prospective clinical data. October —NCI initiated the Patient Navigator Research Program PNRP to assess the impact of patient navigators on providing timely and quality standard cancer care to patients following an abnormal cancer finding. The PNRP was designed to encourage research collaborations and partnerships with organizations serving diverse underserved communities within cancer care delivery systems. October 5, —The Annual Report to the Nation on the Status of Cancer , , showed observed cancer death rates from all cancers combined dropped 1. The report's authors attribute the declines in death rates to progress in prevention, early detection, and treatment. October 11, —NCI announced the Transdisciplinary Research on Energetics and Cancer initiative to study the effects of diet, weight, and physical activity on cancer and to answer critical questions to help guide our nation's public health efforts. November 7, —NCI launched a cancer biorepository pilot project designed to standardize biospecimen collection and management among investigators of Specialized Programs of Research Excellence for prostate cancer to enhance the quality and availability of various biospecimens and associated data for the broader scientific community. December 7, —Results from several studies presented at the San Antonio Breast Cancer Symposium validated that a test called Oncotype DX can predict the risk of breast cancer recurrence in a sizable group of patients. The studies also appeared to identify which of those patients might benefit most from chemotherapy. The studies were heralded by researchers as an important moment in the move toward individualized cancer care. December 13, —NCI and the National Human Genome Research Institute launched a comprehensive effort to accelerate an understanding of the molecular basis of cancer through the application of genome analysis technologies, especially large-scale genome sequencing. The overall effort, called The Cancer Genome Atlas, began with a pilot project to determine the feasibility of a full-scale effort to systematically explore the universe of genomic changes involved in all types of human cancer. April 17, —Initial results of the Study of Tamoxifen and Raloxifene showed that the drug raloxifene, used to prevent and treat osteoporosis in postmenopausal women, works as well as tamoxifen in reducing breast cancer risk for postmenopausal women at increased risk of the disease. May 23, —The Trial Assigning Individualized Options for Treatment Rx or TAILORx was launched to examine whether genes that are frequently associated with risk of recurrence for women with early-stage breast cancer can be used to assign patients to the most appropriate and effective treatment. June 7, —Gene profiling, a molecular technique that examines many genes simultaneously, was shown to accurately distinguish between two types of immune cell tumors, Burkitt's lymphoma and diffuse large B-cell lymphoma DLBCL. Burkitt's lymphoma and DLBCL appear similar when viewed under a microscope but correct diagnosis is critical because each requires very different treatments. June 8, —NCI announced FDA approval of the human papillomavirus HPV vaccine, based on the research of NCI scientists Douglas Lowy, M. Nearly two decades before, researchers at NCI and other institutions began searching for the underlying causes of cervical cancer. That scientific quest led to the vaccine Gardasil human papillomavirus [HPV] vaccine, quadrivalent , which protects against infection from the two types of human papillomavirus that cause the majority of cervical cancers worldwide. June 29, —Researchers at NCI identified a link between inherited and acquired genetic factors that dramatically increase the chance of developing a very common type of melanoma. case-control study, and incidence rates for melanoma in the U. The Melanoma Risk Assessment Tool can be used by health professionals to identify individuals at increased risk of melanoma, help them plan for regular screening, and potential. October 5, —The Biomarkers Consortium, a public-private biomedical research partnership composed of The Foundation for the National Institutes of Health, NIH, FDA, and the Pharmaceutical Research and Manufacturers of America, formed to search for and validate new biomarkers to accelerate the delivery of new technologies, medicines, and therapies for prevention, early detection, diagnosis, and treatment of disease. October 18, —NCI released new data from the Cancer Genetic Markers of Susceptibility CGEMS study on prostate cancer intended to help identify genetic factors that influence the disease and could be integral to the discovery and development of new, targeted therapies. This was the first public release of a whole-genome association study of cancer—such studies examine the entire genome, with no assumptions about which genetic alterations cause cancer. Adding an MRI scan to the diagnostic evaluation effectively doubled the number of cancers immediately found in these women. Age-adjusted breast cancer incidence rates in women in the United States fell 6. Prescriptions for HRT also declined rapidly in and May 8, —Researchers from NCI and Baylor College of Medicine found that people infected with the hepatitis C virus are at an increased risk of developing certain lymphomas cancers of the lymphatic system. June 14, —NCI launched the three-year pilot phase of a new program that will help bring state-of-the-art cancer care to patients in community hospitals across the United States. October 15, — The Annual Report to the Nation on the Status of Cancer , , showed cancer death rates decreased on average 2. November 27, —A new model for calculating invasive breast cancer risk the CARE model was found to give better estimates of the number of breast cancers that would develop in African American women 50 to 79 years of age than an earlier model which was based primarily on data from white women. January —Scientists reported that results of a randomized phase III clinical trial show that a combination of low oral doses of difluoromethylornithine and sulindac greatly reduces the recurrence of colon polyps and is safe and well tolerated. March 6, —DNA mutations found in a type of non-Hodgkin lymphoma that has a poor prognosis led researchers to a better understanding of how the cancer develops and how it might be treated. April 21, —Researchers identified a pattern of gene activity in mice that may help to predict individual risk for breast cancer metastasis and survival in humans. A single gene called bromodomain 4 Brd4 regulates the expression of this pattern, also called a signature. Researchers found that one result of this Brd4 regulation is the suppression of tumor growth and metastasis in a mouse model of cancer. June 23, —NCI researchers found that cells from a blood-borne cancer called multiple myeloma rely on the activity of a single protein, IRF4, for the activation of a wide range of genes responsible for cell survival and spread. Blocking the production of this protein can be strikingly effective in eliminating cancer cells in laboratory models of multiple myeloma. January 1, —Scientists identified mutations in a gene that predict a high likelihood of relapse in children with acute lymphoblastic leukemia ALL. Although further research is needed, the findings are likely to provide the basis for future diagnostic tests to assess the risk of treatment failure. By using a molecular test to identify this genetic marker in ALL patients, physicians should be better able to determine appropriate therapies. February 11, —Researchers established that abnormal white blood cells can be present in patients' blood more than six years prior to the diagnosis of a chronic form of lymphocytic leukemia. This finding may lead to a better understanding of the cellular changes that characterize the earliest stages of the disease and how it progresses. March 18, —A new report from the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, designed to provide answers about the effectiveness of prostate cancer screening, showed that six annual screenings for prostate cancer led to more diagnoses of the disease, but no fewer prostate cancer deaths. August 14, —Results of a randomized phase III clinical trial show that targeted therapy with the drug imatinib mesylate Gleevec reduces disease recurrence following surgery to remove a localized gastrointestinal stromal tumor. October 5, —Researchers identified a gene that may play a role in the growth and spread of a childhood cancer called rhabdomyosarcoma, which develops in the body's soft tissues. The finding revealed a potential new target for the treatment of this disease. October 7, —Researchers found that a small RNA molecule, known as a microRNA, may help physicians identify liver cancer patients who, in spite of their poor prognosis, could respond well to treatment with a biological agent called interferon. December 18, —Initial results from a large, randomized clinical trial for patients with multiple myeloma, a cancer of the blood and bone marrow, showed that patients who received the oral drug lenalidomide Revlimid, also known as CC following a blood stem cell transplant avoided recurrences longer than patients who received a placebo. January 6, —Researchers with a study conducted at NCI, the National Institute for Allergy and Infectious Diseases, and the National Human Genome Research Institute, discovered genetic mutations that may contribute to the development of an aggressive form of non-Hodgkin lymphoma. These findings provided insight into a mechanism that cancer cells may use to survive, thus identifying potential new targets for treatment of the disease. January 19, —Researchers found that the most common form of malignant brain cancer in adults, glioblastoma multiforme, is not a single disease but appears to be four distinct molecular subtypes that respond to aggressive chemotherapy and radiation differently, according to a study by The Cancer Genome Atlas Research Network. April 19, —Long-term results show that Raloxifene, a common osteoporosis drug, prevented breast cancer to the same degree, but with fewer serious side-effects than the drug Tamoxifen that had been in use many years for breast cancer prevention as well as treatment. In particular, there was significantly less endometrial cancer with raloxifene use. November 10, —Researchers discovered mutations in a particular gene that affects the treatment prognosis for some patients with acute myeloid leukemia, an aggressive blood cancer that kills 9, Americans annually. December 23, —NCI announced major changes to the long-established Clinical Trials Cooperative Group Program that conducts many of the nationwide trials of new cancer therapies. In a major transformation, NCI intends to consolidate the nine groups that currently conduct trials in adult cancer patients into four state-of-the-art entities that will design and perform improved trials of cancer therapies. The changes are designed to provide greater benefits for cancer patients and more information for researchers. These moves come in response to an NCI-requested April report from the Institute of Medicine, which called for a series of changes to the cooperative groups program, including restructuring. The NCI Cooperative Group program, founded over 50 years ago, involves more than 3, institutions and 14, investigators, and the program enrolls over 25, patients in clinical trials each year. March 10, —The number of cancer survivors in the United States increased to There were 3 million cancer survivors in and 9. March 31, —Rates of death in the United States from all cancers for men and women continued to decline between and , the most recent reporting period available, according to the latest Annual Report to the Nation on the Status of Cancer. June 5, —NCI announced clinical trial results showing that in a high-risk form of pediatric acute lymphoblastic leukemia, a high-dose schedule of a drug raises already high cure rates even higher. June 29, —An analysis of genomic changes in ovarian cancer has provided the most comprehensive and integrated view of cancer genes for any cancer type to date. Ovarian serous adenocarcinoma tumors from patients were examined by The Cancer Genome Atlas TCGA Research Network. TCGA researchers completed whole-exome sequencing, which examines the protein-coding regions of the genome, on an unprecedented tumors. September, 8, —The NCI-sponsored Costa Rica Vaccine Trial was designed to assess the efficacy of Cervarix in a community-based setting. Two doses of the human papillomavirus vaccine HPV Cervarix were as effective as the current standard three-dose regimen after four years of follow-up. January 19, —A clinical trial has shown that addition of chemotherapy to radiation therapy leads to a near doubling of median survival time in patients with a form of brain tumor oligodendroglioma that carries a chromosomal abnormality called the 1p19q co-deletion. March 2, —The Diesel Exhaust in Miners Study looked at 12, workers at 8 non-metal mining facilities in the United States. Federal government scientists reported that heavy exposure to diesel exhaust increased risk of death from lung cancer. March 8, —In a new examination of United States cancer incidence data, investigators reported that incidence trends have remained roughly constant for glioma, the main type of brain cancer hypothesized to be related to cell phone use. May 21, — In a study that spanned 20 years, the Prostate, Lung, Colorectal, and Ovarian PLCO Cancer Screening Trial, sponsored by NCI, found that sigmoidoscopy is effective in reducing the rates of new cases and deaths due to colorectal cancer. September 27, —Scientists may have discovered why a protein called MYC can provoke a variety of cancers. Like many proteins associated with cancer, MYC helps regulate cell growth. A study carried out by researchers at NIH and colleagues found that, unlike many other cell growth regulators, MYC does not turn genes on or off, but instead boosts the expression of genes that are already turned on. More than 30 percent of all human cancers are driven by mutations of the RAS family of genes. This approach is called a "hub and spoke" model. The Frederick National Laboratory for Cancer Research FNLCR acts as the hub that connects to the larger community of RAS researchers around the world combining efforts and creating new ways to approach the complex issue of RAS. September 19, —A world-wide competition to bring emerging breast cancer technologies to market was launched by the Avon Foundation for Women, in partnership with NCI and the Center for Advancing Innovation. Teams were offered the opportunity to create strategic business plans and the potential to start new companies based on the development of 10 unlicensed breast cancer inventions by turning them into commercially marketed products. Breast cancer inventions include therapeutics, diagnostics, prognostics, one device, one vaccine, and a health IT invention, all from the NCI intramural Center for Cancer Research and Avon Foundation-funded university labs. October 10, —Glioblastoma multiforme GBM was the first cancer type to be systematically studied by The Cancer Genome Atlas Research Network TCGA in In a new, complementary report, TCGA experts examined more than GBM samples—the largest to date utilizing genomic characterization techniques and nearly more than were examined in —to identify several additional significantly mutated genes in GBM. November 13, —A trial conducted by researchers at NCI showed adult patients with a type of cancer known as Burkitt lymphoma had excellent long-term survival rates—upwards of 90 percent—following treatment with low-intensity chemotherapy regimens. Standard treatment for Burkitt lymphoma involves high-dose chemotherapy, which has a high rate of toxicity, including death, and cures only 60 percent of adult patients. November 20, —NCI scientists report that the incidence of oropharyngeal cancer significantly increased during the period among people in countries that are economically developed. Recent studies from several countries have reported rising incidence of oropharyngeal cancers and subsequent studies have shown the human papilloma virus HPV as the potential cause. Researchers note that prophylactic HPV vaccine has been shown to protect against oral HPV infection, suggesting an additional benefit of vaccination programs for both women and men. March 1, —NCI transformed its longstanding Cooperative Group program into the new National Clinical Trials Network NCTN. Recent advances in deciphering the cancer genome have enabled the development of targeted therapies. To explore targeted therapies, cancer clinical trials need to screen large numbers of patients with the same or different histologic tumor types to identify those patients whose tumors contain the distinct molecular targets of the therapies being tested. March 5, — Ten winners of a world-wide competition to bring emerging breast cancer research technologies to market faster were announced today by the Avon Foundation for Women, in partnership with the NCI, and the Center for Advancing Innovation CAI. ALCHEMIST represents three integrated, precision medicine trials that are designed to identify people with early-stage lung cancer who have tumors that harbor EGFR and ALK gene alterations and evaluate whether drug treatments targeted against those molecular changes can lead to improved survival compared to current standard of care therapy alone e. August 30, —In a large international collaborative analysis of risk factors for non-Hodgkin lymphoma NHL , NCI scientists were able to quantify risk associated with medical history, lifestyle factors, family history of blood or lymph-borne cancers, and occupation for 11 different NHL subtypes, including less common subtypes. These findings provide crucial insight into the diverse factors that drive different NHL subtypes and correspond with their biological and clinical characteristics. October 7, —President Obama announced that John Schiller, Ph. The honorees received their medals at a White House ceremony later in as recognition for their outstanding contributions to discoveries that enabled the development of HPV vaccines. NCORP is a national network of investigators, cancer care providers, academic institutions, and other organizations. The National Cancer Institute NCI is the federal government's principal agency for cancer research and training. The NCI coordinates the National Cancer Program, which conducts and supports research, training, health information dissemination, and other programs with respect to the cause, diagnosis, prevention, and treatment of cancer, rehabilitation from cancer, and the continuing care of cancer patients and the families of cancer patients. Novartis Oncology is a global leader in transforming outcomes for people with cancer. We offer a wide range of innovative therapies to help meet patient needs and have one of the strongest, most productive pipelines in the industry. Our research is driven by a distinctive scientific and clinical strategy focused on precision oncology — understanding how cancer develops on a genomic level and developing drugs that hone in on those targets. At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives through the discovery, development and manufacture of healthcare products. We work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. We collaborate with healthcare providers, governments and local communities to support and expand access to reliable, affordable healthcare around the world. Roche is also the world leader in in vitro diagnostics and tissue-based cancer diagnostics, and a frontrunner in diabetes management. Founded in , Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable contribution to society. The company also aims for improving patient access to medical innovations by working with all relevant stakeholders. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. The Roche Group, headquartered in Basel, Switzerland, is active in over countries and in employed more than 94, people worldwide. Sanofi is a global life sciences company committed to improving access to healthcare and supporting the people we serve throughout the continuum of care. From prevention to treatment, Sanofi transforms scientific innovation into healthcare solutions, in human vaccines, rare diseases, multiple sclerosis, oncology, immunology, infectious diseases, diabetes and cardiovascular solutions and consumer healthcare. World Cancer Congress Learn more. World Cancer Day Main navigation About UICC What is cancer control? General Assembly General Assembly Member benefits Learning and development Online learning Master courses. Implementation research for cancer prevention in Europe. Technical Fellowships. Regional Dialogues. Virtual Dialogues Ageing and Cancer series. TNM project structure. World Cancer Day The campaign. World Cancer Leaders' Summit Programme, speakers and awards. Targeted commitments Cancer Resolution. UICC Advocacy Network. About ATOM Coalition. Young Leaders Thematic areas Antimicrobial resistance AMR World AMR Awareness Week WAAW. Breast Cancer Awareness Month. UICC's actions on cervical cancer. Resources for tobacco control. Members Membership categories and fees. Our partners. World Health Organization WHO. Access all resources. Main navigation Who we are About UICC What is cancer control? Union for International Cancer Control UICC About us. Leading global action on cancer. Supporting the cancer community. Driving global impact. What is cancer control? News and updates Five individuals awarded YY Study Grants to further cancer research. Featured resources. Author s : Union for International Cancer Control UICC. Download 9. Download 2. Download 8. |
| Cancer Initiatives | CDC | August — The Cancer Prevention Fellowship Program CPFP , one of the first formal postdoctoral research training programs in cancer prevention, began. This newly discovered function suggests the molecule, a peptide called Beta-defensin 2, may be useful in the development of more effective cancer vaccines. February 7, —Scientists from NCI and FDA reported that patterns of proteins found in patients' blood may reflect the presence of ovarian cancer, even at early stages. Program investigators study cancer-prone families to identify and clone predisposing genes; investigate the prevalence of identified genes in the general population; conduct pharmacogenetic studies to evaluate genetic polymorphisms as determinants of cancer risk and treatment outcomes; conduct integrative analyses of environmental and germline risk factors with comprehensive data on histological and molecular profiling of tumors and their precursors, including somatic genomic analyses; and translate advances in molecular genetics into evidence-based management strategies. December 20, — NCI researchers published the Breast Cancer Risk Assessment Model, a tool to estimate a woman's risk of developing invasive breast cancer, based on data from case-control studies and breast cancer incidence rates in the US. July 27, —A Bureau-level organization was established for NCI, giving the Institute and its components organizational status commensurate with the responsibilities bestowed on it by the National Cancer Act of |
Es hat den Sinn nicht.
der Maßgebliche Standpunkt, neugierig.