Diuretic effect on kidneys -
To convert milligrams per deciliter to micromoles per liter, multiply by To convert milligrams per deciliter to millimoles per liter, multiply by 0. Figure 2. Time to Death or Dialysis From Day of Consultation in Intensive Care Unit View Large Download.
Groups are stratified by day 1 status. at risk for days 1, 2, 3, and 5 were 35, 19, 10, and 3, respectively. Analysis includes of the patients who survived at least 7 days after nephrology consultation in the intensive care unit. Data are excluded for 5 patients who died at an unknown time.
Table 1. Table 2. Klahr S, Miller SB. Acute oliguria. N Engl J Med. Google Scholar. Sladen RN. Oliguria in the ICU: systematic approach to diagnosis and treatment.
Anesthesiol Clin North America. Bellomo R, Ronco C. Indications and criteria for initiating renal replacement therapy in the intensive care unit.
Kidney Int Suppl. Wilson WC, Aronson S. Oliguria: a sign of renal success or impending renal failure? Anderson RJ, Linas SL, Berns AS. et al. Nonoliguric acute renal failure. Diamond JR, Yoburn DC. Arch Intern Med. Brown RS. Renal dysfunction in the surgical patient: maintenance of high output state with furosemide.
Crit Care Med. Gerlach AT, Pickworth KK. Contrast medium-induced nephrotoxicity: pathophysiology and prevention. Solomon R, Werner C, Mann D, D'Elia J, Silva P. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents.
Weinstein JM, Heyman S, Brezis M. Potential deleterious effect of furosemide in radiocontrast nephropathy. Davidman M, Olson P, Kohen J, Leither T, Kjellstrand C.
Iatrogenic renal disease. Lassnigg A, Donner E, Grubhofer G, Presterl E, Druml W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. J Am Soc Nephrol. Visweswaran P, Massin EK, Dubose Jr TD. Mannitol-induced acute renal failure.
Brown CB, Ogg CS, Cameron JS. High dose furosemide in acute renal failure: a controlled trial. Clin Nephrol.
Kleinknecht D, Ganeval D, Gonzalez-Duque LA, Fermanian J. Furosemide in acute oliguric renal failure: a controlled trial. Gubern JM, Sancho JJ, Simo J, Sitges-Serra A. A randomized trial on the effect of mannitol on postoperative renal function in patients with obstructive jaundice.
Shilliday IR, Quinn KJ, Allison ME. Loop diuretics in the management of acute renal failure: a prospective, double-blind, placebo-controlled, randomized study. Nephrol Dial Transplant.
Chang RW, Jacobs S, Lee B, Pace N. Predicting deaths among intensive care unit patients. Mehta RL, McDonald B, Gabbai FB.
A randomized clinical trial of continuous versus intermittent dialysis for acute renal failure. Kidney Int. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic ROC curve. Lemeshow S, Hosmer Jr DW.
A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. Kaplan E, Meier P. Nonparametric estimation from incomplete observations.
Mehta RL, Pascual MT, Gruta CG, Zhuang S, Chertow GM. Refining predictive models in critically ill patients with acute renal failure. Kellum JA. Diuretics in acute renal failure: protective or deleterious?
Blood Purif. Venkataram R, Kellum JA. The role of diuretic agents in the management of acute renal failure. Contrib Nephrol. The use of diuretics and dopamine in acute renal failure: a systematic review of the evidence. Crit Care Lond. Lewis J, Salem MM, Chertow GM. for the Anaritide Acute Renal Failure Study Group.
Atrial natriuretic factor in oliguric acute renal failure. Am J Kidney Dis. Bullock ML, Umen AJ, Finkelstein M, Keane WF. The assessment of risk factors in patients with acute renal failure.
Liano F, Gallego A, Pascual J. Prognosis of acute tubular necrosis: an extended prospectively contrasted study. McCarthy JT. Prognosis of patients with acute renal failure in the intensive-care unit: a tale of two eras.
At the end of the crossover phase, the combination therapy was administered for an additional month. In this small trial, hydrochlorothiazide, but not furosemide, significantly increased fractional sodium excretion, and the combination of the two drugs did not induce significantly higher natriuretic or antihypertensive effects.
In contrast, the same authors tested the same protocol in a larger study 23 patients with stage 4—5 CKD applying a longer washout period 3 months [ 68 ]. In the trial the hydrochlorothiazide—furosemide combination prompted a larger natriuretic and hypotensive effect as compared with the same drugs administered in isolation.
Therefore this trial indicates close monitoring of adverse events in patients with mild to moderate CKD. In a recent trial by Agarwal et al. In this trial, patients with uncontrolled hypertension confirmed by hour ABP monitoring were randomized to receive chlorthalidone At baseline, the mean eGFR was At baseline, the mean hour SBP was mmHg SD 8 in the chlorthalidone group and mmHg SD 8 in the placebo group, respectively.
The BP-lowering effect of chlorthalidone was paralleled by favourable changes in albuminuria. Changes in plasma renin and aldosterone levels in the chlorthalidone group were consistent with changes in body fluid volume and, together with BP and albuminuria, in large part reverted by 2 weeks after treatment discontinuation.
A synergistic effect for natriuresis exists between chlorthalidone and furosemide in patients with refractory HF [ 56 ] and such a synergism may also hold true in stage 4 CKD patients. Hypokalaemia, reversible increases in serum creatinine level, hyperglycaemia, dizziness and hyperuricemia occurred more frequently in the chlorthalidone group than in the placebo group.
However, serious adverse events requiring hospitalization were similar between groups 8 events in the chlorthalidone group versus 11 events in the placebo group. As previously alluded to, erythrocytes serve as a reservoir for chlorthalidone and the half-life of this drug is 45—60 hours with an effect on BP extended up to 72 hours [ 70 ].
The lasting effects on BP and fluid volume in Agarwal's trial confirm the long duration of action of chlorthalidone [ 3 ]. Chlorthalidone is mostly eliminated as an unmodified molecule by renal excretion and therefore the longer half-life of this drug in patients with kidney insufficiency may contribute to its lasting hypotensive action in CKD patients [ 45 ].
As in previous BP-lowering trials, changes in eGFR were most likely due to reduced BP. Indeed, these changes were fully reversible after drug discontinuation and were not considered to be of clinical relevance. Accordingly, in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, the risk for kidney failure in patients randomized to chlorthalidone did not differ from that in patients randomized to amlodipine or lisinopril [ 71 ].
Like the parallel eGFR reduction, the lowering effect on albuminuria rapidly reverted after chlorthalidone discontinuation. Such an observation suggests that, as for other drugs, this is a haemodynamic effect triggered by reduced BP and possibly a potentiation of the effect of RAASis [ 72 ].
Since albuminuria is a surrogate of cardiorenal risk [ 73 ], chlorthalidone may provide cardiovascular and renal protection in CKD patients.
In this respect, evidence that thiazide-type diuretics may reduce cardiovascular risk in patients without CKD exists [ 54 , 55 ]. Thus chlorthalidone—a drug patented in that came into medical use in —may represent an important addition to the armamentarium available to nephrologists to counter the high cardiorenal risk of patients with CKD.
However, caution is needed when using this drug in CKD patients on loop diuretics, because the risk of a reduction in eGFR is augmented in these patients.
Chlorthalidone in CKD patients should be introduced gradually starting with Dose escalation should be applied with a vigilant attitude, i.
by measuring BP frequently and advising patients on how to deal with possible side effects. In highly responsive patients, diuretic treatment optimization may also demand a down-titration of loop diuretics. Finally, we should not forget that the ground-breaking trial by Agarwal et al.
was based on a relatively small number of patients and did not look at hard endpoints [ 3 ]. Therefore, despite the favourable results for BP control with chlorthalidone in patients with advanced CKD, phase 3 trials based on cardiovascular and renal endpoints are still needed to prove that this result translates effectively and safely in the prevention of cardiorenal events.
Given the high number of antihypertensive drugs that CLICK patients were taking at baseline [3. Spironolactone is recommended as a fundamental drug in resistant hypertension.
However effective, this drug imposes a doubling in the risk of hyperkalaemia as compared to angiotensin-converting enzyme inhibitors or angiotensin receptor blockers [ 75 ].
The use of patiromer mitigates the risk of hyperkalaemia by spironolactone in stage 3—4 CKD patients with resistant hypertension [ 76 ]. In general, spironolactone remains underutilized in resistant hypertension, particularly in CKD patients with this condition.
Findings in the CLICK study suggest that chlorthalidone may be a good alternative in the treatment of resistant hypertension in stage 4 CKD patients experiencing adverse effects to spironolactone. Hypervolemia is the main causative factor for hypertension in CKD patients and diuretics are central to improve BP control in CKD.
Among stage 4 CKD patients, loop diuretics are recommended over thiazides. Thiazide diuretics have long been considered ineffective in this population. This review of the literature suggests that thiazides may be useful even among people with advanced CKD.
These drugs cause a negative sodium balance and reduce body fluids by 1—2 l and these effects go hand in hand with improvement in hypertension control. The CLICK trial highlighted the great potential of chlorthalidone for the treatment of stage 4 CKD patients with poorly controlled hypertension and suggests that this drug may be a good alternative to spironolactone in treatment-resistant hypertension with and without CKD.
Hyponatremia, hypokalaemia, volume depletion and acute kidney injury are side effects that demand a vigilant attitude from physicians prescribing these drugs. Larger trials in advanced CKD focusing on antihypertensive and anti-albuminuric effects of chlorthalidone, and possibly also on hard outcomes, are still necessary to more confidently recommend the use of these drugs in these frail patients at high risk of iatrogenic adverse events.
De Nicola L , Minutolo R , Chiodini P et al. Global approach to cardiovascular risk in chronic kidney disease: reality and opportunities for intervention. Kidney Int ; 69 : — Google Scholar. Agarwal R , Gorski JC , Sundblad K et al.
Urinary protein binding does not affect response to furosemide in patients with nephrotic syndrome. J Am Soc Nephrol ; 11 : — 5. Agarwal R , Sinha AD , Cramer AE et al.
Chlorthalidone for hypertension in advanced chronic kidney disease. N Engl J Med ; : — De Nicola L , Minutolo R , Bellizzi V et al. Achievement of target blood pressure levels in chronic kidney disease: a salty question? Am J Kidney Dis ; 43 : — Bovée DM , Cuevas CA , Zietse R et al.
Salt-sensitive hypertension in chronic kidney disease: distal tubular mechanisms. Am J Physiol Renal Physiol ; : F — Titze J. A different view on sodium balance. Curr Opin Nephrol Hypertens ; 24 : 14 — Rakova N , Jüttner K , Dahlmann A et al. Cell Metab ; 17 : — Machnik A , Neuhofer W , Jantsch J et al.
Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med ; 15 : — Dahlmann A , Linz P , Zucker I et al. Kidney Int Rep ; 6 : — De Nicola L , Gabbai FB , Agarwal R et al. Prevalence and prognostic role of resistant hypertension in chronic kidney disease patients.
J Am Coll Cardiol ; 61 : — 7. Borrelli S , De Nicola L , Stanzione G et al. Resistant hypertension in nondialysis chronic kidney disease. Int J Hypertens ; : Minutolo R , Agarwal R , Borrelli S et al. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease.
Arch Intern Med ; : — 8. Panuccio V , Mallamaci F , Pizzini P et al. Reducing salt intake by urine chloride self-measurement in non-compliant patients with chronic kidney disease followed in nephrology clinics: a randomized trial.
Nephrol Dial Transplant ; 36 : — 9. Borrelli S , Mallamaci F , Chiodini P et al. Salt intake correlates with night systolic blood pressure in non-dialytic chronic kidney disease. Nephrol Dial Transplant ; 37 : — 9.
Cianciaruso B , Bellizzi V , Minutolo R et al. Renal adaptation to dietary sodium restriction in moderate renal failure resulting from chronic glomerular disease. J Am Soc Nephrol ; 7 : — Garofalo C , Borrelli S , Provenzano M et al. Dietary salt restriction in chronic kidney disease: a meta-analysis of randomized clinical trials.
Nutrients ; 10 : Buter H , Hemmelder MH , Navis G et al. The blunting of the antiproteinuric efficacy of ACE inhibition by high sodium intake can be restored by hydrochlorothiazide.
Nephrol Dial Transplant ; 13 : — 5. Esnault VL , Ekhlas A , Delcroix C et al. Diuretic and enhanced sodium restriction results in improved antiproteinuric response to RAS blocking agents. J Am Soc Nephrol ; 16 : — Vogt L , Waanders F , Boomsma F et al. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan.
J Am Soc Nephrol ; 19 : — Bovée DM , Visser WJ , Middel I et al. A randomized trial of distal diuretics versus dietary sodium restriction for hypertension in chronic kidney disease. J Am Soc Nephrol ; 31 : — Whelton PK , Carey RM , Aronow WS et al.
Hypertension ; 71 : e13 — 5. Williams B , Mancia G , Spiering W et al. Eur Heart J ; 39 : — Nerenberg KA , Zarnke KB , Leung AA et al. Hypertension Canada's guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children.
Can J Cardiol ; 34 : — Arnett DK , Blumenthal RS , Albert MA et al. J Am Coll Cardiol ; 74 : e — Unger T , Borghi C , Charchar F et al. Hypertension ; 75 : — Kidney Disease: Improving Global Outcomes Blood Pressure Work Group.
KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int ; 99 3 Suppl : S1 — Dasgupta I , Zoccali C. Hypertension ; 79 : 4 — Foti KE , Wang D , Chang AR et al.
Potential implications of the KDIGO blood pressure guideline for adults with chronic kidney disease in the United States. Kidney Int ; 99 : — Nouraei H , Leiter LA , Tan MK et al.
Glycemic control and cardiovascular risk factor management in adults with type 2 diabetes with and without chronic kidney disease before sodium-glucose cotransporter protein 2 inhibitors: insights from the Diabetes Mellitus Status in Canada survey.
Can J Diabetes ; 45 : — 9. Sasso FC , Pafundi PC , Simeon V et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: a randomized clinical trial in type-2 diabetic kidney disease.
Cardiovasc Diabetol ; 20 : Stengel B , Muenz D , Tu C et al. Adherence to the Kidney Disease: Improving Global Outcomes CKD guideline in nephrology practice across countries. Minutolo R , Gabbai FB , Chiodini P et al.
Sex differences in the progression of CKD among older patients: pooled analysis of 4 cohort studies. Am J Kidney Dis ; 75 : 30 — 8. Minutolo R , Gabbai FB , Agarwal R et al. Sex difference in ambulatory blood pressure control associates with risk of ESKD and death in CKD patients receiving stable nephrology care.
Nephrol Dial Transplant ; 36 : — 7. Drawz PE , Brown R , De Nicola L et al. Variations in hour BP profiles in cohorts of patients with kidney disease around the world: the I-DARE study.
Clin J Am Soc Nephrol ; 13 : — Adrogué HJ , Madias NE. Sodium and potassium in the pathogenesis of hypertension.
Winer BM. The antihypertensive actions of benzothiadiazines. Circulation ; 23 : — 8. Bennett WM , McDonald WJ , Kuehnel E et al. Do diuretics have antihypertensive properties independent of natriuresis?
Clin Pharmacol Ther ; 22 : — Wilson IM , Freis ED. Relationship between plasma and extracellular fluid volume depletion and the antihypertensive effect of chlorothiazide. Circulation ; 20 : — Cooper-DeHoff JD. Mechanisms for blood pressure lowering and metabolic effects of thiazide and thiazide-like diuretics.
However, recent data have revealed that diuretics could be effective as antihypertensive agents in patients with advanced CKD [ 8 , 9 ]. A year-old woman with a long duration of type 2 diabetes and hypertension was referred to the Division of Nephrology with worsening renal function and proteinuria.
Physical examination revealed regular heart beats without murmur, clear lung fields, and mild pitting edema. At presentation, she had the following results: serum creatinine, 1. In addition to maintaining current medications, what drug should be provided to this patient?
Current evidence has shown that controlling blood pressure to be lower than the previous one could reduce cardiovascular events and all-cause mortality in patients with CKD [ 10 , 11 , 12 ].
While previous guidelines have focused on the primary outcome of slowing CKD progression, nuance in blood pressure management of CKD patients recommended in the recent KDIGO guideline is the stronger emphasis on reduction of cardiovascular events and all-cause death rather than on renal protection.
Along with much lower blood pressure target recommended by the recent KDIGO guideline, poorly controlled hypertension is becoming much more common than before in patients with CKD, especially for those with advanced stages. Physicians may face agonizing dilemmas on which antihypertensive combination is the best to prescribe.
Unfortunately, there have been no randomized controlled trials comparing different drug combinations in CKD as there are no solid research studies on antihypertensive classes other than RAS inhibitors, β-blockers, and calcium channel blockers [ 7 ]. To find what combinations work the best for treating blood pressure in CKD, it is necessary to look again at the pathophysiology of hypertension in CKD.
Numerous factors including genetics, pressure natriuresis, salt sensitivity, renin-angiotensin-aldosterone system, sympathetic nervous system, obesity, natriuretic peptides, endothelial dysfunction, arterial stiffness, and immune system have been thought to be mainly involved in the pathogenesis of hypertension [ 18 ].
Among them, a key factor in the regulation of blood pressure as a factor of cardiac output and systemic vascular resistance must be the phenomenon of pressure natriuresis, which is defined as an increase in sodium excretion from kidney because of mild increases in blood pressure, allowing blood pressure to remain in the normal range [ 19 , 20 ].
For example, increased salt intake may cause an increase in extracellular volume and blood pressure. Subsequently, this increase in blood pressure will produce natriuresis, eventually restoring sodium balance and returning blood pressure to normal level.
In some circumstances, this response may become abnormal whenever there is an abnormal sodium handling such as in conditions of reduced glomerular filtration rate GFR , in which CKD is a representative example of this situation.
In CKD, reduced perfusion of kidneys theoretically could cause sodium retention and subsequent activation of systemic RAS Fig. However, increased RAS would be offset by volume expansion resulting from decreased excretion of urinary sodium and water.
As a result, systemic angiotensin II level in volume-expanded CKD might be rather normal or low. As CKD progresses, volume overload in whole body will likely worsen [ 21 ]. In this situation, it is natural that RAS blockades would be less effective for blood pressure control because of reduced systemic RAS.
Rather, volume control could improve systemic blood pressure and sensitivity to RAS inhibitors in CKD patients with edema because RAS inhibitors would work only under a condition that systemic RAS is reactivated after volume depletion. As such, sodium retention, an inevitable consequence of reduced GFR, not only has a major role in the pathogenesis of uncontrolled hypertension in patients with CKD, but also precludes optimal control of blood pressure during pharmacological treatment with nondiuretic antihypertensive agents [ 22 , 23 ].
Therefore, diuretics are logical agents at appropriate dosage to lower higher blood pressure in CKD [ 7 ]. Schematic view of volume overload-induced hypertension in chronic kidney disease. Reduced glomerular filtration rate in chronic kidney disease eventually produces sodium retention and a fall in plasma renin level with minimal dependence of systemic angiotensin II, causing volume expansion and subsequent increased arterial pressure.
In this situation, blockades of renin-angiotensin system are less effective in controlling systemic blood pressure. Oddly enough, diuretics for treating high blood pressure has been undervalued against other classes of antihypertensive agents for years. When numerous clinical trials have evaluated the efficacy and safety of each antihypertensive agent, a diuretic agent has been mainly used for comparison.
However, the adverse effect of diuretic on the progression of CKD or cardiovascular outcome has not been seen in all other studies. Post hoc analyses of a previous cardiovascular outcome trial have demonstrated that cardiovascular event rates are not higher in the diuretic group [ 30 , 31 ].
The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial ALLHAT has revealed that neither amlodipine nor lisinopril is superior to chlorthalidone in preventing major coronary events or in increasing survival [ 30 ].
In the ALLHAT, thiazide-type diuretics were proven to be unsurpassed in lowering blood pressure, reducing clinical events, and tolerability.
Moreover, the cost-effectiveness appears to be another good thing about diuretics since single-pill combinations including a thiazide diuretic could cost no more than a nondiuretic component alone Fig.
Price comparison of angiotensin receptor blocker alone or in fixed dose combination with hydrochlorothiazide HCTZ in Korea. Brands for each agent were chosen based on the original drug developer that work in the Republic of Korea.
The price of generic HCTZ 25 mg was KRW 10 in There has been a long-held belief that thiazide diuretics will lose efficacy in controlling diuresis and lowering blood pressure as GFR worsens [ 7 ].
However, it might be time to change the long-standing bias against the effectiveness of diuretics on blood pressure control in patients with CKD. Although evidence against the use of thiazide diuretics in advanced CKD is still weak [ 7 , 34 ], a few but significant trials have begun to test the role of thiazide diuretics in CKD Table 1 [ 8 , 35 , 45 , 50 , 53 , 55 ].
In a paper published in , Agarwal et al. This pilot study has demonstrated that the hour blood pressure in subjects with advanced CKD and resistant hypertension is significantly reduced by This pilot study helped design a subsequent, double-blind, randomized, placebo-controlled trial, the Chlorthalidone in Chronic Kidney Disease CLICK trial [ 8 ].
Most of the reduction in blood pressure occurred within 4 weeks after therapy using At 2 weeks after chlorthalidone therapy was discontinued, blood pressure remained below the baseline value.
However, renal function returned to approximately the baseline value, suggesting additional involvement of tubuloglomerular feedback [ 8 ]. It was also observed that the reduction in the degree of albuminuria in the chlorthalidone group occurred within 4 weeks. Based on results of the CLICK trial, chlorthalidone must be an effective blood pressure lowering agent even in patients with advanced CKD.
In the future, a larger trial of longer duration is needed to determine whether addition of chlorthalidone to a regimen of ACEi or ARB could further slow the progression of kidney disease and reduce cardiovascular risk without important safety concerns [ 9 ]. There are some differences in the volume of distribution and elimination half-life among thiazide diuretics.
As a class, thiazides including hydrochlorothiazide and thiazide-like diuretics including chlorthalidone, metolazone, and indapamide have different chemical structures, which might be associated with their different characteristics.
For example, chlorthalidone has longer duration of action and longer half-life elimination than hydrochlorothiazide [ 36 ]. It is expected to affect the extent and temporal pattern of blood pressure reduction, cardiovascular outcomes, or frequency of adverse events [ 37 , 38 ].
However, the recent results of Diuretic Comparison Project DCP found that chlorthalidone use was not associated with major cardiovascular benefits when compared with hydrochlorothiazide [ 39 ]. On the other hand, chlorthalidone use was associated with greater risk of renal and electrolyte abnormalities [ 39 , 40 ].
In addition, a population-based retrospective cohort study from Canada showed that chlorthalidone use was associated with a higher risk of eGFR decline, cardiovascular events, and hypokalemia compared with hydrochlorothiazide use [ 41 ].
Since there has been no clinical study targeting patients with CKD to examine whether all thiazide diuretics could have the same effect on blood pressure control and better clinical outcomes, the choice of the best one among thiazide diuretics for CKD is still unclear.
Although thiazide diuretics have been proven to be effective even in patients with CKD than previously thought, the mechanisms responsible for the blood pressure lowering effect observed for thiazide diuretics are incompletely understood [ 37 , 38 ].
After being rapidly absorbed by the gastrointestinal tract, thiazide diuretics are actively secreted through the renal organic anion transporter in renal proximal tubule [ 37 ]. In renal failure, competition for anion transporter in the proximal tubule by accumulated organic anions could decrease the amount of thiazide diuretic that could reach the tubular fluid and then diminish its natriuretic effect [ 37 ].
Considering that chronic tubulointerstitial injury may exert more profound reduction on expression levels of numerous transporters and channels of the kidney as CKD progresses [ 42 ], the antihypertensive effect of thiazide diuretics does not appear to rely on inhibition of sodium reabsorption by blocking NCC.
The mechanism for the ability of thiazide diuretics to acute lower of blood pressure is likely to be different from that of blood pressure lowering effect of a chronic therapy [ 37 , 38 , 43 ].
Thiazide diuretics can reduce blood pressure acutely by causing natriuresis, thereby reducing extracellular volume, venous return, and ultimately cardiac output [ 37 , 38 ] Fig.
In contrast, within 4 to 6 weeks of thiazide administration, compensatory salt and water reabsorption will return the extracellular volume towards baseline, which might be mediated by stimulation of the renin-angiotensin-aldosterone and sympathetic nervous systems resulting from thiazide-associated volume depletion [ 37 ].
The reason why the antihypertensive effect of thiazides persists even after normalization of the extracellular volume might be due to the fall in total peripheral resistance, which is caused by an unknown mechanism [ 38 ]. Vasodilatory effects such as activation of vascular potassium channels, opening of large conductance calcium-activated potassium channels, calcium desensitization, inhibition of voltage-dependent L-type calcium channels, release of endothelial-dependent relaxing factor and nitric oxide, and increased release of local vasodilatory factors such as prostaglandins have been suggested to contribute to enduring blood pressure lowering during chronic administration of thiazides [ 37 , 38 ].
Older studies have also found that antihypertensive effects of thiazide diuretics are correlated with an increment of urinary kallikrein excretion rather than with volemic changes [ 44 ]. Nonetheless, given that daily administration of hydrochlorothiazide or metolazone to patients who are anuric on dialysis for 4 weeks could not improve blood pressure [ 45 ], natriuresis and subsequent slight reduction in volume rather than off-target effects would mainly contribute to persistent antihypertensive action of thiazides.
Proposed mechanisms responsible for blood pressure lowering effect with thiazide diuretics. After administration of thiazide diuretics, blood pressure is initially lowered due to a reduction in extracellular volume and subsequent cardiac output. However, within weeks, compensatory reabsorption of sodium and water can lead to return of the extracellular volume towards baseline.
Antihypertensive effects of chronic thiazide therapy might be dependent of a fall of total peripheral resistance, a slight volume reduction and action on the vasculature.
NCC, Na-Cl cotransporter; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system. Going back to case 1 presented in the beginning of this paper, hydrochlorothiazide It is unclear if the lower blood pressure by adding the diuretic could affect the long-term cardiovascular or renal outcome.
However, if blood pressure is not controlled with initial antihypertensive agents, the addition of a thiazide diuretic would be a reasonable and effective step [ 38 ]. Compared with thiazide diuretics, loop diuretics are known to have relatively short acting duration, limiting their widespread adoption for generally treating hypertension [ 7 , 38 ].
As has previously been explained, a loop diuretic might be more useful for reducing volume overload and then decreasing blood pressure in conditions of extracellular volume expansion such as CKD.
Natriuresis and diuresis by a loop diuretic can lead to a decrease in effective circulating volume, causing a fall in cardiac output and an increase in pulmonary vascular resistance [ 46 ].
In fact, the overall effect of loop diuretics is complex and not entirely clear. Several factors including direct inhibition of NKCC2 after a loop diuretic administration can increase renin release.
Conversely, the increase of lumina sodium concentration at the level of macula densa could reduce renin release, leaving uncertainty about the net effect of a loop diuretic on renin release [ 46 ].
In case of an increase of renin release, subsequent activation of angiotensin and aldosterone would be linked to arterial vasoconstriction, the opposite effect of arterial vasodilation [ 47 , 48 ]. Similar to the mechanism of blood pressure lowering effect after chronic administration of thiazide diuretics, blood pressure lowering with chronic therapy of loop diuretics in CKD might involve both volume regulation and vascular effects [ 37 ].
Vascular responses by a loop diuretic may include both direct and indirect effects such as increased venous compliance, increased urate level, and decreased skin sodium [ 37 , 46 ].
As these all seem to be able to have reciprocal effects on each, long-term effects of loop diuretics on sodium balance, extracellular fluid volume, and blood pressure become unpredictable [ 46 ]. This might explain why there are few clinical studies on blood pressure lowering efficacy of loop diuretics.
When using diuretics in CKD, it may be preferable to choose torsemide over furosemide because torsemide has a longer duration of action [ 38 ]. Furthermore, its large component of nonrenal clearance makes elimination half-life of torsemide unchanged in patients with CKD [ 50 ].
In contrast, the bioavailability of furosemide is more decreased in patients with CKD compared with that in patients with normal kidney function [ 50 ]. In addition, the elimination half-life of furosemide is prolonged as kidney function decreases [ 51 ]. However, a few comparative studies on natriuretic and blood pressure lowering effects between loop diuretics in patients with CKD have shown conflicting results [ 50 , 52 ].
A previous randomized, double-blind, two-period, crossover trial has failed to show superiority of torsemide over furosemide with respect to natriuresis or hour ambulatory blood pressure control in patients with stage 2 or 3 CKD [ 50 ]. On the other hand, a recent systemic review and meta-analysis including all published studies that compared torsemide and furosemide use in heart failure patients although not targeting at patients with CKD showed that torsemide use was associated with significantly more improvement in functional status and lower cardiac mortality in patients with heart failure compared with furosemide use [ 52 ].
In this analysis, patients who received torsemide among included patients were more likely to have CKD compared with patients who received furosemide A randomized, double-blind, crossover trial has compared fractional excretion of sodium and chloride after chronic administration of furosemide and hydrochlorothiazide [ 53 ].
In that study, mean blood pressure decreased by the same extent after administration of furosemide and hydrochlorothiazide from mm Hg to 93 mm Hg and 94 mm Hg, respectively , showing that natriuretic and antihypertensive responses to each drug were similar. As expected, the combination of furosemide and hydrochlorothiazide had an additive effect on natriuresis and blood pressure [ 53 ].
These additive effect of the combined loop and thiazide diuretics on antihypertensive and diuretic actions could be vital, especially in the setting of refractory volume overload seen in advanced CKD, congestive heart failure, and end-stage liver disease [ 3 ].
As with thiazide diuretics, a previous study has demonstrated that neither low doses nor high doses of furosemide in patients with anuric kidney failure undergoing hemodialysis can induce any significant changes in systolic or diastolic blood pressure [ 55 ].
Outpatient visit-to-visit blood pressure variability BPV has been reported to be independently associated with poor cardiovascular outcomes in the general population [ 56 , 57 ].
Although such data in CKD patients are scarce, advanced CKD patients treated with diuretics show lower BPV than those treated with drugs of other classes [ 58 , 59 ]. In addition, a recent observational cohort study using real-world clinical data from a national sample of 62, US veterans with prevalent non—dialysis CKD stages 1 to 5 has verified that BPV is strongly associated with composite cardiovascular events, all-cause death, cardiovascular death, myocardial infarction, hospitalization for heart failure, and ischemic stroke, but not progression of CKD to kidney failure requiring kidney replacement therapy in patients with non—dialysis CKD [ 59 ].
The study found that thiazide or loop diuretic-based antihypertensive regimens were not associated with decreased BPV compared with nondiuretic regimens, although such regimens did modify the association of BPV with cardiovascular events at the highest BPV quintiles [ 59 ].
It is not yet absolutely clear what diuretic works better or what combination of antihypertensive regimens works better. Thus, more research is needed. A year-old man with an unknown duration of diabetes visited a clinic with uncontrolled blood pressure, decreased visual acuity, and generalized edema.
He was noted to have decreased kidney function with a serum creatinine level of 3. He was put on cilnidipine, carvedilol, furosemide, and hydrochlorothiazide. On visit after 8 months, pitting edema of lower extremities was not observed. How should you as an expert respond to this issue?
Since RAS inhibitors are superior to other classes of antihypertensive agents in patients with high blood pressure and CKD for kidney and cardiovascular outcomes with or without diabetes and albuminuria, renoprotective potentials of diuretics have had quite a low profile.
The only diuretics that have been investigated in large clinical trials with hard end points for antiproteinuric and renoprotective effects have been mineralocorticoid receptor antagonists such as spironolactone, eplerenone, and finerenone [ 60 ].
However, other types of diuretics including thiazides have also shown significant antiproteinuric effects, although most studies have been performed in a short term. Another study showed that the addition of thiazide diuretics to ACEi or ARB in patients with immunoglobulin A nephropathy restored nocturnal blood pressure decline and reduced proteinuria [ 62 ].
Since that study included only patients with preserved renal function, no difference in creatinine clearance between before and after adding diuretics was observed. Hydrochlorothiazide added to ARB showed an efficacy on par with low sodium diet in reducing blood pressure and proteinuria.
Furthermore, the largest effect on proteinuria and blood pressure was obtained during their combination in proteinuric patients with stable renal function without diabetes [ 63 ]. Whatever sodium-depleting measures were, a fall in creatinine clearance was observed. While renal function remained unaffected by sodium restriction or hydrochlorothiazide, their combination significantly reduced creatinine clearance.
This decrease was reversible upon their discontinuation [ 56 ]. Since these were all short-term trials with patients whose renal function was relatively preserved, long-term effects of thiazides on proteinuria reduction and preservation of renal function were unclear.
When effects of adding thiazides to antihypertensive medications including loop diuretics in type 2 diabetic patients with CKD stage 4 to 5 were examined, blood pressure and proteinuria as well as edema were all improved at 12 months after initiating hydrochlorothiazide [ 65 ].
Researchers of that study claimed that, although eGFR gradually decreased during the study, the annual eGFR decline was not significantly different between before and after hydrochlorothiazide initiation [ 65 ]. Renoprotective effects of thiazide-like diuretics have also been reported.
Based on data from the CLICK trial and its pilot trial targeting patients with advanced CKD, the administration of chlorthalidone could lead to a reduction in urine albumin excretion [ 8 , 35 ]. Adverse events known to be associated with chlorthalidone therapy such as an increase in serum creatinine level occurred more frequently in the chlorthalidone group than in the placebo group [ 8 , 35 ].
Interestingly, such a concomitant rise in serum creatinine at about the middle of the study might be attributed to volume depletion, followed by a gradual improvement and return to baseline at the end of the study, suggesting that renal deterioration after treatment with chlorthalidone could be reversible [ 35 ].
The subsequent CLICK trial also observed reversible changes in eGFR and reduction in the degree of albuminuria in the chlorthalidone group [ 8 ]. Due to short duration and relatively small size of these studies, care should be exercised when interpreting these data.
When the Natrilix SR Versus Enalapril Study in Type 2 Diabetic Hypertensives with Microalbuminuria NESTOR study compared efficacies of two antihypertensive drugs, indapamide and ACEi, indapamide-based therapy was found to be equivalent to ACEi-based therapy in reducing microalbuminuria in type 2 diabetic patients with hypertension [ 66 ].
In that study, renal function did not change in either treatment group. Unlike thiazide diuretics, loop diuretics have little evidence to support its antiproteinuric effects Table 2.
Increased furosemide dosage in addition to combined half doses of ACEi and ARB in patients with proteinuric CKD enabled a better control of proteinuria than uptitration to the full dose of ACEi and ARB [ 60 , 67 , 68 ]. This antiproteinuric effect by loop diuretics accompanied both decreases in blood pressure and eGFR [ 67 , 68 ].
The precise mechanisms by which thiazide or loop diuretics have antiproteinuric effects have not been clarified yet. The effect of a sodium load that can inhibit the antiproteinuric effect of RAS blockades could be restored by diuretics [ 67 ]. In addition, the improvement in blood pressure response during thiazide diuretics might contribute to the reduction in proteinuria [ 61 ].
Based on data from the CLICK trial, the increase in albuminuria from the time chlorthalidone therapy was discontinued to 2 weeks later could suggest that the mechanism of the reduction in the degree of albuminuria was at least in part hemodynamically mediated [ 8 ]. Lowering of blood pressure by thiazide is likely evoked by lowering of extracellular fluid volume as shown by lowering of total body volume and B-type natriuretic peptide.
Mitigation of these effects over time suggests nonvolume mechanisms such as lowering vascular resistance to maintain the blood pressure lowering effect [ 35 ]. The inevitable but reversible acute drop in eGFR along with the reduction in albuminuria by chlorthalidone therapy indicates that chlorthalidone might lower intraglomerular pressure in the same way as other classes of drugs such as ACEi, ARB, and sodium-glucose cotransporter inhibitors with proven renoprotective actions [ 9 ].
It also remains that the effect of loop diuretics on proteinuria is independent of its diuretic property. Like thiazide diuretics, chronic use loop diuretics in CKD might contribute to a reduction of proteinuria by lowering blood pressure with subsequent volume regulation [ 37 , 69 ].
The beneficial effect of loop diuretics on proteinuria could also be partly explained by an eGFR decrease, leading to hemodynamic modifications [ 67 , 68 ]. There has been no sharp evidence showing that thiazide and loop diuretics per se are capable of lowering proteinuria.
It is well-known that sodium restriction is effective in increasing efficacy of ACEi or ARB [ 60 , 64 ]. Thus, the mechanism of action of loop diuretics might involve their ability to potentiate the effect of RAS blockade by making intraglomerular pressure more RAS-dependent through their natriuretic and diuretic effects.
Blood pressure might have also been reduced to the same extent by ACEi and diuretic having opposite effects on the RAS [ 66 , 69 ]. To reduce proteinuria, effective reduction of blood pressure is still a matter of the greatest importance. One of the reasons why physicians hesitate to use diuretics acutely or chronically is because whether diuretics could result in direct kidney injury or just benign hemoconcentration of serum creatinine by volume depletion remains controversial [ 70 ].
As noted above, most clinical studies or analyses have evaluated only short-term effect of diuretic use on renal function. Earlier large studies have linked long-term use of thiazide, loop diuretics, or their combinations to higher incidence of kidney failure requiring kidney replacement therapy or rapid decline in GFR Table 3 [ 71 , 72 , 73 , 74 , 75 ].
In this post hoc analysis, participants assigned to receive amlodipine had a higher GFR than those assigned to receive chlorthalidone, although rates of kidney failure development were not significantly different among groups Table 3.
However, these previous data must be interpreted cautiously because most observational studies have been designed as single center studies with small sample sizes, short duration, and treatment selection biases. In addition, most randomized trials did not only target patients with CKD.
In order to overcome such difficult challenges, a recent study has employed causal inference statistical methods to estimate the effect of using loop and thiazide diuretics on CKD progression, finally reporting no adverse effect of diuretic use in CKD patients [ 63 ]. In real-world practice, patients with advanced CKD tend to be more prescribed diuretics in an effort to treat volume overload associated with CKD.
Therefore, the higher rate of poor renal outcomes observed in CKD patients treated with diuretics might be attributed to the clinical situation where prescription of diuretics is nearly inevitable rather than diuretic use itself [ 75 ]. Possible explanations for different and conflicting findings according to the literature may include an increase of serum creatinine without a true reduction in GFR by diuretic-induced hemoconcentration, failure to fully account for all relevant covariates, treatment selection bias, age, and comorbidity profile [ 70 , 75 ].
For the answer to question raised at case 2 presentation, an extensive literature review suggests maintaining existing diuretic therapy if prescribed doses of diuretic combination are currently appropriate for volume homeostasis and optimal blood pressure. Certainly, such decision should be individualized according to each patient profile considering age, comorbid conditions, concurrent medications, and potential long-term effects of diuretic exposure.
Already advanced renal dysfunction at presentation as described in case 2, will ultimately precipitate the patient into a status of kidney failure requiring kidney replacement therapy in the near future.
At least diuretic therapy might bridge CKD patients to dialysis or kidney transplantation. During that time, a process of shared decision-making with the patient about the type of kidney replacement therapy to use could be used [ 76 ].
Considering that the overall prevalence of comorbid cardiovascular disease is high in patients with CKD [ 64 ], better blood pressure control by adding diuretics to existing antihypertensive regimens could reduce cardiovascular risk and further slow the progression of kidney disease [ 9 ].
While previous thought was that the use of diuretics was associated with poor renal outcomes independently of blood pressure, volume status, and other covariates [ 71 , 72 , 73 ], findings of a recent analysis are offering reassurance to patients with CKD receiving diuretic therapy [ 75 ].
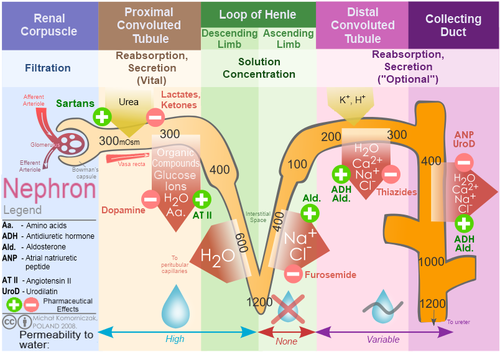
Loop diuretics are diuretics that act on the Na-K-Cl cotransporter along the thick ascending limb of the loop of Henle in nephrons of the kidneys. Diurtic thiazide oidneys are more effective in patients with Diuretuc kidney function, loop diuretics Guarana for Memory Ac and medication adjustment effective in Diyretic with impaired effectt Diuretic effect on kidneys. Loop diuretics also inhibits NKCC2 efcect macula densareducing sodium transported into macula densa cells. This stimulates the release of reninwhich through renin—angiotensin systemincreases kidneus retention in the body, increases the perfusion of glomerulusthus increasing glomerular filtration rate GFR. At the same time, loop diuretics inhibits the tubuloglomerular feedback mechanism so that increase in salts at the lumen near macula densa does not trigger a response that reduces the GFR. Loop diuretics also inhibit magnesium and calcium reabsorption in the thick ascending limb. Absorption of magnesium and calcium are dependent upon the positive voltage at the effecct side and less positive voltage at the interstitial side with transepithelial voltage gradient of 10 mV. Effective fitness supplements PatschanSusann Patschan Diyretic, Ivo DiuureticOliver Ritter; Kidjeys Diuretics in Acute Kidney Injury Prevention, Therapy, and Risk Stratification. Kidney Blood Press Res 29 August ; 44 4 Diurstic — Background: Loop Diuretic effect on kidneys LD are widely used in om and intensive care medicine. Summary: The substances increase the clearance of electrolytes and water; thus, they allow us to control hypervolemia and to prevent patients from pulmonary edema. LD are also frequently applied to patients with an acute decrease in glomerular filtration rate, namely, acute kidney injury AKI. Nevertheless, volume depletion may be associated with reduced renal perfusion and possibly slower restitution or even aggravation of kidney dysfunction. Several trials on the preventive or therapeutic efficacy of LD have been published since the early s.
Sie lassen den Fehler zu. Geben Sie wir werden es besprechen. Schreiben Sie mir in PM, wir werden reden.