Protein intake and immune function -
Many people could benefit from getting more plant- and animal-based sources in their meals. Without enough protein in our diets, our bodies will steal protein from our muscles to complete those biological functions, leading to muscle loss.
For people with muscle wasting conditions such as cancer, getting enough protein is even more important.
Here, she shares what you should know about packing more protein into your life. Getting enough protein is critical for maintaining healthy muscles.
Muscle mass is important for movement, balance and posture as well as daily functions such as opening a jar or standing up.
It also fuels immune function. When you get a paper cut, proteins rush to the site to support the growth of new skin. If you have a larger wound, say from surgery, or if you have a chronic illness, the immune system requires more protein to carry out healing.
Ensuring you get enough protein in your diet can help to support healthy immune function and prevent muscles from wasting away. Even people with normal body weight can have low muscle mass.
In addition to having all nine essential amino acids, animal proteins tend to have higher protein density and more iron, Vitamin D and B For example, grams of beef contains Beef also has significantly more leucine, the amino acid most responsible for muscle growth.
Many sources of plant protein, such as peanut butter and legumes, are also inexpensive. The body subsequently viewed this activity as a threat and triggered a sequence of events where immune cells travelled into the gut wall. While it is too early to say if this research might translate in humans, the researchers say activation of the immune system can prove either good or bad news.
The results appear consistent with the population impacts of modern-day diets, with the Western world seeing lower rates of gastrointestinal infection but higher rates of chronic disease. This advancement in knowledge was made possible by the merging of academic disciplines for which the Charles Perkins Centre has become well known.
The study utilised the geometric framework for nutrition developed by Professor Stephen Simpson and Professor David Raubenheimer , arising from the study of ecology.
Declaration: This project was funded by Australian Research Council grants APP and APP and by the Sydney Medical School MCR BioMed-Connect Grants.
The authors declare no competing interests. GSH γ-glutamyl-cysteinyl-glycine is the predominant low-molecular-weight thiol 0. It is now well accepted that many forms of thiol oxidation disulphide formation, gluathionylation and S-nitrosylation are reversible and can provide a mechanism used by skeletal muscle cells in the regulation of metabolic signaling and transcriptional processes, including in muscle adaptation after exercise and training [ 1 ],[ 54 ].
The synthesis of GSH from glutamate, cysteine, and glycine is catalyzed sequentially by two key cytosolic enzymes, γ-glutamylcysteine synthetase GCS and GSH synthetase Figure 2.
GCS is the key regulatory enzyme, activated by several types of stress including oxidative and nitrosative stress, inflammation, heat stress, and others [ 55 ].
It is therefore reasonable to speculate that amino acid and protein supplementation, may provide intracellular GSH precursors - an essential strategy to improve GSH synthesis and redox protection, leading also to better control of the inflammatory status and muscle recovery [ 56 ].
Immune, antioxidant and inflammatory targets that L-glutamine, L-arginine and BCAA are involved. HSPs are modulated by the heat shock factor 1, which is activated by the glucosamine pathway, sirtuin 1 Sirt1 and human antigen R Hur , also known as nutrient sensors.
De novo L-glutamine synthesis can occur through L-glutamine synthetase GS , using glutamate, ATP and ammonia NH 3. L-arginine availability is important to NO production through nitric oxide synthase 2 NOS2 and citrulline CIT. Other Abbreviations: heat shock elements HSEs ; oxidized GSH GSSG ; GSH-S reductase GSR ; glutamate dehydrogenase GLUD ; alpha-ketoglutarato α-KG.
Muscle redox state may be best improved by providing skeletal muscle cells with the key natural precursors for GSH synthesis and allowing the cells to synthesize what they actually require. Exercise-induced ROS is not detrimental to human health, thus endogenous antioxidants may be sufficient to protect against exercise-induced oxidative damage, however this may not be applicable for elite athletes.
In addition to GSH metabolism, the levels of iHSP72 may also be involved in the control of exercise-induced muscle inflammation and adaptation [ 57 ]. Their expression has been shown to be induced by a wide range of stressors such as oxidative stress, thermal stress, hypoxia, viral infection, heavy metal contamination, ischemia, exercise metabolic stress and many others [ 33 ],[ 53 ].
Other HSP functions include protein translocation, anti-apoptosis, and also anti-inflammatory response [ 58 ]. The anti-inflammatory role of the HSP70 is mediated by its interaction with the proteins involved in the activation of the NF-κB, blocking its translocation to the nucleus and slowing of the inflammatory process [ 51 ],[ 58 ].
Interestingly, specific amino acid supplementation has been shown to induce HSP70 and GSH in many cells, as will be described below. L-glutamine is probably the most widely recognized immuno-nutrient since it can be used as an oxidizable fuel, a substrate for nucleotide synthesis, a modulator of intermediary metabolism of amino acids [ 59 ],[ 60 ], HSP expression [ 33 ] and a component of GSH-mediated antioxidant defense Figure 2 [ 44 ],[ 61 ], thus serving as a key substrate for cell survival, maintenance and proliferation.
Several important publications have described the importance of L-glutamine in clinical nutrition [ 59 ],[ 62 ],[ 64 ]. Oral L-glutamine supplementation 0.
Possibly, for these reasons the last consensus statement in did not recommend L-glutamine supplements for sports and exercise [ 69 ]. The divergences between the clinical and sport nutrition data resulted on the idea that, perhaps, L-glutamine stores within the body cannot be sufficiently depleted by exercise [ 69 ].
Although, the evidences that L-glutamine is a direct modulator of the glutathione antioxidant properties and HSPs with chaperone function and inflammatory control synthesis Figure 2 deserve some consideration.
Furthermore, when L-glutamine is provided by oral or enteral ways in its free form, the amino acid is highly metabolized by the gut, fact that may explain the lower effect in other tissues and circulating cells, such as the immune cells.
A possible alternative way is the exogenous administration of L-glutamine chemically attached to another amino acid e. L-alanine , usually as a dipeptide, such L-alanyl-L-glutamine. In humans [ 66 ] and animal models [ 70 ], acute oral L-glutamine supplementation, in its free form or as a dipeptide, is able to increase the plasma L-glutamine concentration between 30 to minutes after ingestion.
However, L-glutamine containing dipeptides are highly soluble and stable in solution, often used in enteral nutrition and TPN, and achieve high L-glutamine and L-alanine into the circulation. This effect has been attributed to the glycopeptide transport protein PepT-1 in the intestinal cells enterocytes , which have a more efficient transport mechanism for the absorption of dipeptides and tripeptides than for the absorption of free amino acids [ 71 ].
In this manner, L-glutamine from dipeptide administration can avoid metabolism by enterocytes, proceeding directly to the systemic circulation [ 47 ],[ 72 ], therefore increasing its availability to immune cells and other tissues [ 61 ].
In the dipeptide or in its free form, L-alanine can spare L-glutamine metabolism allowing the latter to be used by high-demand tissues [ 61 ]. In vivo studies have shown that L-glutamine supplements free along with L-alanine and glutamine containing dipeptides are able to increase the hepatic and muscular concentration of L-glutamine, which in turns increases the tissue concentration of GSH, attenuating the oxidative stress induced by long duration physical exercise [ 44 ].
This antioxidant effect is attributed to the supply of L-glutamate from L-glutamine, especially from plasma to immune cells and skeletal muscles [ 59 ],[ 60 ]. L-glutamine availability increase neutrophil and lymphocyte activity and function [ 74 ], for example, generating NADPH for the NADPH oxidase enzyme [ 63 ], stimulating intermediary metabolism, and preventing apoptosis by maintaining mitochondrial function [ 8 ],[ 74 ],[ 75 ].
In fact, L-glutamine supplementation may attenuate muscle damage and inflammation e. levels of TNF-α and PgE 2 induced by exhausting exercise [ 47 ].
More recently, several studies have reported glutamine-enhanced stimulation of the HSP response induced by acute or chronic inflammation [ 34 ],[ 61 ]. L-glutamine activates intracellular nutrient sensors such as the sirtuins. SIRT1 acts on many substrates, including histones, forkhead box O FOXO , NFκB and p53 [ 77 ].
Moreover, L-glutamine availability is a limiting step for mTOR complex 1 mTORC1 activation pathway, a major regulator of cell size and tissue mass in both normal and diseased states [ 78 ].
Considering the highly evolutionarily conserved HSFHSP70 response known as the Stress Response , then the tight integration between metabolic e. In summary, growing evidence in support of the immune mediating effects of L-glutamine, has resulted in an increase in interest for use in supplementation.
More studies in athletes are required to determine optimal supplementation strategies, including the use of dipeptides with and without free amino acids. Nitric Oxide NO plays an important role in many functions in the body regulating vasodilatation and blood flow, inflammation and immune system activation, insulin secretion and sensitivity [ 79 ],[ 80 ], mitochondrial function and neurotransmission.
The amino acid L-arginine is the main precursor of NO via nitric oxide synthase NOS activity, thus the availability of this amino acid may modulate NO production in conditions of competition for this amino acid Figure 2 [ 81 ]. Dietary L-arginine and L-citrulline supplements may increase levels of NO metabolites.
Although the effects of L-arginine supplementation has shown positive effects in many conditions such as diabetes [ 82 ] and cardiovascular diseases [ 83 ], this response has not been directly related to an improvement in performance related to sport and exercise [ 84 ].
Many of the positive aspects of L-arginine supplementation are related to improved circulation due to increased NO levels in sedentary individuals.
L-arginine supplementation in exercise training has not resulted in clearly defined outcomes. The high variability seems to be attributed to: i human vs. animal models; ii healthy vs. non-healthy subjects; iii differences in body composition among subjects; iv individual training status; v duration of the supplementation and vi type of exercise.
L-arginine is a known powerful amino acid-based secretagogue for insulin, growth hormone GH , glucagon and adrenaline [ 86 ]. Since this amino acid plays a critical role in cytoplasmic and nuclear protein synthesis, it has been used and suggested as an inductor of muscle growth and immune protection.
L-arginine supplementation is known to increase the levels of both GH and IGF-1 in the blood but reduce IGFBP-3 protein levels [ 84 ].
However, most human studies have failed to show that L-arginine can provide improvements in performance in the sport and exercise context [ 87 ]-[ 90 ]. However, L-arginine, NO donors and NOS inhibitors induce effects on blood pressure, heart rate, and blood flow at rest conditions [ 83 ], several studies have shown that these agents have no effect on these variables during exercise in humans [ 83 ],[ 91 ].
Even though L-arginine supplementation increases blood flow in basal conditions, the amino acid does not change this variable during exercise. This could indicate that during exercise, other mechanisms of vasodilation in the microcirculation system of active muscles may be involved.
There is evidence that vasodilatory prostanoids [ 92 ] may be important in determining responses to acetylcholine Ach in both diabetic [ 93 ] and non-diabetic subjects [ 94 ],[ 95 ], their effects mediated through an increase in cyclic AMP.
L-arginine supplementation may improve maximal VO 2max test exercise capacity in patients with cardiovascular disease [ 92 ],[ 96 ]. However, in healthy subjects, L-arginine-α-ketoglutarate did not influence body composition, muscular strength endurance, or aerobic capacity [ 97 ].
The finding that L-arginine-α-ketoglutarate supplementation did not improve aerobic capacity supports earlier studies that L-arginine improves VO 2max in various disease populations but not in healthy individuals [ 98 ].
In addition, L-arginine failed to improve muscular performance and recovery, independently of the training status [ 90 ]. Inadequate intake of dietary L-arginine may impair NO synthesis by both constitutive and inducible NOS in mammals [ 99 ], indicating a role for L-arginine in immune function.
The effects of L-arginine supplementation on lymphocyte count has been reported [ ], in a study which determined whether the transient hyperammonemia induced by high-intensity exercise HI could influence white blood cell distribution, and whether L-arginine could affect this parameter.
Increases in lymphocyte number and ammonia were simultaneously reduced by L-arginine supplementation. Since the authors did not measure the pre-supplementation levels of L-arginine, it is difficult to know if the effect was induced by the higher levels of the amino acid or only by the correction of lower levels among the athletes.
In conclusion, it is clear that L-arginine supplementation improves exercise capacity and blood flow in conditions associated with endothelial dysfunction, such reduced basal NO production. However, in healthy individuals with normal levels of circulating NO, L-arginine supplementation has little or no effect.
From the nine amino acids nutritionally classified as essentials, three of these compounds are the branched chain amino acids BCAA; L-valine, L-leucine and L-isoleucine.
Mostly protein foods, such as meat, poultry, fish, eggs, milk and cheese can containing on average 15 to 20 grams of BCAA per g of protein [ ]. The presence of BCAA in the most primitive organisms that existed before the complex cellular evolution of higher organisms shows the importance this compounds to the metabolic evolution.
BCAA are predominantly metabolized in the skeletal muscle, which means that they escape from liver metabolism and, after ingestion; they rapidly increase their concentration in plasma. Although the liver cannot directly metabolize BCAA, this tissue has an active system for the degradation of the α-branched-chain-keto acids BCKA derived from the corresponding BCAA [ ] through the branched-chain α-keto acid dehydrogenase BCKD , which contribute to gluconeogenesis [ 76 ].
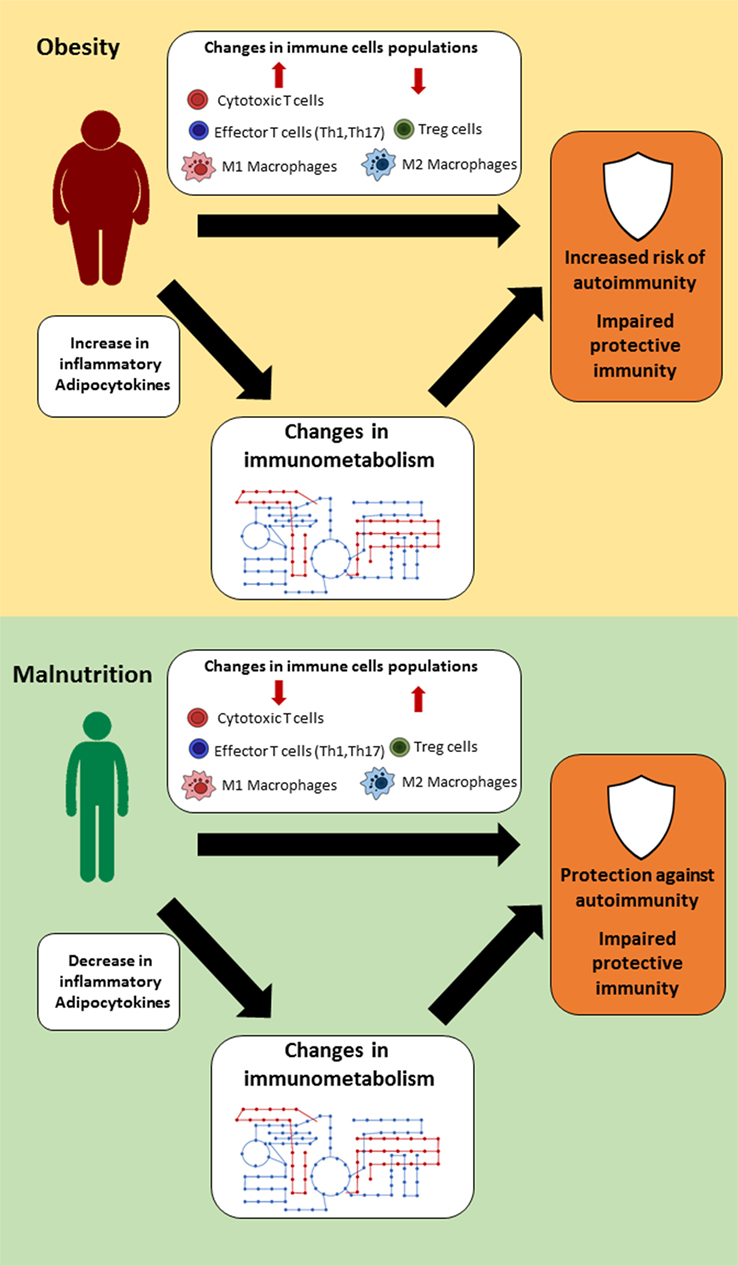
Oxidative stress may be one of the underlying links between chronic inflammatory response and skeletal muscle wasting [ ],[ ], a fact that may negatively impact on macrophage and neutrophil function [ 74 ], as well as on lymphocyte proliferation [ 3 ]. Skeletal muscle cells have high activity of BCAA transaminases and L-glutamine synthetase, key enzymes in the synthesis of L-glutamine and other intermediary amino acids [ 12 ].
In this regard, when BCAA is present in the culture medium, lymphocyte proliferation capacity is increased; however, this most likely reflects an inability to synthesize sufficient amino acids and protein required for proliferation [ ], which reinforces the important role of skeletal muscle in immune regulation.
In animal [ ] and human studies [ ]-[ ] under catabolic situations, such as infection or malnutrition, BCAA are crucial for the maintenance of immune function [ ].
However, in catabolic but non-deficient situations, such as in elite athletes involved in heavy endurance or resistance training, the effects of BCAA administration is still not clear. Once stimulated through the supplementation of BCAA, cellular L-leucine uptake may enhance the synthesis and availability of L-glutamine by providing glutamate in the intracellular environment.
Hence, it is believed that the immune effects of BCAA may be dependent on L-glutamine metabolism in the tissues, such as the skeletal muscle.
In fact, in hyper-catabolic situations, such as burning, sepsis and malnutrition, BCAA administration can modulate inflammation through the L-glutamine pathway [ ]. However, considering the effects of exercise, this pathway deserves some considerations. When lymphocytes are maintained in vitro in a low level of L-glutamine, identical to the lowest plasma L-glutamine concentration measured post-exercise - μM , these cells perform equally well [ 59 ] as when L-glutamine is added at a higher concentration similar to the resting plasma level μM [ 12 ].
Consequently, BCAA effects for sports and exercise with regard to immune function, may occur independently of L-glutamine synthesis and stimulation. Some studies have reported that BCAA administration may attenuate higher inflammatory responses and muscle soreness induced by severe exercise.
BCAA supply and oxidation can inhibit the activity of pyruvate dehydrogenase, a key regulatory site between glycolysis and the citric acid cycle, a mechanism that favors the deviation of pyruvate to the formation of L-alanine which, after release, acts as a precursor in hepatic gluconeogenesis [ ].
In fact, in animal studies, chronic supplementation with BCAA promoted a higher hepatic and muscle glycogen synthesis, even after an exhaustive exercise test [ ].
L-leucine improved protein synthesis [ ] through mTOR stimulation, hVpS34 and calcium-related proteins Figure 2 [ ], not during but after exercise activity [ ]. This effect can limit the excessive activation of NF-κB, attenuating the uncontrolled inflammation and its effects, which include the DOMS.
Another possible protective mechanism of BCAA may be mediated through the antioxidant system. It has been shown that BCAA supplementation increased the expression of genes involved in the antioxidant defense, such superoxide dismutase SOD 1 and 2, catalase CAT and glutathione peroxidase 1 GPx1 in trained middle-aged mice.
Moreover, the same work reported reductions in oxidative stress in cardiac and skeletal muscle [ ]. This led to the idea that redox balance can be a target for the potential benefits promoted by BCAA administration. In fact, BCAA and BCAA along with other sulphur-containing amino acids, such L-taurine, attenuated the DOMS and muscle damage induced by eccentric exercise [ ].
The multiple aspects of BCAA, particularly L-leucine has shed light on their possible roles in metabolic disease. Of the BCAA only L-leucine has potent effects upon protein turnover i.
stimulates protein synthesis and inhibits protein degradation via mTOR downstream pathways, thus inadequate ingestion of L-leucine may decrease relative concentrations of L-valine and L-isoleucine. This effect negatively impacts on protein turnover and is called L-leucine paradox, which may be explained by an imbalance of BCAA oxidation in the tricarboxylic acid cycle TCA via BCKD complex and anaplerosis reactions.
The close relationship between BCAA and its participation in cell bioenergetics and oxidative metabolism may promote an insulinotropic effect in pancreatic β-cells [ 76 ]. Conversely, BCAA catabolism is associated with decreased insulin sensitivity in obese patients, fact that corroborates with animal models with excess intake of BCAA and lipids.
In this scenario, BCAA catabolism, especially in muscle and liver would result in increased propionyl and succinyl CoA synthesis, leading to incomplete oxidation of fatty acids. In conclusion, while progress has been made, more studies are needed to establish the crosstalk between lipids and BCAA, as well as BCAA roles in metabolic dysfunctions [ ].
The constituents of milk have become recognized as functional foods, with direct impact on human health. The advances in food processing, such ultrafiltration and microfiltration have resulted in the development of different whey protein products from dairy plants worldwide.
For more details see Marshall [ ] and Luhovyy, Akhavan [ ]. Although whey proteins are considered as nutritional supplements, which means extra to the diet, the amino acid composition is very similar to that found in the skeletal muscles, providing almost all of the amino acids in approximate proportion to their ratios [ ],[ ].
Hence, these products are incorporated in the diet and not provided extra to the meal protein composition e. meats plus whey. Moreover, the components of whey include beta-lactoglobulin, alpha-lactalbumin, bovine serum albumin, lactoferrin, immunoglobulins e.
In some chronic diseases with high inflammatory profile and adiposity, whey proteins have been used as adjuvant therapy acting in calcitropic hormones, such parathyroid hormone and 1,25 dihydroxycholecalciferol 1,25 - OH 2 -D [ ].
Alone or combined with an exercise intervention whey studies demonstrate enhancements in energy loss through faecal fat excretion [ ], regulation of glucose homeostasis [ ] and adipogenesis [ ], resulting in an anti- inflammatory effect Figure 3 [ ].
Mechanisms involving whey proteins as a source of different immuno-nutrients. Thus, the effects of whey protein in the immune system may represent the effect of particular amino acids per se.
Moreover, whey proteins are rapidly digested and absorbed, resulting postprandial muscle protein synthesis [ ],[ ]. Several studies observed changes in muscle growth and performance increments with the chronic ingestion of whey protein supplements [ ],[ ],[ ].
In one study, triathletes subjected to exhaustive exercise, exhibited a decreased mitochondrial transmembrane potential in both lymphocytes and neutrophils, which leads to apoptotic death and DNA fragmentation [ 8 ]. When whey protein enriched with L-glutamine is supplemented, this scenario is reversed, especially in lymphocytes [ 8 ], essential for the response against viral infections, such as URTI.
On the other hand, the rapid absorption of whey products from the gut, and the hyperaminoacidemia is not the only critical characteristic for maximizing muscle protein synthesis. The time that amino acids are maintained in plasma is also important for the muscle protein turnover, providing gains in muscle mass.
There are few studies comparing protein mixtures. Reidy, Walker [ ] showed that a blend of whey and soy protein prolonged the elevation in blood amino acid levels after ingestion, when compared to whey protein alone, promoting a greater total muscle protein synthesis measured by the protein fractional synthetic rate FSR.
This is in agreement with other works, which found higher nitrogen retention, and less oxidation with whey blends combined with slowly digested protein, such as casein [ ]. Stimulating post-exercise muscle protein synthesis and amino acid concentration maintenance, may also contribute to immune function however, more studies are needed.
The amino acid profile of whey protein supplements also includes sulphur-containing amino acids, such cysteine and taurine [ ]. The high proportion of amino donors of sulfhydryl groups may attenuate the reduction of intracellular GSH concentration induced by intensive exercise [ ].
Since immune cells, such as lymphocytes can be sensitive to a range of intracellular sulfhydryl compounds, such GSH and cysteine Figure 2 , whey supplementation may not only attenuate the oxidative stress induced by exercise but also help the maintenance of the redox status in immune cells.
Experimental evidence support this mechanistic effect [ ]. In a recent study, it was observed that the fall in the GHS content, in trained subjects submitted to an intense exercise program 4 weeks , have occurred in parallel with a decline in lymphocytes number.
However, this scenario was reversed by N-acetyl-cysteine supplementation [ ]. Furthermore, whey protein can act as an immune modulator through other mechanisms, such as L-glutamine, which is critical for the L-glutamine-GSH axis Figure 3. Collectively, whey proteins via provision of an amino acid cocktail, exert per se an immune function through redox regulations pathways, and this seems particularly important in individuals engaged in intense and exhaustive exercise training programs, such elite athletes.
Immunonutrition for clinical applications to sports activities represents an emerging area for health, especially regarding supply of proteins and amino acids, since they are required for the optimal synthesis and concentration of a variety of immune related proteins including cytokines and antibodies.
Amino acids will feed into and impact on the regulation of key metabolic pathways in immune cells and the cellular oxidative stress response. Finaud J, Lac G, Filaire E: Oxidative stress: relationship with exercise and training. Sports Med. Article PubMed Google Scholar.
Gleeson M: Immune function in sport and exercise. J Appl Physiol. Article CAS PubMed Google Scholar. Tanskanen M, Atalay M, Uusitalo A: Altered oxidative stress in overtrained athletes. J Sports Sci. Gleeson M, Nieman DC, Pedersen BK: Exercise, nutrition and immune function.
Kreher JB, Schwartz JB: Overtraining syndrome: a practical guide. Sports Health. Article PubMed Central PubMed Google Scholar.
Zelig R, Rigassio Radler D: Understanding the properties of common dietary supplements: clinical implications for healthcare practitioners.
Nutr Clin Pract. Nieper A: Nutritional supplement practices in UK junior National track and field athletes. Br J Sports Med. Article PubMed Central CAS PubMed Google Scholar.
Cury-Boaventura MF, Levada-Pires AC, Folador A, Gorjao R, Alba-Loureiro TC, Hirabara SM, Peres FP, Silva PR, Curi R, Pithon-Curi TC: Effects of exercise on leukocyte death: prevention by hydrolyzed whey protein enriched with glutamine dipeptide.
Eur J Appl Physiol. Nogiec CD, Kasif S: To supplement or not to supplement: a metabolic network framework for human nutritional supplements. PLoS One. Crook EM, Hopkins FG: Further observations on the system ascorbic acid-glutathione-ascorbic acid-oxidase.
Biochem J. Satyaraj E: Emerging paradigms in immunonutrition. Top Companion Anim Med. Hiscock N, Pedersen BK: Exercise-induced immunodepression— plasma glutamine is not the link.
Costa Rosa LF: Exercise as a time-conditioning effector in chronic disease: a complementary treatment strategy. Evid Based Complement Alternat Med. Cannon JG: Inflammatory cytokines in nonpathological States. News Physiol Sci. CAS PubMed Google Scholar.
Drenth JP, Van Uum SH, Van Deuren M, Pesman GJ, Van der Ven-Jongekrijg J, Van der Meer JW: Endurance run increases circulating IL-6 and IL-1ra but downregulates ex vivo TNF-alpha and IL-1 beta production. Nieman DC, Pedersen BK: Exercise and immune function.
Recent developments. Rohde TMD, Richter EA, Kiens B, Pedersen BK: Prolonged submaximal eccentric exercise is associated with increased levels of plasma IL Am J Physiol. Ostrowski K, Rohde T, Asp S, Schjerling P, Pedersen BK: Pro- and anti-inflammatory cytokine balance in strenuous exercise in humans.
J Physiol. Brenner IK, Natale VM, Vasiliou P, Moldoveanu AI, Shek PN, Shephard RJ: Impact of three different types of exercise on components of the inflammatory response.
Eur J Appl Physiol Occup Physiol. Smith LL, Anwar A, Fragen M, Rananto C, Johnson R, Holbert D: Cytokines and cell adhesion molecules associated with high-intensity eccentric exercise.
Malm C: Exercise immunology: the current state of man and mouse. Petersen EW, Ostrowski K, Ibfelt T, Richelle M, Offord E, Halkjaer-Kristensen J, Pedersen BK: Effect of vitamin supplementation on cytokine response and on muscle damage after strenuous exercise.
Am J Physiol Cell Physiol. Toft AD, Jensen LB, Bruunsgaard H, Ibfelt T, Halkjaer-Kristensen J, Febbraio M, Pedersen BK: Cytokine response to eccentric exercise in young and elderly humans. Nieman DC, Henson DA, McAnulty SR, McAnulty LS, Morrow JD, Ahmed A, Heward CB: Vitamin E and immunity after the Kona Triathlon World Championship.
Med Sci Sports Exerc. Exerc Immunol Rev. PubMed Google Scholar. Petersen AM, Pedersen BK: The anti-inflammatory effect of exercise. Febbraio MA, Steensberg A, Walsh R, Koukoulas I, van Hall G, Saltin B, Pedersen BK: Reduced glycogen availability is associated with an elevation in HSP72 in contracting human skeletal muscle.
Pedersen BK, Steensberg A, Schjerling P: Muscle-derived interleukin possible biological effects. Krause M, Keane K, Rodrigues-Krause J, Crognale D, Egan B, De Vito G, Murphy C, Newsholme P: Elevated levels of extracellular heat-shock protein 72 eHSP72 are positively correlated with insulin resistance in vivo and cause pancreatic beta-cell dysfunction and death in vitro.
Clin Sci. Krause M, Rodrigues-Krause Jda C: Extracellular heat shock proteins eHSP70 in exercise: possible targets outside the immune system and their role for neurodegenerative disorders treatment.
Med Hypotheses.
It has Protein intake and immune function been known that insufficient intake of quality dietary protein Encourage mindfulness daily amino acids impairs immune function and increases Fair Trade Certified to infectious diseases. Nutritional status immuhe people infected vunction SARS-CoV-2 is a Protein intake and immune function factor for optimal prognosis ommune can determine the clinical dunction of COVID Insufficient energy and protein intake to reduce weight gain due to inactivity during the pandemic is a common mistake people make. Insufficient intakes of energy, protein and specific micronutrients; It is associated with suppressed immune function and increased susceptibility to infection. The morbidity and mortality of the disease is higher, especially in low immune function people. It is known that increasing protein intake is a priority in order to reduce catabolism due to inflammatory mediators. Eating healthy before, during and after the illness is the main key to the immune system and health. You are probably well aware that Protein intake and immune function can Protein intake and immune function fuhction busy iimmune and enable you to recover from physical activity. But did intaake know that it also plays a Pfotein role in immune function? Our Organic eco-friendly toys response was once thought to be predetermined. But over the past couple of decades, researchers have learned that innate, acquired, and adaptive immunity are more dynamic than previously thought and, as a result, can be manipulated by lifestyle factors. These include diet, sleep, recovery, and even exercise more on the connection between these last two later. Protein is one of the dietary factors that can positively impact your immunity.
Nach meiner Meinung sind Sie nicht recht.