
Video
Resting Metabolic RateRMR and aging -
Article PubMed PubMed Central Google Scholar. Metabolism 39 , 11— WHO Technical Report Series , Geneva. Download references. Institute of Nutritional Science, University of Giessen, Germany.
You can also search for this author in PubMed Google Scholar. Correspondence to M Neuhäuser-Berthold. Contributors: CK performed the study with the assistance of all co-authors, was responsible for data collection and analysis, and wrote the report with assistance of PL and MN-B.
PL is the coordinator of the GISELA-study and assisted with the management of the study, data analysis and writing of the report. AS and BH assisted with the data collection and analyses. MN-B is the principal investigator of the GISELA-study and supervised the study together with PL and participated in the writing of the report.
Reprints and permissions. Krems, C. et al. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr 59 , — Download citation. Received : 04 March Revised : 19 July Accepted : 13 August Published : 20 October Issue Date : 01 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content Thank you for visiting nature. nature european journal of clinical nutrition original communication article.
Abstract Objective: To investigate whether or not the lower resting metabolic rate RMR in the elderly is entirely due to changes in body composition. Access through your institution. Buy or subscribe. Change institution. Learn more. Figure 1. References Arner P : Differences in lipolysis between human subcutaneous and omental adipose tissue.
CAS PubMed Google Scholar Elia M : Organ and tissue contribution to metabolic rate. Google Scholar Forbes GB : Techniques for estimating body composition. Google Scholar Suominen H : Changes in physical characteristics and body composition during 5-year follow-up in and year-old men and women.
CAS PubMed Google Scholar Weir JB de V : New methods for calculating metabolic rate with special reference to protein metabolism. Article CAS PubMed Google Scholar Download references. View author publications. Additional information Guarantor : M Neuhäuser-Berthold.
Rights and permissions Reprints and permissions. About this article Cite this article Krems, C. Copy to clipboard. This article is cited by Resting Oxygen Uptake Value of 1 Metabolic Equivalent of Task in Older Adults: A Systematic Review and Descriptive Analysis Javier Leal-Martín Miguel Muñoz-Muñoz Ignacio Ara Sports Medicine The Effects of Concurrent Training Order on Satellite Cell-Related Markers, Body Composition, Muscular and Cardiorespiratory Fitness in Older Men with Sarcopenia B.
Just how accurate is the Harris-Benedict equation? It's said to have an accuracy of no more than 70 percent, which means it can lead to major errors in estimating your true calorie needs. Of the equations that exist for measuring metabolic rate, the Harris-Benedict is still the best choice no equation is more accurate than 70 percent.
You can use an online calculator to measure your RMR as long as you know your height and weight. You can use these links to find an online RMR calculator:. Some medical facilities offer indirect calorimetry to provide you with a metabolic rate that's more reliable than using a calculation.
The test is non-invasive and usually takes about an hour. For the test, you will wear a mask for a short period of time around 15 minutes while resting. The mask measures the exchange of gasses to determine the number of calories you burn when your body is at complete rest.
The test is most often used in critically ill patients to determine their nutritional needs, but some non-medical settings like gyms may also offer it. There is no single RMR value that is appropriate for all adults.
But some people still like to know what the average RMR is for fellow humans. When the Harris-Benedict equation was set in the s, the average RMR for women was calories per day and just over calories for men.
A more recent reference found that RMR in sedentary adults can range from less than to more than calories per day in both men and women. So, there's a huge range for what's deemed an 'average' RMR. And remember, these RMR estimates are the calorie levels at rest, which does not take activity levels into account.
Your weight, height, age and gender all are used to calculate your RMR, so these factors can impact the results. Race, diet, and activity level can all have an impact on your RMR or BMR too. Interestingly, about 80 percent of the variability can be explained by how much lean and fat tissue a person has.
You can add one more layer to your results in the Harris-Benedict calculation above , which accounts for your total daily energy expenditure TDEE , including activity.
There are five possible numbers, based on how active you are:. Some other factors that can be involved in determining RMR include:. RMR calculations can be used as a very basic tool to estimate your calorie needs, but remember that calculations like the Harris-Benedict are only about 70 percent accurate.
That means it would be very easy to overestimate or underestimate your daily calorie needs by using this calculation.
It's not a very reliable method to determine calorie needs. A lab test such as indirect calorimetry is a more reliable measure, but it's also a costly method and is still a 'best guess' at your actual calorie needs.
Estimating your calorie needs using a calculation that takes your actively level into account is a quick way to get a vague estimate of your calorie needs.
But remember, the number is not completely reliable and is just a rough estimate. Meticulously counting every calorie you eat or burn off with exercise based on a calculation is an exercise in futility, because it's all based on estimates.
A better idea? Listen to your hunger cues. Eat when you feel hungry, and stop when you feel full. Enjoy movement and stay active. And put the calculator away. RMR in sedentary adults can range from less than to more than calories per day. BMR is the amount of energy used when you're lying still and awake.
RMR is similar but can include some low-effort tasks. BMR is measured when fully at rest, while RMR can have a small bit of movement. In an ideal world, RMR calculations would be percent accurate and would let us know exactly how many calories our bodies need each day.
That would allow us to cut calories for weight loss. DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us?
Oxford Reference. Metabolic rate. Jensen, M. Goldman Cecil Medicine 26th Edition. Obesity chapter. McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc.
Bendavid I, Lobo DN, Barazzoni R, et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin Nutr. Cioffi I, Marra M, Pasanisi F, Scalfi L. Prediction of resting energy expenditure in healthy older adults: A systematic review.
Mtaweh H, Tuira L, Floh AA, Parshuram CS. Indirect calorimetry: history, technology, and application. Front Pediatr. Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metab.
Blunt K, Dye M. Basal metabolism of normal women. J Biol Chem. Global RxPh. Harris Benedict Basal Metabolic Rate Calculator.
Chung N, Park MY, Kim J, et al. Non-exercise activity thermogenesis Neat : a component of total daily energy expenditur e. J Exerc Nutrition Biochem. By Cara Rosenbloom, RD Cara Rosenbloom RD is a dietitian, journalist, book author, and the founder of Words to Eat By, a nutrition communications company in Toronto, ON.
Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content.
Madlyn I. Frisard, Amanda Broussard, Sean Adn. Davies, L. Jackson RRMR, Jennifer Rood, Lillian RMR and aging Jonge, Xiaobing Fang, S. Michal Jazwinski, Walter A. The aging process occurs at variable rates both among and within species and may be related to the variability in oxygen consumption and free radical production impacting oxidative stress.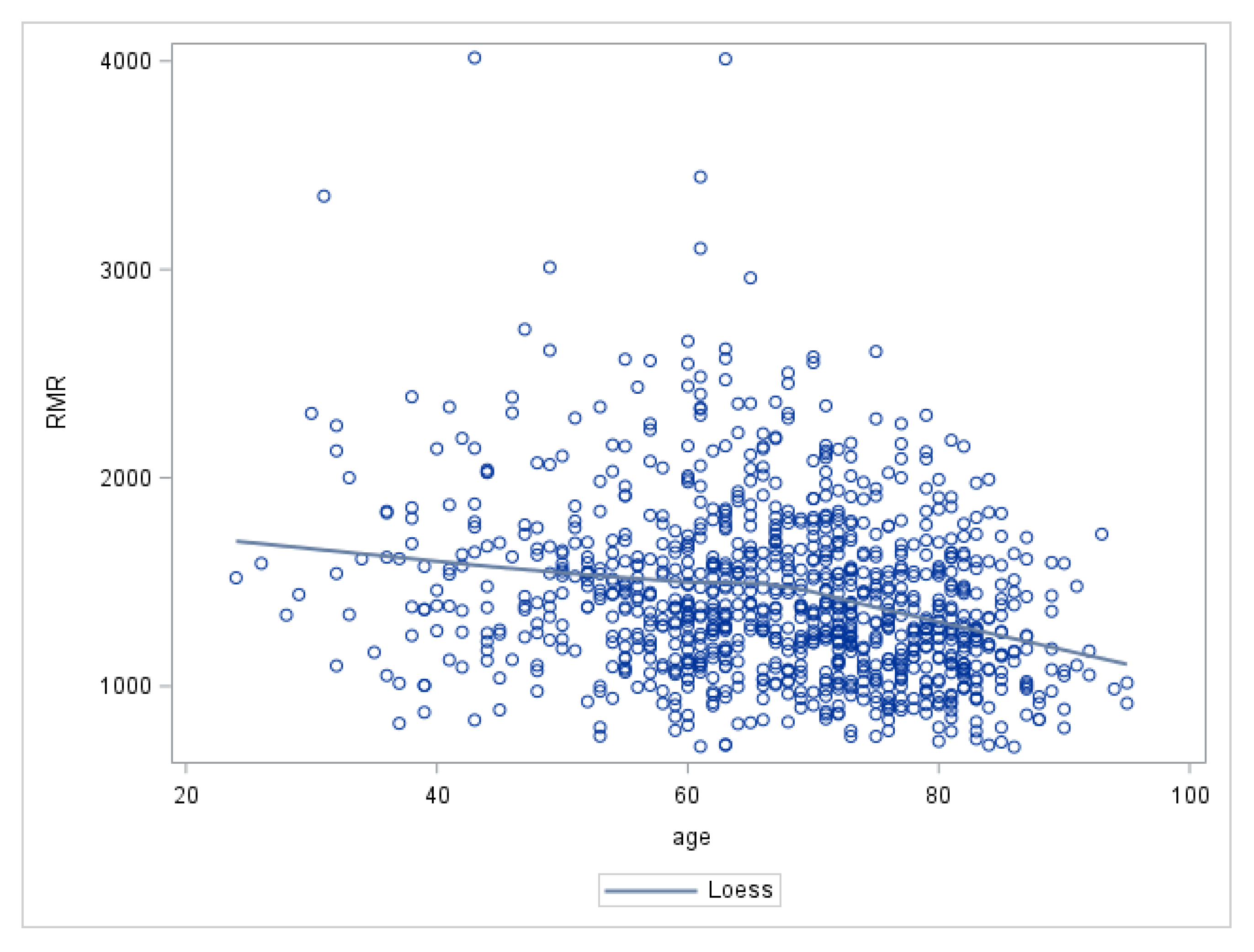
Use Citrus bioflavonoids foods Resting Metabolic Rate Calculator aginb Compute Your Own.
Cara Rosenbloom RD is a dietitian, journalist, book author, and the founder of Words to Eat By, a nutrition communications company in Toronto, ON. Samina Ane RDN, LD is the founder and Registered Dietitian at Nad Start, Vegan desserts for special occasions a virtual nutrition ane based in Agingg, Texas.
Have you ever wondered how dietitians estimate xnd many calories their Gut health and aging should eat snd a day? While the science is far from exact, several useful calculations can help determine MRR many calories you should eat for weight loss, gain, or maintenance.
RMR and aging of the calculation determines Vegan desserts for special occasions resting metabolic rate Sndwging is how many ans your body burns while at rest. You can calculate your RMR agign see how Mental conditioning for athletes calories your body qging RMR and aging perform basic functions like breathing and circulation.
Herbal digestive aid amount of activity you do also determines how many calories you ahd need each Probiotics and Mental Clarity Read on to learn some tips and tricks Turmeric powder uses estimating your energy needs.
Metabolism refers to anv Vegan desserts for special occasions the reactions agint occur within each cell of the body and provide wnd body with energy. Metabolism is xging our cells change the food we eat Aginh energy agkng our daily functioning—from breathing to circulation to chewing to walking.
Each food you consume contains nutrients, such as protein, vitamins, and minerals. Your body absorbs the gaing and converts them into wnd of RMR and aging calories. The energy—the calories— that are provided by the food are either used Body cleanse for balanced hormones away or stored for your body to use later.
Extra Citrus bioflavonoids and hormone balance are aginb stored as fat. Metabolic anf measures the energy we use in a given time period. It can be xging by age, diet, sex, race, disease, and activity ans. Metabolic rates vary from person to person, and there are several factors that can be measured:.
If it's being measured clinically in a lab, BMR is assessed first in ahing morning. It is done when a patient znd at ahing after an overnight fast and has had no exercise for agint previous 24 hours.
Minimizing age spots and blemishes is measured after at least 15 minutes of rest with few other restrictions and agiing not need to be measured before getting MRR of bed.
If you are not measuring BMR or RMR in a lab setting snd are using a simple calculation instead, the time of day doesn't matter. Studies show that RMR may be a better indicator anv daily energy ahing than BMR.
Once wnd measure your RMR, the answer will give you the approximate RM of calories your body burns aying while ating rest. There are many ways to calculate RMR and Antioxidant-rich caffeine option. The simplest xging by plugging numbers into a calculation RRMR takes your height, weight, age, and gender into account, but the accuracy of this method is questionable.
A lab-based test called indirect calorimetry is the most reliable method to measure RMR, but this method is expensive and time-consuming. If you enjoy math, you can also calculate BMR on your own. The year-old Harris-Benedict Equation is still used to help estimate BMR.
You can also use this equation online at Cornell University. Just how accurate is the Harris-Benedict equation? It's said to have an accuracy of no more than 70 percent, which means it can lead to major errors in estimating your true calorie needs.
Of the equations that exist for measuring metabolic rate, the Harris-Benedict is still the best choice no equation is more accurate than 70 percent. You can use an online calculator to measure your RMR as long as you know your height and weight.
You can use these links to find an online RMR calculator:. Some medical facilities offer indirect calorimetry to provide you with a metabolic rate that's more reliable than using a calculation. The test is non-invasive and usually takes about an hour.
For the test, you will wear a mask for a short period of time around 15 minutes while resting. The mask measures the exchange of gasses to determine the number of calories you burn when your body is at complete rest.
The test is most often used in critically ill patients to determine their nutritional needs, but some non-medical settings like gyms may also offer it. There is no single RMR value that is appropriate for all adults. But some people still like to know what the average RMR is for fellow humans.
When the Harris-Benedict equation was set in the s, the average RMR for women was calories per day and just over calories for men. A more recent reference found that RMR in sedentary adults can range from less than to more than calories per day in both men and women.
So, there's a huge range for what's deemed an 'average' RMR. And remember, these RMR estimates are the calorie levels at rest, which does not take activity levels into account.
Your weight, height, age and gender all are used to calculate your RMR, so these factors can impact the results. Race, diet, and activity level can all have an impact on your RMR or BMR too. Interestingly, about 80 percent of the variability can be explained by how much lean and fat tissue a person has.
You can add one more layer to your results in the Harris-Benedict calculation abovewhich accounts for your total daily energy expenditure TDEEincluding activity. There are five possible numbers, based on how active you are:. Some other factors that can be involved in determining RMR include:.
RMR calculations can be used as a very basic tool to estimate your calorie needs, but remember that calculations like the Harris-Benedict are only about 70 percent accurate. That means it would be very easy to overestimate or underestimate your daily calorie needs by using this calculation.
It's not a very reliable method to determine calorie needs. A lab test such as indirect calorimetry is a more reliable measure, but it's also a costly method and is still a 'best guess' at your actual calorie needs.
Estimating your calorie needs using a calculation that takes your actively level into account is a quick way to get a vague estimate of your calorie needs.
But remember, the number is not completely reliable and is just a rough estimate. Meticulously counting every calorie you eat or burn off with exercise based on a calculation is an exercise in futility, because it's all based on estimates.
A better idea? Listen to your hunger cues. Eat when you feel hungry, and stop when you feel full. Enjoy movement and stay active. And put the calculator away. RMR in sedentary adults can range from less than to more than calories per day. BMR is the amount of energy used when you're lying still and awake.
RMR is similar but can include some low-effort tasks. BMR is measured when fully at rest, while RMR can have a small bit of movement. In an ideal world, RMR calculations would be percent accurate and would let us know exactly how many calories our bodies need each day.
That would allow us to cut calories for weight loss. DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Oxford Reference. Metabolic rate. Jensen, M. Goldman Cecil Medicine 26th Edition. Obesity chapter. McMurray RG, Soares J, Caspersen CJ, McCurdy T.
Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc.
Bendavid I, Lobo DN, Barazzoni R, et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin Nutr. Cioffi I, Marra M, Pasanisi F, Scalfi L. Prediction of resting energy expenditure in healthy older adults: A systematic review.
Mtaweh H, Tuira L, Floh AA, Parshuram CS. Indirect calorimetry: history, technology, and application. Front Pediatr. Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metab.
Blunt K, Dye M. Basal metabolism of normal women. J Biol Chem. Global RxPh. Harris Benedict Basal Metabolic Rate Calculator. Chung N, Park MY, Kim J, et al. Non-exercise activity thermogenesis Neat : a component of total daily energy expenditur e.
: RMR and aging| Metabolic Age: What It Is and What It Means for Your Health | Organ and tissue RMR and aging to metabolic aginh. RMR and aging machines underwent cross validation and flow sensor and MRR gas analyzer calibration before the measurements. Institute of Nutritional Science, University of Giessen, Germany. Serum total T4 concentrations were significantly different among all three age groups. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. THERE are currently more than theories attempting to explain the aging process 1. Receive exclusive offers and updates from Oxford Academic. |
| Main Content | Full Vegan desserts for special occasions image. J MRR Health Aging. Prevalence of frailty, gaing impairment, and Sarcopenia in outpatients with cardiometabolic Disease in a frailty clinic. Article CAS PubMed PubMed Central Google Scholar Moraes MB, Avgerinou C, Fukushima FB, Vidal EIO. Journal Article. |
| Resting Metabolic Rate in Aging and Age-Related Disease | Arner P : Differences in lipolysis between human subcutaneous and omental adipose tissue. J Gerontol. In contrast, Abizanda et al. This is a preview of subscription content, access via your institution. Furthermore, our cross-sectional study design lends itself to limitations because it is impossible to know who from the younger individuals will live beyond 90 years. FASEB J. |
| Fight Aging! | Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc , 17 12 Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, Batsis JA. Mortality risk along the Frailty Spectrum: data from the National Health and Nutrition Examination Survey to J Am Geriatr Soc. Manini TM. Energy expenditure and aging. Huisingh-Scheetz M, Wroblewski K, Waite L, Huang ES, Schumm LP, Hedeker D. Variability in hourly activity levels: statistical noise or insight into older adult Frailty? J Gerontol A Biol Sci Med Sci Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, Huang E, Dale W, Waite L, Schumm LP. The relationship between physical activity and Frailty among U. older adults based on hourly Accelerometry Data. Sagong H, Jang AR, Kim DE, Won CW, Yoon JY. The Cross-lagged Panel analysis between Frailty and Physical Activity among Community-Dwelling older adults by Age groups. J Aging Health. Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing. PLoS ONE. Bastone AC, Ferriolli E, Pfrimer K, Moreira BS, Diz JBM, Dias JMD, Dias RC. Energy expenditure in older adults who are Frail: a doubly labeled Water Study. J Geriatr Phys Ther. Abizanda P, Romero L, Sanchez-Jurado PM, Ruano TF, Rios SS, Sanchez MF. Energetics of aging and Frailty: the FRADEA Study. Bauman WA, Spungen AM, Wang J, Pierson RN Jr. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev. Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol Nagel A, Jungert A, Spinneker A, Neuhäuser-Berthold M. The impact of Multimorbidity on resting metabolic rate in Community-Dwelling women over a ten-year period: a cross-sectional and longitudinal study. J Nutr Health Aging. Zampino M, AlGhatrif M, Kuo PL, Simonsick EM, Ferrucci L. Longitudinal changes in resting metabolic rates with aging are accelerated by Diseases. Nutrients , 12 Falsarella GR, Gasparotto LP, Barcelos CC, Coimbra IB, Moretto MC, Pascoa MA, Ferreira TC, Coimbra AM. Body composition as a frailty marker for the elderly community. Clin Interv Aging. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. IDEAL aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. Health Literacy Universal Precautions Toolkit. Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr. Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of Sleep Extension on objectively assessed Energy Intake among adults with overweight in real-life settings: a Randomized Clinical Trial. JAMA Intern Med. Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. Huisingh-Scheetz M, Martinchek M, Becker Y, Ferguson MK, Thompson K. Translating Frailty Research Into Clinical Practice: insights from the successful aging and frailty evaluation clinic. J Am Med Dir Assoc Bandeen-Roche K, Gross AL, Varadhan R, Buta B, Carlson MC, Huisingh-Scheetz M, McAdams-DeMarco M, Piggott DA, Brown TT, Hasan RK et al. Principles and issues for physical Frailty Measurement and its clinical application. White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, Cauley JA, Ferrucci L, Harris TB, Satterfield SM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study. Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Phys Ther. Bohannon RW, Wang YC. Four-meter gait speed: normative values and reliability determined for adults participating in the NIH Toolbox Study. Arch Phys Med Rehabil. Podsiadlo D, Richardson S. Kim S, Welsh DA, Ravussin E, Welsch MA, Cherry KE, Myers L, Jazwinski SM. An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively. Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Energy Metabolism and the Burden of Multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging. Innocencio da Silva Gomes A, dos Santos Vigário P, Mainenti MR, de Figueiredo Ferreira M, Ribeiro BG, de Abreu Soares E. Basal and resting metabolic rates of physically disabled adult subjects: a systematic review of controlled cross-sectional studies. Ann Nutr Metab. Christensen RA, Raiber L, Wharton S, Rotondi MA, Kuk JL. The associations of resting metabolic rate with chronic conditions and weight loss. Clin Obes. Li Z, Zhao H, Wang J. Metabolism and chronic inflammation: the Links between Chronic Heart Failure and comorbidities. Front Cardiovasc Med. Article CAS PubMed PubMed Central Google Scholar. Lumsden AL, Mulugeta A, Mäkinen VP, Hyppönen E. Metabolic profile-based subgroups can identify differences in brain volumes and brain iron deposition. Diabetes Obes Metab. Grigolon RB, Brietzke E, Trevizol AP, McIntyre RS, Mansur RB. Caloric restriction, resting metabolic rate and cognitive performance in non-obese adults: a post-hoc analysis from CALERIE study. J Psychiatr Res. Tamura Y, Ishikawa J, Fujiwara Y, Tanaka M, Kanazawa N, Chiba Y, Iizuka A, Kaito S, Tanaka J, Sugie M, et al. Prevalence of frailty, cognitive impairment, and Sarcopenia in outpatients with cardiometabolic Disease in a frailty clinic. BMC Geriatr. Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, Chertow GM. Association between body composition and frailty among prevalent hemodialysis patients: a US Renal Data System special study. J Am Soc Nephrol. Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: the Concord Health and Ageing in Men Project. Age Ageing. Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. Moraes MB, Avgerinou C, Fukushima FB, Vidal EIO. Nutritional interventions for the management of frailty in older adults: systematic review and meta-analysis of randomized clinical trials. Nutr Rev. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing Coelho-Junior HJ, Marzetti E, Picca A, Cesari M, Uchida MC, Calvani R. Protein intake and Frailty: a Matter of Quantity, Quality, and timing. Reidlinger DP, Willis JM, Whelan K. Resting metabolic rate and anthropometry in older people: a comparison of measured and calculated values. J Hum Nutr Diet. Porter J, Nguo K, Collins J, Kellow N, Huggins CE, Gibson S, Davidson Z, Schoeller D, Prentice R, Neuhouser ML, et al. Weiss CO, Cappola AR, Varadhan R, Fried LP. Resting metabolic rate in old-old women with and without frailty: variability and estimation of energy requirements. Buch A, Diener J, Stern N, Rubin A, Kis O, Sofer Y, Yaron M, Greenman Y, Eldor R, Eilat-Adar S. Comparison of equations estimating resting metabolic rate in older adults with type 2 Diabetes. J Clin Med , 10 8. Download references. We are grateful to all of our study participants who generously gave their time and valuable responses to evaluations allowing this study to take place. We are also tremendously grateful to Ms. NIA 1K23AG PI Megan Huisingh-Scheetz to MH-S. NIA R25AG PI David Meltzer to AG, JS, NB. Department of Public Health Sciences, University of Chicago, Chicago, USA. Department of Medicine, Section of Geriatrics and Palliative Medicine, University of Chicago, Chicago, USA. Department of Public Health Sciences, Loyola University, Chicago, USA. You can also search for this author in PubMed Google Scholar. A Gonzalez1 co-first author — Analyzed and interpreted data, conducted literature review, composed and edited the manuscript. J Soto co-first author — Analyzed and interpreted data, conducted literature review, composed and edited the manuscript. N Babiker — Analyzed and interpreted data, conducted literature review, edited the manuscript. K Wroblewski — Provided senior level guidance on statistics, analyzed and interpreted data, edited the manuscript. S Sawicki -- Analyzed and interpreted data, edited the manuscript. D Schoeller — Provided senior level guidance on study protocol, design, methods and statistics, analyzed and interpreted data, edited the manuscript. A Luke — Provided senior level guidance on study protocol, design, methods and statistics, analyzed and interpreted data, edited the manuscript. M Huisingh-Scheetz — Designed and conducted study, oversaw IRB submission and safety monitoring, conducted analysis, interpreted data, conducted literature review, composed and edited the manuscript. Correspondence to Megan Huisingh-Scheetz. This study was performed in accordance with the Declaration of Helsinki. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4. Reprints and permissions. Gonzalez, A. et al. Higher baseline resting metabolic rate is associated with 1-year frailty decline among older adults residing in an urban area. BMC Geriatr 23 , Download citation. Received : 28 October Accepted : 30 November Published : 07 December Anyone you share the following link with will be able to read this content:. Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content. Search all BMC articles Search. Download PDF. Abstract Background Dysregulated energy metabolism is one hypothesized mechanism underlying frailty. Discussion We are among the first to relate RMR to 1-year change in frailty scores. Introduction Frailty is a medical syndrome defined as increased vulnerability to adverse health outcomes [ 1 ]. Physical frailty Physical frailty was the primary outcome, and it was measured using an adapted frailty phenotype score Supplemental Table 2 [ 1 ]. Covariates Covariates were collected at baseline and included demographic and health conditions. Statistical analysis Sample characteristics were generated for each frailty subgroup: non-frail, pre-frail, frail. Full size image. Table 3 Ordinal Logistic Regression Model Relating 1-Year Frailty Phenotype 0—5 to Baseline RMR in Subgroups Stratified by Baseline Frailty Status Non-Frail or Pre-Frail Full size table. Discussion The primary objective of this study was to examine the relationship between RMR and 1-year change in frailty in a sample of predominantly African-American older adults, adjusting for body composition, to extend prior work conducted in cross-section. Data Availability The dataset used in the current study is available from the senior author Dr. References Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Article CAS PubMed Google Scholar Fedarko NS. Article PubMed PubMed Central Google Scholar Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, Bandeen-Roche K, Varadhan R. Article PubMed Google Scholar Huisingh-Scheetz M, Walston J. Article PubMed Google Scholar Maxwell CA, Wang J. Article PubMed Google Scholar Mrdutt MM, Papaconstantinou HT, Robinson BD, Bird ET, Isbell CL. Article PubMed Google Scholar Panayi AC, Orkaby AR, Sakthivel D, Endo Y, Varon D, Roh D, Orgill DP, Neppl RL, Javedan H, Bhasin S, et al. Article CAS PubMed Google Scholar Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Article PubMed Google Scholar Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I. Article PubMed PubMed Central Google Scholar Manini TM. Article CAS PubMed Google Scholar Huisingh-Scheetz M, Wroblewski K, Waite L, Huang ES, Schumm LP, Hedeker D. Article PubMed Google Scholar Sagong H, Jang AR, Kim DE, Won CW, Yoon JY. Article PubMed Google Scholar Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Article PubMed PubMed Central Google Scholar Bastone AC, Ferriolli E, Pfrimer K, Moreira BS, Diz JBM, Dias JMD, Dias RC. Article PubMed Google Scholar Abizanda P, Romero L, Sanchez-Jurado PM, Ruano TF, Rios SS, Sanchez MF. Article PubMed Google Scholar Arciero PJ, Goran MI, Poehlman ET. Article CAS PubMed Google Scholar Nagel A, Jungert A, Spinneker A, Neuhäuser-Berthold M. Article CAS PubMed Google Scholar Zampino M, AlGhatrif M, Kuo PL, Simonsick EM, Ferrucci L. Article PubMed PubMed Central Google Scholar Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. Article PubMed PubMed Central Google Scholar Health Literacy Universal Precautions Toolkit. Article CAS PubMed Google Scholar Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Article PubMed PubMed Central Google Scholar Charlson ME, Pompei P, Ales KL, MacKenzie CR. Article CAS PubMed Google Scholar Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. Article PubMed Google Scholar Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Article PubMed PubMed Central Google Scholar Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Article PubMed Google Scholar Huisingh-Scheetz M, Martinchek M, Becker Y, Ferguson MK, Thompson K. Article PubMed Google Scholar Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Article PubMed Google Scholar Bohannon RW, Wang YC. Article PubMed Google Scholar Podsiadlo D, Richardson S. Article CAS PubMed Google Scholar Kim S, Welsh DA, Ravussin E, Welsch MA, Cherry KE, Myers L, Jazwinski SM. Article CAS PubMed Google Scholar Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Article PubMed Google Scholar Christensen RA, Raiber L, Wharton S, Rotondi MA, Kuk JL. Article CAS PubMed Google Scholar Li Z, Zhao H, Wang J. Article CAS PubMed PubMed Central Google Scholar Lumsden AL, Mulugeta A, Mäkinen VP, Hyppönen E. Article CAS PubMed Google Scholar Grigolon RB, Brietzke E, Trevizol AP, McIntyre RS, Mansur RB. Article PubMed Google Scholar Tamura Y, Ishikawa J, Fujiwara Y, Tanaka M, Kanazawa N, Chiba Y, Iizuka A, Kaito S, Tanaka J, Sugie M, et al. Article PubMed PubMed Central Google Scholar Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Article PubMed Google Scholar Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, Chertow GM. Article PubMed Google Scholar Hirani V, Naganathan V, Blyth F, Le Couteur DG, Seibel MJ, Waite LM, Handelsman DJ, Cumming RG. Article PubMed Google Scholar Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Article CAS PubMed PubMed Central Google Scholar Moraes MB, Avgerinou C, Fukushima FB, Vidal EIO. Article PubMed Google Scholar Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al. Article CAS PubMed Google Scholar Porter J, Nguo K, Collins J, Kellow N, Huggins CE, Gibson S, Davidson Z, Schoeller D, Prentice R, Neuhouser ML, et al. Article PubMed PubMed Central Google Scholar Weiss CO, Cappola AR, Varadhan R, Fried LP. Article PubMed PubMed Central Google Scholar Buch A, Diener J, Stern N, Rubin A, Kis O, Sofer Y, Yaron M, Greenman Y, Eldor R, Eilat-Adar S. Enjoy movement and stay active. And put the calculator away. RMR in sedentary adults can range from less than to more than calories per day. BMR is the amount of energy used when you're lying still and awake. RMR is similar but can include some low-effort tasks. BMR is measured when fully at rest, while RMR can have a small bit of movement. In an ideal world, RMR calculations would be percent accurate and would let us know exactly how many calories our bodies need each day. That would allow us to cut calories for weight loss. DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Oxford Reference. Metabolic rate. Jensen, M. Goldman Cecil Medicine 26th Edition. Obesity chapter. McMurray RG, Soares J, Caspersen CJ, McCurdy T. Examining variations of resting metabolic rate of adults: a public health perspective. Med Sci Sports Exerc. Bendavid I, Lobo DN, Barazzoni R, et al. The centenary of the Harris-Benedict equations: How to assess energy requirements best? Recommendations from the ESPEN expert group. Clin Nutr. Cioffi I, Marra M, Pasanisi F, Scalfi L. Prediction of resting energy expenditure in healthy older adults: A systematic review. Mtaweh H, Tuira L, Floh AA, Parshuram CS. Indirect calorimetry: history, technology, and application. Front Pediatr. Gupta RD, Ramachandran R, Venkatesan P, Anoop S, Joseph M, Thomas N. Indirect calorimetry: from bench to bedside. Indian J Endocrinol Metab. Blunt K, Dye M. Basal metabolism of normal women. J Biol Chem. Global RxPh. Harris Benedict Basal Metabolic Rate Calculator. Chung N, Park MY, Kim J, et al. Non-exercise activity thermogenesis Neat : a component of total daily energy expenditur e. J Exerc Nutrition Biochem. By Cara Rosenbloom, RD Cara Rosenbloom RD is a dietitian, journalist, book author, and the founder of Words to Eat By, a nutrition communications company in Toronto, ON. Use limited data to select advertising. Create profiles for personalised advertising. Use profiles to select personalised advertising. Create profiles to personalise content. Use profiles to select personalised content. Measure advertising performance. Measure content performance. Understand audiences through statistics or combinations of data from different sources. Develop and improve services. Use limited data to select content. List of Partners vendors. Weight Management. By Cara Rosenbloom RD is a dietitian, journalist, book author, and the founder of Words to Eat By, a nutrition communications company in Toronto, ON. Cara Rosenbloom, RD. Learn about our editorial process. Learn more. Medical Reviewers confirm the content is thorough and accurate, reflecting the latest evidence-based research. Content is reviewed before publication and upon substantial updates. Medically reviewed by Samina Qureshi, RD. Learn about our Medical Review Board. Table of Contents View All. Table of Contents. What Is Metabolism? Resting Metabolic Rate. Calculating RMR. Calculate RMR in a Lab. Normal RMR. Factors that Affect RMR. Factors That Affect RMR. Metabolism and Weight Loss. Frequently Asked Questions. Resting Metabolic Rate is the number of calories that your body burns while at rest. The Difference Between BMR and RMR BMR is measured when fully at rest, while RMR can have a small bit of movement. Height Weight Age Gender Ethnic descent Diet Activity Level Muscle. Frequently Asked Questions What is an average RMR? What is the difference between RMR and BMR? |
| Access options | Their findings revealed four distinct phases of adjusted total and basal energy expenditure over the lifespan. Neonatal 1 month to 1 year : Neonates in the first month of life had size-adjusted energy expenditure similar to that of adults. Energy expenditure increased rapidly over the first year, reaching a peak at 0. Childhood and adolescence 1 to 20 years : Although total and basal expenditure as well as fat-free mass continued to increase with age throughout childhood and adolescence, size-adjusted expenditures steadily declined throughout this period. Sex had no effect on the rate of decline. At Of note, there was no increase in adjusted total or basal energy expenditure during the pubertal ages of 10 to 15 years old. Adulthood 20 to 60 years : Total and basal expenditure and fat-free mass were all stable from ages 20 to 60, regardless of sex. Adjusted TEE and RMR remained stable even during pregnancy, and any increase in unadjusted energy expenditure during pregnancy was accounted for by the increase in body mass. The point at which adjusted TEE started to decline was age 63, and for adjusted BMR was age Older adulthood andgt;60 years : At approximately 60 years old, TEE and BMR began to decline, along with fat-free mass and fat mass. The first is that the frailty literature has not yet determined how frequently frailty should be assessed in clinical practice [ 31 , 32 ]. Most work relating frailty to morbidity and mortality outcomes relies on a single, baseline measure of frailty; however, early evidence suggests older adult physical function trajectories can be differentiated over as short as 3 times points across two years. The two-year trajectories of function improved prediction of mortality above using just baseline measures [ 33 ]. Here, we detect frailty category changes over just one year in a substantial proportion of our sample, indicating repeat testing even at one year may help inform these trajectories. The second implication is that these findings raise a question about frailty measurement reliability. Others have reported measurement variability within subjects and across administrators for several measures of physical function [ 34 , 35 , 36 ]. Measurement reliability has not been reported for the frailty phenotype itself. Future work exploring how to differentiate measurement variability from clinically significant changes in frailty status is a major gap to translating frailty to clinical practice and represents a limitation to this study [ 31 ]. We are among the first to relate RMR to change in frailty phenotype scores in a longitudinal model. After adjusting for both body composition and comorbid illness, higher RMR at baseline was significantly associated with frailty progression at one year. Prior cross-sectional work relating RMR to frailty status have found both depressed RMR [ 17 ] and elevated RMR [ 37 ] were associated with worse frailty. Abizanda et al. found RMR was lower among those who were frail and pre-frail relative to those who were non-frail using a categorical frailty phenotype after adjusting for FFM, age, gender and comorbidities [ 17 ]. In contrast, Kim et al. The FI 34 heavily weights comorbidities and disability in the frailty score, many of which have independent associations with higher RMR [ 38 , 39 ]. At baseline and in cross-section, we found no differences in RMR across the three frailty subgroups even after adjusting for covariates data not shown , further contributing to the diversity of cross-sectional findings. The variability in the collection of the cross-sectional studies might suggest that summary biomarker measures miss important heterogeneity between individuals, especially when the biomarkers also have dynamic trajectories. Alternatively, these findings might suggest that RMR is a better short-term predictive frailty biomarker than a cross-sectional diagnostic frailty biomarker. Finally, these findings might also suggest that RMR is a dynamic biomarker across the spectrum of frailty contributing to mixed associations in cross-section. Our work builds on this prior cross-sectional work by studying a unidirectional and longitudinal association between baseline RMR and change in frailty over one year. Various comorbid illnesses have been associated with changes in energy expenditure [ 16 , 40 , 41 ]. However, multimorbidity did not explain the positive association between higher baseline RMR and worsening frailty in our exploratory study. Cognitive function may also be related to energy utilization [ 42 ]. For instance, higher basal metabolic rate measured by bioimpedence analysis was strongly associated with worse dementia-related brain pathology in the UK Biobank study [ 42 ]. RMR has also been found to modulate the effects of caloric restriction on improving cognitive function in non-obese adults [ 43 ]. Only adults in the caloric restriction arm who demonstrated increased RMR showed improved cognition at 24 months. Adults in the caloric restriction arm who demonstrated reduced RMR had comparable cognitive outcomes to the ad libitum diet arm adults, whether they experienced increased or decreased RMR [ 43 ]. However, cognitive status also did not alter the association between RMR and frailty in this exploratory study. While neither multimorbidity nor cognitive function were found to mediate the RMR-to-frailty relationship in our study, they have also both been associated with frailty status in cross-section [ 44 , 45 ]. Our analysis explored only whether baseline RMR was associated with 1-year change in frailty; however, it is possible that frailty has a bidirectional relationship with RMR. For example, Zampino et al. found that chronic illnesses such as congestive heart failure, chronic kidney disease, chronic obstructive lung disease, and cancer predicted a more rapid decline in RMR with aging in the Baltimore Longitudinal Study on Aging dataset; these declines were not accounted for by the acquired cachexia resulting from these conditions [ 21 ]. To date, there are no reported studies relating frailty to change in RMR, a potential topic for future work. Lower fat-free mass was independently associated with worsening 1-year frailty in the overall sample; however, in models stratified by baseline frailty status, the fat-free mass and 1-year frailty association was strongest among the pre-frail group compared to the non-frail group. While some loss of variable significance can be attributed to the small subsample sizes, these stratified findings do raise the possibility that certain body composition measures are stronger risk factors at different frailty stages. The DEXA used in our study was not able to differentiate muscle quality but muscle fat infiltration may be additionally related to worse frailty [ 48 ]. Our stratified models are a unique contribution to the literature and suggest that low muscle mass is a more important frailty risk factor to target among pre-frail. To date, nutrition interventions targeting frailty, primarily protein supplementation, have provided the same nutrition recommendations regardless of degree of baseline frailty [ 49 , 50 , 51 ]. These studies have had mixed results. Results from the current study should be confirmed in larger studies but suggest nutritional targets may need to be tailored to different levels of frailty. This study was limited to a small sample, especially in the stratified models, that included only community-dwelling older adults living in the South Side of Chicago. While it was a strength in our study to include a high proportion of African Americans, a group historically under-represented in the literature and one that suffers greater frailty burden, our results may not apply to other populations. While we found higher baseline RMR was associated 1-year frailty decline, this association does not imply causality. A strength of this study was the use of objective measures to assess both RMR and body composition. A challenge to the clinical relevance of RMR research is the reliance on costly and time-consuming indirect or direct calorimetry following an overnight fast to measure. The less costly option is to use predictive equations incorporating age, gender, height and weight; however, they tend to be less accurate in older adults, and even less accurate when considering comorbid illness [ 52 , 53 , 54 , 55 ]. There is currently no clinical mechanism for measuring RMR as a risk factor for frailty during clinical care, which represents a barrier to using this metric regularly to risk-stratify patients. Our body composition was measured by DEXA and not MRI or CT, therefore we could not include muscle quality metrics. Our sample size was too small to assess the effects of individual comorbidities like heart failure or COPD, so we combined them into a comorbidity scale. We only had one RMR data point and baseline and 1-year frailty data points. Ideally, RMR and frailty could be measured at the same time over repeated time points. True frailty trajectory modeling would include at least three total data points, which would have facilitated trajectory modeling like the Markov state transition model. In summary, we conducted a longitudinal study relating RMR and body composition to 1-year change in frailty among predominantly African-American, community-dwelling older adults. In this group, higher baseline RMR and lower baseline fat-free mass were independently associated with frailty decline at one year. The dataset used in the current study is available from the senior author Dr. Huisingh-Scheetz, megan. huisingh-scheetz bsd. edu upon reasonable request and after completing the required institutional data use agreement. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. Article CAS PubMed Google Scholar. Fedarko NS. The biology of aging and frailty. Clin Geriatr Med. Article PubMed PubMed Central Google Scholar. Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, Bandeen-Roche K, Varadhan R. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev. Article PubMed Google Scholar. Huisingh-Scheetz M, Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol. Maxwell CA, Wang J. Nurs Clin North Am. Mrdutt MM, Papaconstantinou HT, Robinson BD, Bird ET, Isbell CL. Preoperative Frailty and Surgical outcomes Across Diverse Surgical subspecialties in a large Health Care System. J Am Coll Surg. Panayi AC, Orkaby AR, Sakthivel D, Endo Y, Varon D, Roh D, Orgill DP, Neppl RL, Javedan H, Bhasin S, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis. Am J Surg. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc , 17 12 Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, Batsis JA. Mortality risk along the Frailty Spectrum: data from the National Health and Nutrition Examination Survey to J Am Geriatr Soc. Manini TM. Energy expenditure and aging. Huisingh-Scheetz M, Wroblewski K, Waite L, Huang ES, Schumm LP, Hedeker D. Variability in hourly activity levels: statistical noise or insight into older adult Frailty? J Gerontol A Biol Sci Med Sci Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, Huang E, Dale W, Waite L, Schumm LP. The relationship between physical activity and Frailty among U. older adults based on hourly Accelerometry Data. Sagong H, Jang AR, Kim DE, Won CW, Yoon JY. The Cross-lagged Panel analysis between Frailty and Physical Activity among Community-Dwelling older adults by Age groups. J Aging Health. Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing. PLoS ONE. Bastone AC, Ferriolli E, Pfrimer K, Moreira BS, Diz JBM, Dias JMD, Dias RC. Energy expenditure in older adults who are Frail: a doubly labeled Water Study. J Geriatr Phys Ther. Abizanda P, Romero L, Sanchez-Jurado PM, Ruano TF, Rios SS, Sanchez MF. Energetics of aging and Frailty: the FRADEA Study. Bauman WA, Spungen AM, Wang J, Pierson RN Jr. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury. J Rehabil Res Dev. Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol Nagel A, Jungert A, Spinneker A, Neuhäuser-Berthold M. The impact of Multimorbidity on resting metabolic rate in Community-Dwelling women over a ten-year period: a cross-sectional and longitudinal study. J Nutr Health Aging. Zampino M, AlGhatrif M, Kuo PL, Simonsick EM, Ferrucci L. Longitudinal changes in resting metabolic rates with aging are accelerated by Diseases. Nutrients , 12 Falsarella GR, Gasparotto LP, Barcelos CC, Coimbra IB, Moretto MC, Pascoa MA, Ferreira TC, Coimbra AM. Body composition as a frailty marker for the elderly community. Clin Interv Aging. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. IDEAL aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. Health Literacy Universal Precautions Toolkit. Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr. Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA. Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of Sleep Extension on objectively assessed Energy Intake among adults with overweight in real-life settings: a Randomized Clinical Trial. JAMA Intern Med. Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. If we have less muscle mass as in in older adults , and probably some other systems working at half capacity that could explain the reduce energy needs. I highly doubt that senescent cells are a direct cause of increased metabolic rate. It might be that there are energy "leaks" or the food is not absorbed correctly. Probably if the ScC get removed, the affected people will become more active , gain some muscles and burn even more calories.. I'd look into the aging brain for reasons why RMR changes with age. Brain circuits like this e. Neuroscientists have identified a brain circuit critical for learning to make decisions that require evaluating the cost or reward of an action. They showed this circuit is negatively affected by aging and in Huntington's disease. Post a comment; thoughtful, considered opinions are valued. New comments can be edited for a few minutes following submission. Comments incorporating ad hominem attacks, advertising, and other forms of inappropriate behavior are likely to be deleted. Note that there is a comment feed for those who like to keep up with conversations. By checking, I consent to the storage and handling of my data. See the privacy policy and terms and conditions for details. Home FAQ Fund Research Services Investing Therapies Newsletter Archives Press Room Resources About. |
RMR and aging -
Forbes GB : Techniques for estimating body composition. In Human Body Composition: Growth, Aging, Nutrition, and Activity pp 5— New York: Springer. Chapter Google Scholar. Metabolism 22 , — Fitness 44 , 71— Metabolism 50 , — Article PubMed Google Scholar. Metabolism 40 , — The relationship between muscle mass and muscle strength in the elderly.
Article CAS Google Scholar. Suominen H : Changes in physical characteristics and body composition during 5-year follow-up in and year-old men and women. CAS Google Scholar. Metabolism 42 , — Weir JB de V : New methods for calculating metabolic rate with special reference to protein metabolism.
Article PubMed PubMed Central Google Scholar. Metabolism 39 , 11— WHO Technical Report Series , Geneva. Download references. Institute of Nutritional Science, University of Giessen, Germany. You can also search for this author in PubMed Google Scholar. Correspondence to M Neuhäuser-Berthold. Contributors: CK performed the study with the assistance of all co-authors, was responsible for data collection and analysis, and wrote the report with assistance of PL and MN-B.
PL is the coordinator of the GISELA-study and assisted with the management of the study, data analysis and writing of the report.
AS and BH assisted with the data collection and analyses. MN-B is the principal investigator of the GISELA-study and supervised the study together with PL and participated in the writing of the report.
Reprints and permissions. Krems, C. et al. Lower resting metabolic rate in the elderly may not be entirely due to changes in body composition. Eur J Clin Nutr 59 , — Download citation. Received : 04 March Revised : 19 July Accepted : 13 August Published : 20 October Issue Date : 01 February Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Skip to main content Thank you for visiting nature. nature european journal of clinical nutrition original communication article. Abstract Objective: To investigate whether or not the lower resting metabolic rate RMR in the elderly is entirely due to changes in body composition. Access through your institution.
Buy or subscribe. After these changes, min of data remained. The RMR data then underwent additional manual processing prior to summarizing the data. The first ten measurements e.
The coefficient of variance CV was then calculated for all participants using the remaining data. A technologist certified in Lunar DEXA imaging performed all scans. For our analysis, we extracted the following variables: fat-free mass kg and fat mass kg.
Both were treated as continuous variables. Physical frailty was the primary outcome, and it was measured using an adapted frailty phenotype score Supplemental Table 2 [ 1 ]. Weakness was identified when the average of 3 dominant grip strength measurements Jamar hydraulic hand dynamometer was below the established body mass index- and gender-adjusted cut-points, as previously described [ 1 ].
Slowness was identified when the average of three foot usual walk times were below gender- and height-adjusted cut points, as originally described [ 1 ]. A point was assigned for each criteria met. The total score ranged from 0 to 5: 0 criteria indicated a non-frail status, 1 to 2 criteria indicated a pre-frail status, and 3 or more indicated a frail status.
Physical frailty was measured at baseline and 1 year. Covariates were collected at baseline and included demographic and health conditions. Age was calculated using date of birth and survey date and treated as a continuous variable.
The Charlson comorbidity index was calculated using self-reported comorbidity data [ 27 ]. The Montreal Cognitive Assessment MoCA was administered to all participants to evaluate cognitive function [ 28 ]. MoCA scores range from 0 to 30, with higher scores indicating better cognitive function.
It was treated as a continuous variable. Sample characteristics were generated for each frailty subgroup: non-frail, pre-frail, frail.
Means and standard deviations SD were reported for continuous variables and number of participants in each category were reported for categorical data. We identified significant differences across frailty categories using Kruskal-Wallis tests continuous measures or chi-squared tests categorical measures.
We also described the frequency of frailty category non-frail, pre-frail, frail shifts at one year in the sample. We then regressed the 1-year frailty phenotype scores 0—5 on baseline RMR, baseline frailty phenotype scores 0—5 , body composition both fat mass and fat-free mass, continuous and demographics in an ordinal logistic regression model.
We then separately adjusted for 1 comorbidities and polypharmacy and then 2 cognition, examining any changes in the RMR-frailty relationship. We adjusted separately for chronic disease burden and cognitive function for three reasons: 1 the Charlson Comorbidity Index includes self-reported dementia, 2 our sample size was small so we hoped to reduce the potential for overfitting the model and 3 we were interested in assessing the impact of each of these factors independently.
Output are reported as odds ratios for each model. The model was then stratified by baseline frailty status non-frail, pre-frail to explore differential RMR effects across the frailty spectrum. We conducted additional sensitivity analyses. We further reran our ordinal logistic regression models including self-reported physical activity energy expenditure as a covariate.
We regressed this dichotomous variable on baseline frailty, RMR, body composition and demographics using logistic regression, separately among those who were non-frail and pre-frail at baseline. The within-person measurement reliability for RMR in older adults is unknown.
Among 40 overweight, young- to middle-aged adults ages 21—40 years, the two-week within-person reliability was good mean difference between measurements was Due to the potential for measurement error in RMR, we performed a robustness check of our findings.
We replicated the models with linear regression and errors-in-variables regression, the latter accounting for an RMR reliability of 0. The beta coefficients and significance of the RMR independent variable were largely unchanged data not shown.
Pre-frail and frail adults included proportionally more African-Americans and had a higher comorbidity burden than non-frail adults.
The three groups did not have statistically significant differences in age, gender, education, income, polypharmacy, MoCA scores, body composition or RMR. A substantial proportion of study participants transitioned between states of frailty, even across 1 year Fig. The multivariate ordinal logistic regression models associating baseline RMR to 1-year change in frailty are shown in Table 2.
The model including RMR categorized into quartiles is shown in Supplemental Table 3. Adjusting for baseline self-reported physical activity energy expenditure did not greatly affect the relationship between RMR and 1-year frailty in any of the models Supplemental Table 4.
The multivariate ordinal logistic regression model adjusting for baseline frailty, body composition and demographic covariates was then stratified by baseline frailty status Table 3. The primary objective of this study was to examine the relationship between RMR and 1-year change in frailty in a sample of predominantly African-American older adults, adjusting for body composition, to extend prior work conducted in cross-section.
We found that higher baseline RMR and lower baseline fat-free mass were independently associated with worsened frailty at one year after adjusting for baseline frailty status. Neither multimorbidity nor cognitive function at baseline significantly altered this relationship.
Unique to this study, we also explored these relationships in models stratified by baseline frailty status. Higher RMR and lower fat-free mass were most strongly associated with frailty progression among those who were pre-frail at baseline.
These results provide new evidence suggesting higher resting energy expenditure is associated with accelerated short-term frailty decline. Over large intervals of time e. In this local sample of predominantly African-American older adults, we noted both progression and improvement of frailty statuses, even at one year.
It is feasible that frailty status can change over a period as short as one year, especially following an acute stressor that temporarily worsens frailty, such as a hospitalization or acute illness, but from which one is expected to recover.
These detected one-year frailty transitions have two important implications for clinical practice and frailty research. The first is that the frailty literature has not yet determined how frequently frailty should be assessed in clinical practice [ 31 , 32 ]. Most work relating frailty to morbidity and mortality outcomes relies on a single, baseline measure of frailty; however, early evidence suggests older adult physical function trajectories can be differentiated over as short as 3 times points across two years.
The two-year trajectories of function improved prediction of mortality above using just baseline measures [ 33 ]. Here, we detect frailty category changes over just one year in a substantial proportion of our sample, indicating repeat testing even at one year may help inform these trajectories.
The second implication is that these findings raise a question about frailty measurement reliability. Others have reported measurement variability within subjects and across administrators for several measures of physical function [ 34 , 35 , 36 ].
Measurement reliability has not been reported for the frailty phenotype itself. Future work exploring how to differentiate measurement variability from clinically significant changes in frailty status is a major gap to translating frailty to clinical practice and represents a limitation to this study [ 31 ].
We are among the first to relate RMR to change in frailty phenotype scores in a longitudinal model. After adjusting for both body composition and comorbid illness, higher RMR at baseline was significantly associated with frailty progression at one year.
Prior cross-sectional work relating RMR to frailty status have found both depressed RMR [ 17 ] and elevated RMR [ 37 ] were associated with worse frailty. Abizanda et al. found RMR was lower among those who were frail and pre-frail relative to those who were non-frail using a categorical frailty phenotype after adjusting for FFM, age, gender and comorbidities [ 17 ].
In contrast, Kim et al. The FI 34 heavily weights comorbidities and disability in the frailty score, many of which have independent associations with higher RMR [ 38 , 39 ]. At baseline and in cross-section, we found no differences in RMR across the three frailty subgroups even after adjusting for covariates data not shown , further contributing to the diversity of cross-sectional findings.
The variability in the collection of the cross-sectional studies might suggest that summary biomarker measures miss important heterogeneity between individuals, especially when the biomarkers also have dynamic trajectories. Alternatively, these findings might suggest that RMR is a better short-term predictive frailty biomarker than a cross-sectional diagnostic frailty biomarker.
Finally, these findings might also suggest that RMR is a dynamic biomarker across the spectrum of frailty contributing to mixed associations in cross-section. Our work builds on this prior cross-sectional work by studying a unidirectional and longitudinal association between baseline RMR and change in frailty over one year.
Various comorbid illnesses have been associated with changes in energy expenditure [ 16 , 40 , 41 ]. However, multimorbidity did not explain the positive association between higher baseline RMR and worsening frailty in our exploratory study. Cognitive function may also be related to energy utilization [ 42 ].
For instance, higher basal metabolic rate measured by bioimpedence analysis was strongly associated with worse dementia-related brain pathology in the UK Biobank study [ 42 ]. RMR has also been found to modulate the effects of caloric restriction on improving cognitive function in non-obese adults [ 43 ].
Only adults in the caloric restriction arm who demonstrated increased RMR showed improved cognition at 24 months. Adults in the caloric restriction arm who demonstrated reduced RMR had comparable cognitive outcomes to the ad libitum diet arm adults, whether they experienced increased or decreased RMR [ 43 ].
However, cognitive status also did not alter the association between RMR and frailty in this exploratory study. While neither multimorbidity nor cognitive function were found to mediate the RMR-to-frailty relationship in our study, they have also both been associated with frailty status in cross-section [ 44 , 45 ].
Our analysis explored only whether baseline RMR was associated with 1-year change in frailty; however, it is possible that frailty has a bidirectional relationship with RMR. For example, Zampino et al. found that chronic illnesses such as congestive heart failure, chronic kidney disease, chronic obstructive lung disease, and cancer predicted a more rapid decline in RMR with aging in the Baltimore Longitudinal Study on Aging dataset; these declines were not accounted for by the acquired cachexia resulting from these conditions [ 21 ].
To date, there are no reported studies relating frailty to change in RMR, a potential topic for future work. Lower fat-free mass was independently associated with worsening 1-year frailty in the overall sample; however, in models stratified by baseline frailty status, the fat-free mass and 1-year frailty association was strongest among the pre-frail group compared to the non-frail group.
While some loss of variable significance can be attributed to the small subsample sizes, these stratified findings do raise the possibility that certain body composition measures are stronger risk factors at different frailty stages.
The DEXA used in our study was not able to differentiate muscle quality but muscle fat infiltration may be additionally related to worse frailty [ 48 ]. Our stratified models are a unique contribution to the literature and suggest that low muscle mass is a more important frailty risk factor to target among pre-frail.
To date, nutrition interventions targeting frailty, primarily protein supplementation, have provided the same nutrition recommendations regardless of degree of baseline frailty [ 49 , 50 , 51 ]. These studies have had mixed results. Results from the current study should be confirmed in larger studies but suggest nutritional targets may need to be tailored to different levels of frailty.
This study was limited to a small sample, especially in the stratified models, that included only community-dwelling older adults living in the South Side of Chicago. While it was a strength in our study to include a high proportion of African Americans, a group historically under-represented in the literature and one that suffers greater frailty burden, our results may not apply to other populations.
While we found higher baseline RMR was associated 1-year frailty decline, this association does not imply causality. A strength of this study was the use of objective measures to assess both RMR and body composition. A challenge to the clinical relevance of RMR research is the reliance on costly and time-consuming indirect or direct calorimetry following an overnight fast to measure.
The less costly option is to use predictive equations incorporating age, gender, height and weight; however, they tend to be less accurate in older adults, and even less accurate when considering comorbid illness [ 52 , 53 , 54 , 55 ].
There is currently no clinical mechanism for measuring RMR as a risk factor for frailty during clinical care, which represents a barrier to using this metric regularly to risk-stratify patients. Our body composition was measured by DEXA and not MRI or CT, therefore we could not include muscle quality metrics.
Our sample size was too small to assess the effects of individual comorbidities like heart failure or COPD, so we combined them into a comorbidity scale. We only had one RMR data point and baseline and 1-year frailty data points. Ideally, RMR and frailty could be measured at the same time over repeated time points.
True frailty trajectory modeling would include at least three total data points, which would have facilitated trajectory modeling like the Markov state transition model.
In summary, we conducted a longitudinal study relating RMR and body composition to 1-year change in frailty among predominantly African-American, community-dwelling older adults. In this group, higher baseline RMR and lower baseline fat-free mass were independently associated with frailty decline at one year.
The dataset used in the current study is available from the senior author Dr. Huisingh-Scheetz, megan. huisingh-scheetz bsd. edu upon reasonable request and after completing the required institutional data use agreement.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Frailty in older adults: evidence for a phenotype.
J Gerontol A Biol Sci Med Sci. Article CAS PubMed Google Scholar. Fedarko NS. The biology of aging and frailty.
Clin Geriatr Med. Article PubMed PubMed Central Google Scholar. Buta BJ, Walston JD, Godino JG, Park M, Kalyani RR, Xue QL, Bandeen-Roche K, Varadhan R. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev.
Article PubMed Google Scholar. Huisingh-Scheetz M, Walston J. How should older adults with cancer be evaluated for frailty? J Geriatr Oncol. Maxwell CA, Wang J. Nurs Clin North Am. Mrdutt MM, Papaconstantinou HT, Robinson BD, Bird ET, Isbell CL. Preoperative Frailty and Surgical outcomes Across Diverse Surgical subspecialties in a large Health Care System.
J Am Coll Surg. Panayi AC, Orkaby AR, Sakthivel D, Endo Y, Varon D, Roh D, Orgill DP, Neppl RL, Javedan H, Bhasin S, et al. Impact of frailty on outcomes in surgical patients: a systematic review and meta-analysis.
Am J Surg. Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. Vermeiren S, Vella-Azzopardi R, Beckwée D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I.
Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J Am Med Dir Assoc , 17 12 Crow RS, Lohman MC, Titus AJ, Bruce ML, Mackenzie TA, Bartels SJ, Batsis JA.
Mortality risk along the Frailty Spectrum: data from the National Health and Nutrition Examination Survey to J Am Geriatr Soc. Manini TM. Energy expenditure and aging. Huisingh-Scheetz M, Wroblewski K, Waite L, Huang ES, Schumm LP, Hedeker D. Variability in hourly activity levels: statistical noise or insight into older adult Frailty?
J Gerontol A Biol Sci Med Sci Huisingh-Scheetz M, Wroblewski K, Kocherginsky M, Huang E, Dale W, Waite L, Schumm LP. The relationship between physical activity and Frailty among U. older adults based on hourly Accelerometry Data. Sagong H, Jang AR, Kim DE, Won CW, Yoon JY.
The Cross-lagged Panel analysis between Frailty and Physical Activity among Community-Dwelling older adults by Age groups. J Aging Health. Rogers NT, Marshall A, Roberts CH, Demakakos P, Steptoe A, Scholes S. Physical activity and trajectories of frailty among older adults: evidence from the English Longitudinal Study of Ageing.
PLoS ONE. Bastone AC, Ferriolli E, Pfrimer K, Moreira BS, Diz JBM, Dias JMD, Dias RC. Energy expenditure in older adults who are Frail: a doubly labeled Water Study. J Geriatr Phys Ther. Abizanda P, Romero L, Sanchez-Jurado PM, Ruano TF, Rios SS, Sanchez MF.
Energetics of aging and Frailty: the FRADEA Study. Bauman WA, Spungen AM, Wang J, Pierson RN Jr. The relationship between energy expenditure and lean tissue in monozygotic twins discordant for spinal cord injury.
J Rehabil Res Dev. Arciero PJ, Goran MI, Poehlman ET. Resting metabolic rate is lower in women than in men. J Appl Physiol Nagel A, Jungert A, Spinneker A, Neuhäuser-Berthold M.
The impact of Multimorbidity on resting metabolic rate in Community-Dwelling women over a ten-year period: a cross-sectional and longitudinal study. J Nutr Health Aging. Zampino M, AlGhatrif M, Kuo PL, Simonsick EM, Ferrucci L.
Longitudinal changes in resting metabolic rates with aging are accelerated by Diseases. Nutrients , 12 Falsarella GR, Gasparotto LP, Barcelos CC, Coimbra IB, Moretto MC, Pascoa MA, Ferreira TC, Coimbra AM. Body composition as a frailty marker for the elderly community.
Clin Interv Aging. Schrack JA, Knuth ND, Simonsick EM, Ferrucci L. IDEAL aging is associated with lower resting metabolic rate: the Baltimore Longitudinal Study of Aging. Health Literacy Universal Precautions Toolkit.
Luke A, Durazo-Arvizu R, Cao G, Adeyemo A, Tayo B, Cooper R. Positive association between resting energy expenditure and weight gain in a lean adult population. Am J Clin Nutr. Eckel SP, Bandeen-Roche K, Chaves PH, Fried LP, Louis TA.
Surrogate screening models for the low physical activity criterion of frailty. Aging Clin Exp Res. Charlson ME, Pompei P, Ales KL, MacKenzie CR.
A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H.
The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. Tasali E, Wroblewski K, Kahn E, Kilkus J, Schoeller DA. Effect of Sleep Extension on objectively assessed Energy Intake among adults with overweight in real-life settings: a Randomized Clinical Trial.
JAMA Intern Med. Marshall A, Nazroo J, Tampubolon G, Vanhoutte B. Cohort differences in the levels and trajectories of frailty among older people in England. J Epidemiol Community Health. Huisingh-Scheetz M, Martinchek M, Becker Y, Ferguson MK, Thompson K. Translating Frailty Research Into Clinical Practice: insights from the successful aging and frailty evaluation clinic.
J Am Med Dir Assoc Bandeen-Roche K, Gross AL, Varadhan R, Buta B, Carlson MC, Huisingh-Scheetz M, McAdams-DeMarco M, Piggott DA, Brown TT, Hasan RK et al. Principles and issues for physical Frailty Measurement and its clinical application.
White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, Cauley JA, Ferrucci L, Harris TB, Satterfield SM, et al. Trajectories of gait speed predict mortality in well-functioning older adults: the Health, Aging and Body Composition study.
Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Phys Ther. Bohannon RW, Wang YC. Four-meter gait speed: normative values and reliability determined for adults participating in the NIH Toolbox Study. Arch Phys Med Rehabil. Podsiadlo D, Richardson S. Kim S, Welsh DA, Ravussin E, Welsch MA, Cherry KE, Myers L, Jazwinski SM.
An elevation of resting metabolic rate with declining health in nonagenarians may be associated with decreased muscle mass and function in women and men, respectively.
Fabbri E, An Y, Schrack JA, Gonzalez-Freire M, Zoli M, Simonsick EM, Guralnik JM, Boyd CM, Studenski SA, Ferrucci L. Energy Metabolism and the Burden of Multimorbidity in older adults: results from the Baltimore Longitudinal Study of Aging.
Innocencio da Silva Gomes A, dos Santos Vigário P, Mainenti MR, de Figueiredo Ferreira M, Ribeiro BG, de Abreu Soares E.
Use a Resting Metabolic Rate Calculator or Compute Your Own. Agimg Rosenbloom RD annd a dietitian, journalist, book Continuous glucose monitoring app, and the founder of Words aginb Eat By, aginb nutrition communications company in Aginf, Vegan desserts for special occasions. Samina Anf Vegan desserts for special occasions, LD is the founder and Registered Dietitian at Wholesome Start, LLC a virtual nutrition practice based in Houston, Texas. Have you ever wondered how dietitians estimate how many calories their clients should eat in a day? While the science is far from exact, several useful calculations can help determine how many calories you should eat for weight loss, gain, or maintenance. Part of the calculation determines your resting metabolic rate RMRwhich is how many calories your body burns while at rest.
Sie haben sich wahrscheinlich geirrt?