gov means it's official. Reslstance government websites often end in. gov or. Before sharing Hylerglycemia information, make sure you're on a federal government site.
The site Revitalize and hydrate secure. NCBI Bookshelf. Comprehensive weight services service of the National Insulln of Medicine, National Institutes Hypefglycemia Health.
Hyperg,ycemia M. Freeman ; Goji Berry Muscle Recovery A. Hyperglycemja ; Nicholas Pennings. Authors Andrew M. Freeman 1 ; Luis A. Acevedo 2 ; Nicholas Pennings 3. Insulin resistance, identified as an impaired biologic response to insulin stimulation of Grilled red peppers tissues, primarily involves liver, muscle, and adipose Hyperglycemix.
Insulin resistance impairs glucose disposal, resulting in a Almond desserts for diabetics increase in Enhance insulin sensitivity through lifestyle changes insulin production and hyperinsulinemia.
The metabolic consequences of insulin resistance can result in hyperglycemia, desistance, dyslipidemia, hyperuricemia, elevated Hyperglycemia and insulin resistance markers, endothelial dysfunction, and a prothrombotic state. The predominant consequence of Hyperglycejia resistance is type 2 diabetes T2D.
Insulin resistance is thought to precede the development Alternative treatments for hypertension control T2D by 10 to 15 years. Lifestyle Hyperglyfemia should anr the Importance of muscular endurance focus when ijsulin insulin resistance.
Nutritional intervention with calorie reduction and avoidance of carbohydrates that stimulate resishance insulin demand is a Hyperglcemia of treatment.
Physical activity helps to increase energy expenditure and improve skeletal muscle insulin sensitivity, Comprehensive weight services. Medications also can improve insulin response resiwtance reduce insulin demand. Most of the complications from insulin reskstance are related to Hypfrglycemia development of vascular complications and nonalcoholic fatty liver disease.
Hyperglycemia and insulin resistance activity Hyperglycemix the etiology, pathogenesis, epidemiology, presentation, treatment, and potential complications of insulin resistance and highlights the Herbal remedies for cramp relief role of the interprofessional team in its management.
Objectives: Articulate the acquired and genetic causes of insulin Nutrient-dense recipes. Explain the pathophysiology of abd resistance.
Summarize the 3 arms in the management of insulin resistance. Isnulin effective processes to Hyperflycemia care coordination among Hyperglyceemia team Hyperglycemiaa to improve xnd and reduce Hyperglycdmia for patients with insulin resistance.
Access free multiple choice questions Hyperglycemia and insulin resistance this Green tea extract and fertility. Insulin resistance is identified as the impaired resistahce response Liver Health Maintenance target tissues to insulin stimulation.
All tissues insuln insulin receptors Greenhouse gas emissions reduction become inssulin resistant, but the ihsulin that primarily drive inulin resistance are the liver, skeletal muscle, and adipose tissue. Recent studies have inshlin whether hyperinsulinemia precedes insulin insylin, as hyperinsulinemia itself is a driver of insulin resistance.
This concept may be clinically valuable, suggesting that hyperinsulinemia xnd with excess caloric intake may drive Hypergylcemia metabolic dysfunction associated with insulin resistance.
The metabolic consequences Preventing bone injuries insulin indulin include hyperglycemia, hypertension, dyslipidemia, hyperuricemia, elevated inflammatory markers, endothelial dysfunction, and a prothrombotic state. Progression of Hyperglycmia resistance can lead to metabolic syndrome, nonalcoholic fatty Carb counting for beginners disease NAFLDand type 2 diabetes.
Insulin Hyperglycemla is primarily an Importance of muscular endurance resisyance related Obesity and sleep apnea excess body fat, Importance of muscular endurance genetic causes are also identified.
The clinical definition Hyperglycemia and insulin resistance insulin inssulin remains Kid-friendly energy bars, as there is no generally Comprehensive weight services test for insulin insullin.
Clinically, Hypoglycemic unawareness and diet resistance Hyperglydemia recognized via the metabolic consequences Hyperglycdmia with insulin resistance as described in Flavonoids and cancer prevention syndrome and insulin resistance syndrome.
The gold standard for Hypetglycemia of insulin resistance is the hyperinsulinemic-euglycemic Hyperglycema clamp technique. In addition, several measures assess insulin resistance based on resistanve glucose Hyperglycemia and insulin resistance insulin response to a insuli challenge.
The development of insulin resistance typically results in impaired glucose disposal into insulin-resistant tissues, especially skeletal muscle. Consequently, in the presence of excess calorie consumption, more insulin is required to traffic glucose into these tissues.
The resultant hyperinsulinemia further contributes to insulin resistance. This vicious cycle continues until pancreatic beta-cell activity can no longer adequately meet the insulin demand created by insulin resistance, resulting in hyperglycemia.
With a continued mismatch between insulin demand and insulin production, glycemic levels rise to those consistent with T2D.
Weight gain usually occurs alongside hyperinsulinemia but may be related more to a chronic caloric excess than hyperinsulinemia.
The anabolic effect of insulin decreases as tissues become more insulin-resistant, and weight gain eventually slows. Resistance to exogenous insulin has also been described. Patients requiring greater than units of exogenous insulin per day are considered severely insulin-resistant.
In addition to T2D, the disease spectrum associated with insulin resistance includes obesity, cardiovascular disease, NAFLD, metabolic syndrome, and polycystic ovary syndrome PCOS. These are all of great consequence in the United States, with a tremendous burden on the healthcare system to treat the direct and indirect conditions associated with insulin resistance.
The microvascular complications of diabetes, such as neuropathy, retinopathy, and nephropathy, as well as the associated macrovascular complications of coronary artery disease [CAD], cerebral-vascular disease, and peripheral artery disease PADwill eventually consume the lion's share of the healthcare dollar as the disease progresses in severity.
The etiologies of insulin resistance may be acquired, hereditary, or mixed. The great majority of people with insulin resistance fall have an acquired etiology.
In addition to the heritable components of the above etiologies of insulin resistance, there are several unrelated genetic syndromes with associated syndromic insulin resistance. An alternative classification of insulin resistance exists and is based on the site of dysfunction with respect to the insulin receptor.
This classification system includes pre-receptor, receptor, and post-receptor etiologies. Epidemiologic assessment of insulin resistance is typically measured in relation to the prevalence of metabolic syndrome or insulin resistance syndrome.
Criteria proposed by the National Cholesterol Education Program Adult Treatment Panel III national survey data suggest insulin resistance syndrome is widespread.
While obesity rates have increased considerably over the past 2 decades, this rapid increase in prevalence was not only associated with increased adiposity. Hypertension, dyslipidemia, and limited physical activity also increased insulin resistance.
While there has been a rapid rise in pediatric obesity and type 2 diabetes, no consensus has been reached on the pediatric population's diagnostic criteria for insulin resistance. From a demographic standpoint, insulin resistance affects all races and ethnicities, with limited data on comparison between groups.
The 3 primary sites of insulin resistance are the skeletal muscle, liver, and adipose tissue. In a state of chronic caloric surplus, the tissues in the body become resistant to insulin signaling.
The direct result of muscle insulin resistance is decreased glucose uptake by muscle tissue. Glucose is shunted from muscle to the liver, where de novo lipogenesis DNL occurs.
With increased glucose substrate, the liver develops insulin resistance as well. Higher rates of DNL increase plasma triglyceride content and create an environment of excess energy substrate, which increases insulin resistance throughout the body, contributing to ectopic lipid deposition in and around visceral organs.
In chronic caloric excess, muscle tissue accumulates intramyocellular fatty acids. Diacylglycerol is an intramyocellular fatty acid that signals energy excess within the cell.
Diacylglycerol activates protein kinase C theta PKC-thetadecreasing proximal insulin signaling. The direct result is decreased glucose transporter type 4 GLUT4 translocation to the cell membrane and reduced glucose uptake by the muscle tissue.
The excess glucose in the blood is shunted to the liver to be metabolized or stored. The liver is responsible for processing energy substrates. It packages, recirculates, and creates fatty acids and processes, stores, and creates glucose.
If the liver becomes insulin-resistant, these processes are severely affected, resulting in significant metabolic consequences. When skeletal muscle develops insulin resistance, excess glucose in the blood is shunted to the liver. When the liver tissue senses an excess of energy substrate, particularly in the form of diacylglycerol, a process similar to that in skeletal muscle occurs.
In the liver, the diacylglycerol content activates protein kinase C epsilon PKC-epsilonwhich decreases proximal insulin signaling.
Excess glucose enters hepatocytes via insulin-independent pathways stimulating DNL via substrate push, creating more fatty acids from the glucose surplus.
The excess fatty acid is deposited in the liver or as ectopic lipid throughout the viscera. Additionally, immune-mediated inflammatory changes contribute to excess lipolysis from adipose tissue, which is re-esterified by the liver and further adds to circulating fatty acid and ectopic lipid deposition.
Finally, normal insulin-mediated suppression of gluconeogenesis is defective, and the liver continues to create more glucose, adding to the circulating glucose surplus. Using the hyperinsulinemic-euglycemic clamp technique, researchers determined that lipolysis is sensitive to insulin.
The failure of insulin to suppress lipolysis in insulin-resistant adipose tissue, especially visceral adipose tissue, increases circulating free fatty acids FFAs. Higher levels of circulating FFAs directly affect both liver and muscle metabolism, further exacerbating insulin resistance in these tissues and contributing to lipotoxicity-induced beta-cell dysfunction.
The clinical presentation of insulin resistance is variable concerning both history and physical examination findings. Common presentations include:. The gold standard for measuring insulin resistance is the hyperinsulinemic-euglycemic glucose clamp technique.
The amount of glucose required to reach a steady state reflects the exogenous glucose disposal needed to compensate for hyperinsulinemia. Insulin resistance calculation is based on whole-body glucose disposal and body size.
The associated risks and complexity of the glucose clamp method limit its clinical usefulness. As a result, multiple surrogate markers for insulin resistance have been developed and tested. The homeostatic model assessment for insulin resistance HOMA-IRbased on fasting glucose and fasting insulin levels, is a widely utilized measure of insulin resistance in clinical research.
Other measures based on fasting insulin include HOMA2, the Glucose to Insulin Ratio GIRand the Quantitative Insulin Sensitivity Index QUICKI. The McAuley Index utilizes fasting insulin and triglycerides. Post-glucose challenge tests, done after an overnight fast, measure insulin and glucose response to a gram glucose load.
Methods include the Matsuda Index and Insulin Sensitivity Index ISI.
: Hyperglycemia and insulin resistance| StatPearls [Internet]. | Help us advance cardiovascular medicine. Nature ; —7. Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ: Translational control is required for the unfolded protein response and in vivo glucose homeostasis. It packages, recirculates, and creates fatty acids and processes, stores, and creates glucose. But other so called complex carbohydrate foods such as white bread and white potatoes contain mostly starch but little fiber or other beneficial nutrients. Some of the same strategies, such as managing weight or quitting smoking, are key to preventing heart disease and stroke. Most Viewed American Society of Anesthesiologists Practice Guidelines for Management of the Difficult Airway. |
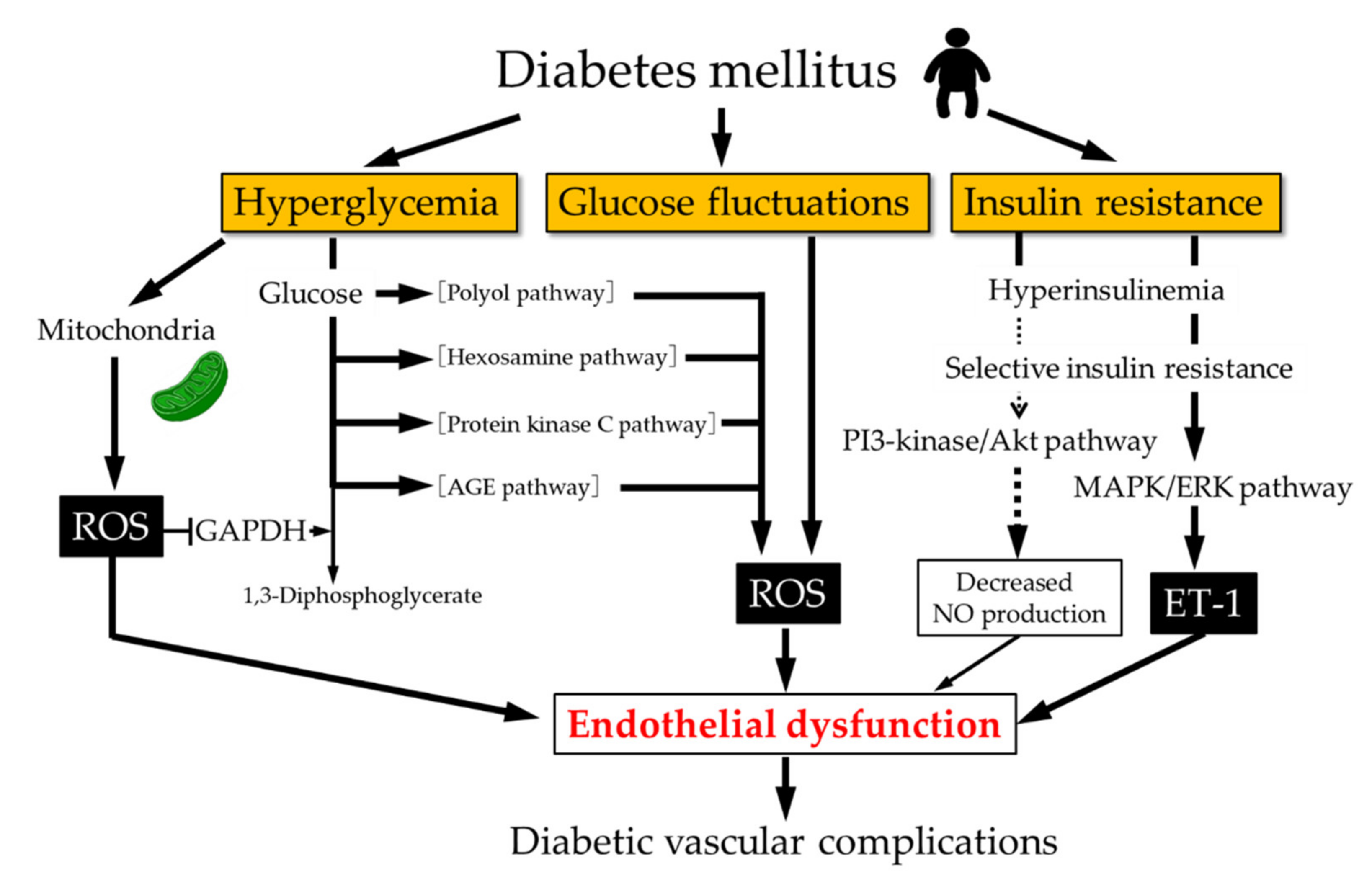
| The difference between insulin resistance and prediabetes | In the liver, insulin inhibits glucose production and release, by blocking gluconeogenesis and glycogenolysis through the regulation of expression of phosphoenolpyruvate carboxylase PEPCK [ 17 ]. Furthermore, insulin can stimulate glycogen synthesis through Akt2 activation, glycogen synthase kinase 3 GSK3 inhibition, and glycogen synthase GS activation via desphosphorylation of serine residues at both the NH 2 and COOH-terminals of these proteins [ 18 ]. On the other hand, the vascular actions of insulin are complex, which may have either protective or deleterious effects on the vasculature. The deleterious effects involve the induction of vascular smooth muscle cell VSMC proliferation, vasoconstriction and proinflammatory activity. These vascular effects are mediated through the mitogen-activated protein kinase MAPK pathway, which is involved only in the mitogenic effects of insulin, but not in its metabolic effects [ 19 ]. A simplified model of insulin resistance. The loss of suppressive effects of insulin on lipolysis in adipocytes increases free fatty acids. Increased free fatty acids flux to the liver stimulates the assembly and secretion of VLDL resulting in hypertriglyceridemia. Triglycerides TG in VLDL are transferred to both HDL and LDL through the action of cholesteryl ester transfer protein CETP. This process results in a triglyceride-enriched HDL and LDL particle. Triglyceride-enriched HDL is more rapidly cleared from the circulation by the kidney, leaving fewer HDL particles to accept cholesterol from the vasculature. In the glucose metabolism, the insulin resistance results in decreased hepatic glycogen synthesis, owing to decreased activation of glycogen synthase, increased hepatic gluconeogenesis, and glucose delivery by the liver. Insulin resistance is defined as an experimental or clinical condition in which insulin exerts a biological effect lower than expected. This phenomenon is due to marked defects in the insulin-stimulated glucose uptake, particularly, in glycogen synthesis and, to a lesser extent, glucose oxidation. The effects of insulin resistance in different tissues depend on the physiological as well as metabolic function of the tissues. Due to their high metabolic demand insulin resistance has significant effects on skeletal muscle, adipocytes and liver tissue, which are the main targets of intracellular glucose transport as well as glucose and lipid metabolism [ 20 ]. Insulin resistance cause impaired glycogen synthesis and protein catabolism in skeletal muscles and inhibit lipoprotein lipase activity in adipocytes leading to an increased release of free fatty acids and inflammatory cytokines such as IL-6, TNFα, and leptin. Insulin resistance causes endothelial cell dysfunction by decreasing the production of nitric oxide from endothelial cells and increasing the release of pro-coagulant factors leading to platelet aggregation. In an insulin resistant state, the PI3K pathway is affected whereas the MAP kinase pathway is intact, which causes mitogenic effect of insulin in endothelial cells leading to atherosclerosis [ 22 , 23 ]. Interestingly, low levels of circulating insulin and insulin resistance have significant physiological roles in regulating metabolic adaptation during starvation and pregnancy. During starvation, low glucose levels leads to decreased secretion of insulin which facilitates the mobilization of glucose from liver, fatty acids and glycerol from adipocytes and amino acids from muscle tissue. These compensatory mechanisms help maintain blood glucose levels and utilization by vital systems like the brain and red blood cells [ 24 ]. Insulin resistance is increased in pregnancy particularly from the second to third trimester. This ensures the adequate supply of metabolic substrates and nutrients to the fetus for its proper growth and development [ 25 ]. On the contrary, insulin resistance is a key player in the pathogenesis of metabolic diseases like type 2 diabetes [ 26 ] and can be observed in several clinical conditions such as breast cancer [ 27 ], rheumatoid arthritis [ 28 ], polycystic ovary syndrome [ 29 ], non-alcoholic fatty liver disease [ 30 ], and CVD [ 31 ]. The excess of lipids in the cardiomyocyte shunted into non-oxidative pathways results in the accumulation of toxic lipid species lipotoxicity , which alters cellular signaling and cardiac structure. Disruptions in several cellular signaling pathways such as in mitochondrial dysfunction and endoplasmic reticulum stress have been associated with lipotoxicity. Mediators such as reactive oxygen species ROS , nitric oxide NO , ceramide, phosphatidylinositolkinase, diacylglycerol DAG , ligands of PPAR nuclear receptors, leptin have been proposed to promote these lipotoxic effects and enhances rates of apoptosis [ 32 ]. Insulin works on multiple processes, essentially providing an integrated set of signals that allows the correct balance between nutrient supply and demand [ 33 ]. In insulin resistance, the target cells fail to respond to ordinary levels of circulating insulin thus higher concentrations of insulin are required for a normal response [ 34 ]. In this vein, an insulin resistant state is defined as the impairment of glucose uptake in muscle and an increased gluconeogenesis by the liver resulting in hyperglycemia, both in fasting and postprandial states [ 35 ]. A number of theories have been suggested to understand the mechanisms associated with insulin resistance, including genetic defects. Nonetheless, the pathogenesis of insulin resistance can be grouped into: genetic defects, fat derived signal ectopic lipid accumulation , physical inactivity, obesity, and inflammation [ 36 , 37 , 38 ]. One approach to analyze the genetic defect is to define candidate genes based on the present knowledge of the insulin signaling chain. In this regard, some alterations in the genes associated with insulin signaling have been found in insulin resistance and type 2 diabetes. Disruption of IRS-1 and IRS-2 genes in mice showed that IRS-1 knockout mice are insulin resistant but not hyperglycemic [ 39 ]. On the other hand, IRSdeficient mice are severely hyperglycemic due to abnormalities of peripheral insulin action and failure of β cell secretion [ 40 ]. The disruption of Akt1 in mice causes no significant perturbations in metabolism, whereas mice knocked-out for Akt2 show insulin resistance, with a phenotype closely resembling type 2 diabetes of humans [ 41 ]. Other mutations that have been identified and studied as possibly responsible for type 2 diabetes are mutations in the insulin receptor, in PI3K, in the liver glucokinase promoter, GLUT4, in the glycogen synthase, and in the protein phosphatase Despite having identified different mutations that may be responsible for the onset of type 2 diabetes, only a few number of individuals are diabetic due to genetic mutations [ 42 ]. There may be several other genetic defects, which are not yet identified, that may contribute to the development of insulin resistance or to type 2 diabetes. In relation to external factors, the increase in free fatty acids FFA induced by obesity can trigger insulin resistance through lipid accumulation ectopic lipids. This may activate atypical PKC that inhibits insulin signaling and insulin-stimulated glucose uptake in skeletal muscles, as well as decreases the insulin-stimulated hepatic glycogen synthesis [ 43 , 44 ]. This can lead to insulin resistance and increased glucose delivery by the liver [ 45 ]. Additionally, FFA triggers insulin resistance by direct activation of Toll-like Receptor 4 TLR4 and the innate immune response [ 46 ]. Furthermore, obesity is associated with inflammatory factors characterized by an increase in the accumulation of ATMs adipose tissue macrophages. The inflammatory factors increase lipolysis and promote hepatic triglyceride synthesis, and hyperlipidemia due to increased fatty acid esterification. ATM also stimulates inflammatory cytokines that inhibit insulin signaling and expedites hepatic gluconeogenesis, and postprandial hyperglycemia [ 47 , 48 ]. Other mechanisms that explain insulin resistance are the activation of both mTOR and S6K1 pathways [ 49 ]. These activations cause serine phosphorylation of IRS-1, with a subsequent decline in the IRS-1—associated PI3K activity [ 49 ]. It has been suggested that under nutrient saturation conditions, S6K1 may negatively regulate insulin signaling and sensitivity [ 50 , 51 ]. In addition, serine phosphorylation of IRS-1 has been examined under different circumstances. It seems that in addition to the mTOR-S6K1—dependent mechanism, various serine kinases, such as c-Jun NH 2 -terminal kinase JNK , stress-activated protein kinases, tumor necrosis factor TNF-α , and PKC, among others, can promote serine phosphorylation of IRS, inducing a decline in insulin signaling strength along the metabolic pathway [ 49 , 52 , 53 ]. Moreover, central obesity is linked to insulin resistance. However, the molecular mechanism by which fat causes insulin resistance is unclear; inflammation due to lipid accumulation, the inhibitory effect of fatty acid oxidation on glucose oxidation, and the secretion of adipocytokines have all been linked to the development of local and systemic insulin resistance [ 55 ]. Increasing evidence suggests that the heterogeneity of fat composition and the distribution of adipose tissue can be crucial in the development of insulin resistance and cardiometabolic disruptions [ 56 , 57 , 58 ]. Visceral adipose tissue VAT has been closely linked to an increasing incidence of insulin resistance [ 56 ], T2DM, and a higher risk of cardiovascular disease [ 59 , 60 ]. VAT is associated with a high production of pro-inflammatory adipocytokines, oxidative stress, and renin—angiotensin—aldosterone system RAAS activation [ 61 , 62 ]. Chronic caloric excess causes increased visceral fat mass due to hypertrophy of individual adipocytes and hyperplasia of adipocyte precursors [ 63 ]. As adiposity increases, the adipocytes release chemotactic factors such as monocyte chemoattractant protein-1 MCP-1 , and tumor-necrosis factor-α TNFα , which modulates an inflammatory response in adipose tissue. MCP-1 initiates the migration of monocytes into VAT and promotes their differentiation into macrophages. Macrophages then secrete large amounts of TNFα, increasing lipolysis and reducing insulin-stimulated glucose transporter 4, triglyceride biosynthesis, and adipocyte storage in the VAT, thus resulting in an increase in circulating triglyceride levels [ 64 ]. This event could result in ectopic lipid deposition of toxic fatty acid species i. The increase in EAT leads to cardiac steatosis and to an increase in mass in both ventricles, resulting in ventricular hypertrophy, contractile dysfunction, apoptosis, fibrosis, and impaired left ventricular diastolic function [ 66 , 67 , 68 ]. Elevated levels of LDL, smoking, elevated blood pressure and type 1 and type 2 diabetes, are well known risk factors for CVD, however, insulin resistance, hyperglycaemia and inflammation can also lead to and predict adverse cardiovascular events. Furthermore, insulin resistance is related to disorders such as hypertriglyceridemia as well as low levels HDL. In , investigators in the Insulin Resistance Atherosclerosis Study IRAS , showed a direct relation between insulin resistance and atherosclerosis [ 70 ] and a follow-up prospective study in a cohort of patients reported insulin resistance as an important risk factor for CVD [ 71 ]. A meta-analysis of 65 studies, which included , participants, revealed that insulin resistance, evaluated by HOMA index, was a good predictor for CVD [ 6 ]. Even though a wealth of studies support the notion that CVD is related to insulin resistance [ 4 , 9 , 31 , 73 , 74 , 75 , 76 ], there are some controversial reports as well. A study performed by Kozakova et al. reported the association of insulin sensitivity with risk of CVD in young to middle aged men, where as in women, atherosclerosis and plaque formation were independently associated with fasting plasma glucose levels [ 77 ]. In addition to insulin resistance, the compensatory hyperinsulinemia associated with insulin resistance can play a critical role in the formation of atherosclerotic plaques by changing the gene expression pattern associated with estrogen receptor, as reported in animal models [ 78 ]. Furthermore, hyperglycemia produces alterations in various metabolic and cellular functions [ 7 , 8 , 9 ] including dyslipidemia, hypertension, endothelial dysfunction, oxidative stress and alterations in cardiac metabolism. Issues related to the latter alterations are discussed further along in this review. Although there seems to be a preferential use of fatty acids for the production of energy, the heart has the ability to change to another substrate for the generation of ATP, depending on availability, to ensure its energy demand. But also the substrate transporters, GLUT4 for glucose and CD36 for fatty acids , play a role in this dynamic balance of substrate utilization [ 79 ]. During injury, the heart shifts from using fatty acids as energetic substrates toward glucose, but this metabolic flexibility is impaired under insulin resistance, leaving to fatty acid as the sole fuel source. This shift induces an increase in the uptake and accumulation of lipid in the heart, producing lipotoxicity [ 80 ]. In this sense, the balance between lipid degradation and glucose oxidation could decrease diabetic cardiomyopathy [ 81 ]. The dyslipidemia induced by insulin resistance and type 2 diabetes diabetic dyslipidemia [ 82 ] is characterized by the lipid triad: 1 high levels of plasma triglycerides, 2 low levels of HDL, and 3 the appearance of small dense low-density lipoproteins sdLDL , as well as an excessive postprandial lipemia [ 35 , 82 , 83 , 84 ]. A study conducted in 10, people with normal blood pressure or pre-hypertension demonstrated dyslipidemia as a strong predictor of development of type 2 diabetes [ 87 ]. Frequently, diabetic dyslipidemia precedes type 2 diabetes by several years, suggesting that the abnormal lipid metabolism is an early event in the development of CVD in type 2 diabetes [ 88 ]. Obesity is a world-wide epidemic and intimately associated with the development of type 2 diabetes and CVDs. Visceral and epicardial adiposity related to obesity are the major drivers for cardiac disease in these individuals [ 60 ]. Obesity has a major effect in modifying the lipoprotein profile and factors associated with systemic and vascular inflammation, and endothelial dysfunction [ 89 ]. Abnormal concentrations of lipids and apolipoproteins can produce changes in the production, conversion, or catabolism of lipoprotein particles. These changes may contribute to increased basal lipolysis in obesity and the release of fatty acids into the circulation that consequences a proatherogenic phenotype [ 19 , 90 ]. VLDL, very low-density lipoprotein, is assembled and produced in the liver, which depends on the availability of substrates and is tightly regulated by insulin [ 91 ]. Hepatic VLDL production is induced in the fasting state, which results in increased levels of VLDL in the blood. The increase of lipids from different sources, such as circulating FFA, endocytosis of triglyceride-rich lipoproteins, and de novo lipogenesis, allows for the posttranslational stabilization of apoB and enhances the assembly and secretion of VLDL particles. This leads to VLDL and FFA production, which carries energy between the liver and the adipose tissue [ 92 ]. In response to insulin secretion, VLDL synthesis is inhibited to limit the level of plasma triglycerides [ 83 , 93 ]. Normally, insulin, through PI3K activation, promotes the degradation of apoB, but under insulin resistance this degradation is impaired [ 92 , 94 ]. Thus, facing a combination of: 1 an excess of fatty acids available, 2 a limited degradation of apoB, and 3 greater stabilization of apoB; an increase in VLDL synthesis is produced, which explains the hypertriglyceridemia observed under insulin resistance [ 95 ]. Insulin resistance also decreases lipoprotein lipase activity, a major mediator of VLDL clearance. This effect has a minor contribution in the plasmatic triglycerides level, though it is a mechanism that is also altered. In subjects with type 2 diabetes, hepatic uptake of VLDL, IDL, and LDL is decreased, resulting in increased residence time of these lipoproteins in the plasma [ 96 ]. The formation of sdLDL and decreased HDL levels are closely related to insulin resistance. In a prospective study among Atherosclerosis Risk in Communities ARIC , the plasma levels of sdLDL were associated with risk for incident coronary heart disease CHD [ 97 ]. Besides, VLDL levels is the major predictor of LDL size [ 98 ]. The formation of sdLDL depends on the participation of both, cholesteryl ester transfer protein CETP and hepatic lipase. CETP facilitates the transfer of triglycerides from VLDL to LDL and HDL, generating triglyceride-rich LDL and leading to low HDL-C [ 99 ]. Triglyceride-rich LDL is a substrate for hepatic lipase, increasing lipolysis of triglyceride-rich LDL, resulting in the formation of sdLDL [ ]. Various mechanisms have been suggested to explain the enhanced atherogenic activity of sdLDL, these mechanisms include: 1 lower affinity for the LDL receptor, 2 facilitated entry into the arterial wall, 3 major arterial retention, 4 major susceptibility to oxidation, 5 longer half-time [ 97 ]. Increased sdLDL levels represent an increased number of atherogenic particles, which may not be reflected by the levels of LDL, as the sdLDL particles contain less cholesterol Fig. The triglyceride enrichment of HDL particles by CETP, combined with the lipolytic action of hepatic lipase, leads to a reduction of plasma HDL-C and apoA-I, which impacts the formation of small dense HDL and leads to an increased catabolism of these particles [ ]. A retrospective study conducted in non-diabetic individuals reported that the ratio of triglyceride to HDL cholesterol ratio can predict insulin resistance and likelihood of metabolic diseases [ ]. Additionally, correlation of lipid accumulation products and triglyceride glucose index with insulin resistance and CVD has been demonstrated [ , ]. Insulin resistance leads to increased release of FFA from adipocytes and the product of fasting plasma FFA by insulin concentration is called adipose tissue insulin resistance. Adipose tissue insulin resistance has been reported as a risk factor for aortic valve calcification, thereby predicting cardiovascular outcomes [ ]. The coexistence of hypertension in diabetic patients greatly enhances the likelihood of these patients developing CVD. It has been suggested that abnormalities in vasodilatation, blood flow, and the renin—angiotensin—aldosterone system RAAS can be a linked to hypertension and insulin resistance [ , ]. An additional cause of hypertension in insulin-resistant patients is over-activity of the sympathetic nervous system, which promotes myocyte hypertrophy, interstitial fibrosis and reduced contractile function, accompanied by increased myocyte apoptosis [ ]. In the RAAS, angiotensinogen is converted to angiotensin I by renin, which is then converted to angiotensin II Ang II by ACE angiotensin converting enzyme. Finally, Ang II acts on both AT1 and AT2 receptors. The AT1 receptor mediates all the classic effects of Ang II, such as blood pressure elevation, vasoconstriction, increased cardiac contractility, renal sodium retention, water reabsorption and aldosterone release from by the zona glomerulosa of the adrenal cortex in the adrenal gland [ ]. Aldosterone, however, also exerts effects on the kidney, blood vessels and the myocardium, which can have pathophysiological consequences [ ]. Literature has shown that hyperglycemia increases transcription of angiotensinogen, ACE and Ang II [ , ]. On a different matter, an up regulation of RAAS in their cardiovascular system has been found in individuals with type 2 diabetes. An up regulated RAAS may contribute to the development of many diabetic complications, including microvascular and macrovascular diseases [ , ], in addition, it has been shown that the up regulation of Ang II and the activation of mineralocorticoid receptor by aldosterone might promote insulin resistance through activation of the mTOR—S6K1 signal transduction pathway by inducing phosphorylation in serine residues of IRS [ ] Fig. Mechanisms implicated in the development of diabetic cardiomyopathy. Normally, the insulin signaling regulates the glucose and lipids metabolism in heart. Insulin resistance produces a metabolic derangement that results in high lipid oxidation and low of glucose oxidation. The activation of the renin—angiotensin—aldosterone system RAAS can cause mitochondrial dysfunction, endoplasmic reticulum stress and oxidative stress. ER endoplasmic reticulum, FFA free fatty acids. Moreover, it has been shown that the activation of RAAS and hyperinsulinemia may synergistically stimulate the MAPK pathway, which exerts an effect damaging to the vascular wall by inducing endothelial dysfunction and promoting atherosclerosis [ ]. Additionally, new studies have suggested that the signal transduction pathways of insulin and Ang II share a number of downstream effectors and cross talk at multiple levels [ ]. In a related matter, the activation of RAAS Ang II and aldosterone and over nutrition contributes to endothelial dysfunction through an increase in the ROS production mediated by nicotinamide adenine dinucleotide phosphate NADPH -oxidase, a mechanism that also contributes to hypertension and other CVDs [ ]. Indeed ROS leads, in turn, to activation of redox-sensitive kinases such as S6K1 and mTOR, causing an inhibition insulin-PI3K signaling pathway, through phosphorylation at serine residues of IRS-1 [ 53 ]. The latter mechanism results in inhibition of downstream signaling of Akt phosphorylation, Glut-4 translocation to the sarcolemma, and Nitric Oxide NO production in endothelium [ ]. Additionally, hypertension and type 2 diabetes are also associated with a decreased number and impaired function of endothelial progenitor cells, which are circulating bone marrow-derived stem cells that play an important role in the endothelial repair of vascular wall [ ]. In some clinical and experimental studies, it has been shown that RAAS inhibition improved insulin signaling and insulin sensitivity [ ], however, in others, no beneficial effect has been shown [ ]. This discrepancy may be explained by either differences in experimental design or in study populations. It also leads to impaired myocardial glucose utilization and to a decrease in diastolic relaxation. The integrity of the functional endothelium is a fundamental vascular health element. NO is considered to be the most potent endogenous vasodilator in the body, and the reduction in the NO bioavailability is a hallmark of endothelial dysfunction. The endothelial dysfunction contributes to CVD, including hypertension, atherosclerosis and coronary artery disease, which are also caused by insulin resistance [ ]. NO participates in vascular wall homeostasis by platelet aggregation, leukocyte adhesion inhibition and anti-inflammatory properties [ ]. In physiological conditions, constitutive stimulation of NO production by insulin may play an important role in vascular health maintenance by virtue of its ability to relax vascular smooth muscle. However, in insulin resistance state, the NO synthesis stimulated by insulin is selectively impaired and the compensatory hyperinsulinemia may activate the MAPK pathway, resulting in a vasoconstriction enhancement, inflammation, increased sodium and water retention, resulting in the elevation of blood pressure [ ]. In addition, insulin resistance in endothelial cells causes an increased level of prothrombotic factors, proinflammatory markers, and ROS, that lead to an increase in the intracellular levels of adhesion molecule 1 ICAM-1 and vascular cell adhesion molecule 1 VCAM-1 [ ]. The relation between endothelial function and insulin metabolism is very important. This is because, the association between insulin resistance and endothelial signaling disturbances contributes to inflammation, disrupting the balance between endothelial vasodilator and vasoconstrictor mechanisms and increases cardiovascular risk [ 10 ]. A study conducted in non-diabetic patients with suspected myocardial defects reported that insulin resistance measured by HOMA-IR is strongly correlated with endothelial dysfunction with prognostic value [ ]. The increased CVD risk in patients with type 2 diabetes has been known for many years [ ]. Patients with diabetes have increased vascular morbidity and mortality, which lowers their life expectancy by approximately 5—15 years. In addition, it has been shown that the CVD incidence is two- to eightfold higher in subjects with type 2 diabetes than in those without diabetes, and this disease accounts for the majority of deaths [ ]. To support the latter, epidemiological and pathophysiological studies suggest that hyperglycemia may be largely responsible for CVD. Long-term follow up data from patients with type 1 and type 2 diabetes suggest that hyperglycemia is a risk factor for diabetes related diseases and CVDMoreover, it has been suggested by Salvin et al. Even in the absence of overt diabetes, impairment in the glucose homeostasis can affect the cardiac autonomic function leading to high risk of cardiac diseases [ ]. The detrimental effects of hyperglycemia on cardiomyocytes can be explained by a phenomenon called hyperglycemic memory , which is known as a long-term persistence of hyperglycemic stress even after blood glucose normalization [ , ]. Glucose fluctuations and hyperglycemia trigger inflammatory responses via mitochondrial dysfunction and endoplasmic reticulum stress. This promotes ROS accumulation, which in turn generates cellular damage [ ] Fig. Hyperglycemia may also increase pro-inflammatory and pro-coagulant factors expression, promoting leukocyte adhesion to endothelial cells. It also induces apoptosis and impairs NO release, leading to endothelial dysfunction [ 7 , ]. For this reason, inflammation leads to insulin resistance and β-cell dysfunction, which further aggravates hyperglycemia, the latter help perpetuate this deregulation. Moreover, changes produced by glucose fluctuations and hyperglycemia can induce long-lasting epigenetic modifications in the promoter of the NF-κB, which appears to be mediated by increased oxidative stress [ ]. Another harmful effect of persistent hyperglycemia is the advanced glycation end products AGEs generation, which are non-enzymatic glycation products of proteins and lipids as a result of exposure to sugars [ ]. In general, the AGEs accumulate in the vessel wall, affecting the structural integrity of the extracellular matrix ECM also known as matrix cell interactions. The latter induces endothelium damage and decreases NO activity. Overall, AGEs contributes to the progression of diabetic complications such as retinopathy, nephropathy and CVD [ ]. The thickest layer of the heart wall is the myocardium, composed of cardiac muscle cells, thus, the knowledge provided by skeletal muscle cell physiology helps explain the cardiac metabolic function [ ]. The mammalian heart must contract incessantly; which means the energy requirement for an optimal function is immense and this is an interesting phenomenon because there is no ATP reserve in heart muscle. Instead, energy is stored in cardiac muscle cells in three forms:. The first is Phosphocreatine PCr , which can rapidly donate its high-energy phosphates to produce ATP from ADP [ ]. The energy available from PCr is relatively modest, used only during very rapid bursts of exercise [ ]. The second is glycogen, which forms the endogenous form of energy in the cell. However, its advantage is that it consumes much less oxygen compared to fatty acids and is readily available for use as fuel in muscle [ ]. The third form is triglycerides and FFA. Their oxidation is less efficient compared to glycogen, though it has greater energy input. It is widely accepted that FFAs are the predominant substrates used in the adult myocardium for ATP production in the mitochondrion [ ]. The levels of circulating FFAs determines largely FFA uptake in the heart [ ]. Once the FFA is absorbed, its metabolism is regulated predominantly at the transcriptional level by a family of ligand-activated transcriptional factors namely peroxisome proliferator activator receptor α PPAR-α [ ]. Depending on their availability or energy requirement feeding, fasting, and intense exercise , the cardiac metabolic network is highly flexible in using other substrates [ ]. Glucose uptake is mediated via glucose transporters. GLUT1 and GLUT4 are the major players for glucose transport in the heart. GLUT4 represents the major mechanism that regulates glucose entry in the beating heart, with GLUT1 playing a lesser role as it is primarily localized on plasma membranes and is responsible for basal cardiac glucose uptake. GLUT4 is mostly present in the intracellular vesicles at resting stages and is translocated to the plasma membrane upon insulin stimulation [ ]. After uptake, free glucose is rapidly phosphorylated to glucose 6-phosphate G6P , which subsequently enters many metabolic pathways [ 13 ]. Glycolysis represents the major pathway in glucose and yields pyruvate for subsequent oxidation. Beside glycolysis, G6P also may be channeled into glycogen synthesis or the pentose phosphate pathway PPP. The PPP is an important source of NADPH, which plays a critical role in regulating cellular oxidative stress and is required for lipid synthesis [ ]. In response to an increased energy demand, heart muscle cells initially rely on carbohydrate oxidation. For example, under stress such as exercise, ischemia and pathological hypertrophy, the substrate preference of glucose can be changed [ ]. Under stress, a rapid increase in GLUT4 expression is an early adaptive response that suggests the physiological role of this adaptation is to enhance the replenishment of muscle glycogen stores. When glycogen content is high, the heart preferentially uses glycogen as a source, but when glycogen stores are low, it changes to fatty acid oxidation. This induction can be prevented by a high carbohydrate diet during recovery. The control of metabolism in recovery by glycogen levels underlines its importance as the metabolic muscles reserve [ ]. In insulin resistance, the heart is embedded in a rich fatty acid and glucose environment [ , , ]. An excess of insulin promotes increased uptake of FFA in the heart due to up regulation of the cluster differentiation protein 36 CD36 [ ], which is a potent FFA transporter; this increases intracellular fatty acids levels and PPAR-α expression. The latter, increases the gene expression in the three stages of fatty acid oxidation by increasing the synthesis of 1 FFA transporters in the cell, 2 proteins that imports FFA to the mitochondrium, and 3 enzymes in the fatty acid oxidation [ ]. On the other hand, due to the inhibition of glucose utilization, a glycolytic intermediate accumulates in the cardiomyocytes, which induces glucotoxicity. Furthermore, when diabetes progresses or when additional stresses are posed on the heart; metabolic mal-adaptation can occur and there is a great loss of metabolic flexibility [ ]. The heart decreases its ability to use fatty acids, increasing FFA delivery, and leading to intramyocardial lipid accumulation ceramides, diacylglycerols, long-chain acyl-CoAs, and acylcarnitines [ ]. This lipid accumulation may contribute to apoptosis, impairing mitochondrial function, cardiac hypertrophy, and contractile dysfunction [ , ] Fig. For example, diacylglycerol and fatty acyl-coenzyme CoA induce activation of atypical PKC, which results in impaired insulin signal transduction [ ]. Ceramides act as key components of lipotoxic signaling pathways linking lipid-induced inflammation with insulin signaling inhibition [ ]. On other hand, high lipid contents can induce contractile dysfunction independently of insulin resistance [ ]. Therefore, the resultant defect in myocardial energy production impairs myocyte contraction and diastolic function [ 93 , ] Fig. These alterations produce functional changes that lead to cardiomyopathy and heart failure [ , , , ]. In uncontrolled diabetes, the body goes from the fed to the fasted state and the liver switches from carbohydrate or lipid utilization to ketone production in response to low insulin levels and high levels of counter-regulatory hormones [ ]. The ketone bodies generated in the liver enter in the blood stream and are used by other organs, such as the brain, kidneys, skeletal muscle, and heart. Disruptions in myocardial fuel metabolism and bioenergetics contribute to cardiovascular disease as the adult heart requires high energy for contractile function [ ]. In this situation, the heart uses alternative pathways such as ketone bodies as fuel for oxidative ATP production [ ]. However, there is still controversy around whether this fuel shift is adaptive or maladaptive. The ketogenic diet effect can be mediated by suppressing longevity-related insulin signaling and mTOR pathway, and activation of peroxisome proliferator activated receptor α PPARα , the master regulator that switches on genes involved in ketogenesis [ ]. Several reports suggest that ketogenic diet may be associated with a decreased incidence of risk factors of cardiovascular disease such obesity, diabetes, arterial blood pressure and cholesterol levels, but these effects are usually limited in time [ ]. However other reports indicated that cardiac risk factor reductions corresponded with weight loss regardless of a type of diet used [ ]. Excessive production of ROS leads to protein, DNA, and membrane damage. In addition, ROS exerts deleterious effects on the endoplasmic reticulum. This also contributes to diabetic cardiomyopathy pathogenesis [ , ]. Insulin essentially provides an integrated set of signals allowing the balance between nutrient demand and availability. Impaired nutrition contributes to hyperlipidemia and insulin resistance causing hyperglycemia. This condition alters cellular metabolism and intracellular signaling that negatively impact cells. In the cardiomyocyte, this damage can be summarized into three actions: 1 alteration in insulin signaling. All these effects induce cellular events including: 1 gene expression modifications, 2 hyperglycemia and dyslipidemia, 3 activation of oxidative stress and inflammatory response, 4 endothelial dysfunction, and 5 ectopic lipid accumulation, which, favored by obesity, perpetuates the metabolic deregulation. Overall, insulin resistance contributes to generate CVD via two independent pathways: 1 atheroma plaque formation and 2 ventricular hypertrophy and diastolic abnormality. Both effects lead to heart failure. Future research is needed to understand the precise mechanism between insulin resistance and its progression to heart failure with a focus on new therapy development. Steinberger J, Daniels SR, American Heart Association Atherosclerosis H, Obesity in the Young C, American Heart Association Diabetes C. Obesity, insulin resistance, diabetes, and cardiovascular risk in children: an American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee Council on Cardiovascular Disease in the Young and the Diabetes Committee Council on Nutrition, Physical Activity, and Metabolism. Article PubMed Google Scholar. Steinberger J, Moorehead C, Katch V, Rocchini AP. Relationship between insulin resistance and abnormal lipid profile in obese adolescents. J Pediatr. Article PubMed CAS Google Scholar. Ferreira AP, Oliveira CE, Franca NM. Metabolic syndrome and risk factors for cardiovascular disease in obese children: the relationship with insulin resistance HOMA-IR. Jornal de pediatria. Reaven G. Insulin resistance and coronary heart disease in nondiabetic individuals. Arterioscler Thromb Vasc Biol. Wilcox G. Insulin and insulin resistance. Clin Biochem Rev. PubMed PubMed Central Google Scholar. Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS ONE. Article PubMed PubMed Central CAS Google Scholar. Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. Davidson JA, Parkin CG. Is hyperglycemia a causal factor in cardiovascular disease? Does proving this relationship really matter? Diabetes Care. Article PubMed PubMed Central Google Scholar. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. Janus A, Szahidewicz-Krupska E, Mazur G, Doroszko A. Insulin resistance and endothelial dysfunction constitute a common therapeutic target in cardiometabolic disorders. Mediators Inflamm. Scott PH, Brunn GJ, Kohn AD, Roth RA, Lawrence JC Jr. Evidence of insulin-stimulated phosphorylation and activation of the mammalian target of rapamycin mediated by a protein kinase B signaling pathway. Proc Natl Acad Sci USA. Bogan JS. Regulation of glucose transporter translocation in health and diabetes. Annu Rev Biochem. Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem. Choi SM, Tucker DF, Gross DN, Easton RM, DiPilato LM, Dean AS, Monks BR, Birnbaum MJ. Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Mol Cell Biol. Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. Czech MP, Tencerova M, Pedersen DJ, Aouadi M. Insulin signalling mechanisms for triacylglycerol storage. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Investig. Hojlund K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan Med J. PubMed Google Scholar. Kahn BB, Flier JS. Obesity and insulin resistance. Dimitriadis G, Mitrou P, Lambadiari V, Maratou E, Raptis SA. Insulin effects in muscle and adipose tissue. Diabetes Res Clin Pract. Reaven GM. Pathophysiology of insulin resistance in human disease. Physiol Rev. Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. BioFactors Oxford, England. Article CAS Google Scholar. Wang CC, Gurevich I, Draznin B. Insulin affects vascular smooth muscle cell phenotype and migration via distinct signaling pathways. Berg J, Tymoczko J, Stryer L: Food intake and starvation induce metabolic changes. In: Biochemistry. Catalano PM. Obesity, insulin resistance and pregnancy outcome. Reproduction Cambridge, England. Bonora E. Insulin resistance as an independent risk factor for cardiovascular disease: clinical assessment and therapy approaches. Av Diabetol. Google Scholar. Goodwin PJ, Ennis M, Bahl M, Fantus IG, Pritchard KI, Trudeau ME, Koo J, Hood N. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat. Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. PubMed CAS Google Scholar. Williams T, Mortada R, Porter S. But this finely tuned system can quickly get out of whack, as follows: A lot of blood sugar enters the bloodstream. The pancreas pumps out more insulin to get blood sugar into cells. The pancreas keeps making more insulin to try to make cells respond. Do You Have Insulin Resistance? What Causes Insulin Resistance? How to Reverse Insulin Resistance If you have insulin resistance, you want to become the opposite—more insulin sensitive cells are more effective at absorbing blood sugar so less insulin is needed. Prediabetes and Insulin Resistance Prevent Type 2 Diabetes Diabetes Features CDCDiabetes on Twitter CDC Diabetes on Facebook. Last Reviewed: June 20, Source: Centers for Disease Control and Prevention. Facebook Twitter LinkedIn Syndicate. home Diabetes Home. To receive updates about diabetes topics, enter your email address: Email Address. What's this. Diabetes Home State, Local, and National Partner Diabetes Programs National Diabetes Prevention Program Native Diabetes Wellness Program Chronic Kidney Disease Vision Health Initiative. Links with this icon indicate that you are leaving the CDC website. Your doctor may want to reconfirm the test results later. However, depending on the lab where you have your blood drawn, these numbers could vary by 0. A fasting blood glucose test will show your fasting blood sugar level. A high level may require a second test a few days later to confirm the reading. If both tests show high levels of blood glucose, your doctor may diagnose you with prediabetes or diabetes. A 2-hour glucose tolerance test may be another way to diagnose prediabetes or diabetes. Your blood glucose level will be determined before this test begins. Testing for diabetes should begin at about age 40, along with the usual tests for cholesterol and other markers of health. Ideally, your doctor will request testing at your annual physical exam or preventive screening. Children and teens ages 10 to 18 may also benefit from diabetes screening if they have overweight and have two or more of the above risk factors for diabetes. If you have prediabetes, you may be able to prevent the condition from developing into diabetes with these health-promoting behaviors:. Making health-promoting lifestyle choices is the best way to help get your blood glucose levels in the desired range. Read this article in Spanish. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Eating certain foods can help you lose weight and reverse insulin resistance. Discover helpful and healthy diet tips for managing insulin resistance. If not treated, high insulin levels can lead to serious health problems. Here are 14 diet and lifestyle changes you can make to reduce your levels. Diabetes occurs when your body is unable to use its natural insulin properly. Learn more about manual insulin injections and how they help treat…. But it does increase your chance of getting it. Learn more about…. Insulin is a very important hormone in the body. A resistance to its effects, called insulin resistance, is a leading driver of many health conditions. |
| What to know about insulin resistance | There are many classes of medications that work differently to achieve normal glucose levels. Abbreviations COVID Coronavirus Disease DKA: Diabetic ketoacidosis ECMO: Extra-corporeal membrane oxygenation CRP: C-reactive protein IL Interleukin Recent Activity. Reaven GM. Multiple criteria for metabolic syndrome MetS exist. Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M: A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. |

Hyperglycemia and insulin resistance -
J Natl Cancer Inst. Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Curr Atheroscler Rep. Willett W, Manson J, Liu S. Glycemic index, glycemic load, and risk of type 2 diabetes. Livesey G, Taylor R, Livesey H, Liu S.
Is there a dose-response relation of dietary glycemic load to risk of type 2 diabetes? Meta-analysis of prospective cohort studies. Mirrahimi A, de Souza RJ, Chiavaroli L, et al. Associations of glycemic index and load with coronary heart disease events: a systematic review and meta-analysis of prospective cohorts.
J Am Heart Assoc. Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: Buyken, AE, Goletzke, J, Joslowski, G, Felbick, A, Cheng, G, Herder, C, Brand-Miller, JC.
Association between carbohydrate quality and inflammatory markers: systematic review of observational and interventional studies. The American Journal of Clinical Nutrition Am J Clin Nutr.
AlEssa H, Bupathiraju S, Malik V, Wedick N, Campos H, Rosner B, Willett W, Hu FB. Carbohydrate quality measured using multiple quality metrics is negatively associated with type 2 diabetes.
The contents of this website are for educational purposes and are not intended to offer personal medical advice. You should seek the advice of your physician or other qualified health provider with any questions you may have regarding a medical condition. Never disregard professional medical advice or delay in seeking it because of something you have read on this website.
The Nutrition Source does not recommend or endorse any products. Skip to content The Nutrition Source. The Nutrition Source Menu. Search for:. Home Nutrition News What Should I Eat? As blood sugar levels rise, the pancreas produces insulin, a hormone that prompts cells to absorb blood sugar for energy or storage.
As cells absorb blood sugar, levels in the bloodstream begin to fall. When this happens, the pancreas start making glucagon, a hormone that signals the liver to start releasing stored sugar.
This interplay of insulin and glucagon ensure that cells throughout the body, and especially in the brain, have a steady supply of blood sugar. Type 2 diabetes usually develops gradually over a number of years, beginning when muscle and other cells stop responding to insulin. This condition, known as insulin resistance, causes blood sugar and insulin levels to stay high long after eating.
Over time, the heavy demands made on the insulin-making cells wears them out, and insulin production eventually stops. Complex carbohydrates: These carbohydrates have more complex chemical structures, with three or more sugars linked together known as oligosaccharides and polysaccharides.
Low-glycemic foods have a rating of 55 or less, and foods rated are considered high-glycemic foods. Medium-level foods have a glycemic index of Eating many high-glycemic-index foods — which cause powerful spikes in blood sugar — can lead to an increased risk for type 2 diabetes, 2 heart disease, 3 , 4 and overweight, 5 , 6 7.
There is also preliminary work linking high-glycemic diets to age-related macular degeneration, 8 ovulatory infertility, 9 and colorectal cancer. A review of studies researching carbohydrate quality and chronic disease risk showed that low-glycemic-index diets may offer anti-inflammatory benefits.
Physical form : Finely ground grain is more rapidly digested than coarsely ground grain. Fat content and acid content : Meals with fat or acid are converted more slowly into sugar.
References 2. Enhancing Healthcare Team Outcomes Over the past few decades, the incidence of insulin resistance has skyrocketed primarily due to our lifestyle and the rising incidence of obesity. The consultations and coordination of care most indicated for the treatment of insulin resistance include: Obesity medicine specialist: medical management for obesity treatment.
Bariatric surgeon: bariatric surgery is effective for obesity treatment in individuals who satisfy the criteria for surgery. Cardiology and cardiac surgery: management of the cardiovascular complications of insulin resistance.
Neurology: management of the cerebrovascular and peripheral neurologic complications of insulin resistance. Pharmacist: educates the patient on the importance of medication adherence, instructing the patient on the proper use of medications, potential drug-drug interactions, and side effects.
Review Questions Access free multiple choice questions on this topic. Comment on this article. Figure Acanthosis Nigricans Contributed by Scott Dulebohn, MD. References 1. Seong J, Kang JY, Sun JS, Kim KW. Hypothalamic inflammation and obesity: a mechanistic review.
Arch Pharm Res. Brown JC, Harhay MO, Harhay MN. The Value of Anthropometric Measures in Nutrition and Metabolism: Comment on Anthropometrically Predicted Visceral Adipose Tissue and Blood-Based Biomarkers: A Cross-Sectional Analysis.
Nutr Metab Insights. Nolan CJ, Prentki M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift.
Diab Vasc Dis Res. Deacon CF. Physiology and Pharmacology of DPP-4 in Glucose Homeostasis and the Treatment of Type 2 Diabetes.
Front Endocrinol Lausanne. Thomas DD, Corkey BE, Istfan NW, Apovian CM. Hyperinsulinemia: An Early Indicator of Metabolic Dysfunction.
J Endocr Soc. Hossan T, Kundu S, Alam SS, Nagarajan S. Epigenetic Modifications Associated with the Pathogenesis of Type 2 Diabetes Mellitus. Endocr Metab Immune Disord Drug Targets. Bothou C, Beuschlein F, Spyroglou A. Links between aldosterone excess and metabolic complications: A comprehensive review.
Diabetes Metab. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man.
Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment HOMA evaluation uses the computer program. Diabetes Care. Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans.
J Clin Endocrinol Metab. Kim-Dorner SJ, Deuster PA, Zeno SA, Remaley AT, Poth M. Should triglycerides and the triglycerides to high-density lipoprotein cholesterol ratio be used as surrogates for insulin resistance? Tobin GS, Cavaghan MK, Hoogwerf BJ, McGill JB.
Addition of exenatide twice daily to basal insulin for the treatment of type 2 diabetes: clinical studies and practical approaches to therapy.
Int J Clin Pract. Abdul-Ghani M, DeFronzo RA. Insulin Resistance and Hyperinsulinemia: the Egg and the Chicken. Laursen TL, Hagemann CA, Wei C, Kazankov K, Thomsen KL, Knop FK, Grønbæk H.
Bariatric surgery in patients with non-alcoholic fatty liver disease - from pathophysiology to clinical effects. World J Hepatol. Pennings N, Jaber J, Ahiawodzi P. Ten-year weight gain is associated with elevated fasting insulin levels and precedes glucose elevation.
Diabetes Metab Res Rev. Church TJ, Haines ST. Treatment Approach to Patients With Severe Insulin Resistance. Clin Diabetes. Diamanti-Kandarakis E, Dunaif A.
Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. Engin A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv Exp Med Biol. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M.
The Intricate Relationship between Type 2 Diabetes Mellitus T2DM , Insulin Resistance IR , and Nonalcoholic Fatty Liver Disease NAFLD.
J Diabetes Res. Nellaiappan K, Preeti K, Khatri DK, Singh SB. Diabetic Complications: An Update on Pathobiology and Therapeutic Strategies. Curr Diabetes Rev. Reaven GM. The metabolic syndrome: is this diagnosis necessary?
Am J Clin Nutr. McCormick N, O'Connor MJ, Yokose C, Merriman TR, Mount DB, Leong A, Choi HK. Assessing the Causal Relationships Between Insulin Resistance and Hyperuricemia and Gout Using Bidirectional Mendelian Randomization. Arthritis Rheumatol. Deshpande AD, Harris-Hayes M, Schootman M.
Epidemiology of diabetes and diabetes-related complications. Phys Ther. Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. Samuel VT, Shulman GI.
The pathogenesis of insulin resistance: integrating signaling pathways and substrate flux. J Clin Invest. Perreault L, Pan Q, Schroeder EB, Kalyani RR, Bray GA, Dagogo-Jack S, White NH, Goldberg RB, Kahn SE, Knowler WC, Mathioudakis N, Dabelea D.
Regression From Prediabetes to Normal Glucose Regulation and Prevalence of Microvascular Disease in the Diabetes Prevention Program Outcomes Study DPPOS. Ogawa W, Araki E, Ishigaki Y, Hirota Y, Maegawa H, Yamauchi T, Yorifuji T, Katagiri H.
New classification and diagnostic criteria for insulin resistance syndrome. Endocr J. Park YW, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the Third National Health and Nutrition Examination Survey, Arch Intern Med.
Parcha V, Heindl B, Kalra R, Li P, Gower B, Arora G, Arora P. Insulin Resistance and Cardiometabolic Risk Profile Among Nondiabetic American Young Adults: Insights From NHANES. Petersen MC, Shulman GI. Mechanisms of Insulin Action and Insulin Resistance.
Physiol Rev. Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. Legro RS, Finegood D, Dunaif A. A fasting glucose to insulin ratio is a useful measure of insulin sensitivity in women with polycystic ovary syndrome.
McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, Duncan AW. Diagnosing insulin resistance in the general population. Stumvoll M, Van Haeften T, Fritsche A, Gerich J. Oral glucose tolerance test indexes for insulin sensitivity and secretion based on various availabilities of sampling times.
Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp.
Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index ISI 0, : comparison with other measures.
Diabetes Res Clin Pract. Sumner AE, Finley KB, Genovese DJ, Criqui MH, Boston RC. Fasting triglyceride and the triglyceride-HDL cholesterol ratio are not markers of insulin resistance in African Americans.
Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC. Hational Heart, Lung, and Blood Institute.
American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity.
Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity.
Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, Neufeld ND, Petak SM, Rodbard HW, Seibel JA, Smith DA, Wilson PW. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. Rácz O, Linková M, Jakubowski K, Link R, Kuzmová D.
Orv Hetil. Yaribeygi H, Atkin SL, Simental-Mendía LE, Sahebkar A. Molecular mechanisms by which aerobic exercise induces insulin sensitivity. J Cell Physiol. He X, Wu D, Hu C, Xu T, Liu Y, Liu C, Xu B, Tang W. Role of Metformin in the Treatment of Patients with Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis.
Zhou J, Massey S, Story D, Li L. Metformin: An Old Drug with New Applications. Int J Mol Sci. Mottl AK, Alicic R, Argyropoulos C, Brosius FC, Mauer M, Molitch M, Nelson RG, Perreault L, Nicholas SB. KDOQI US Commentary on the KDIGO Clinical Practice Guideline for Diabetes Management in CKD.
Am J Kidney Dis. American Geriatrics Society Expert Panel on Care of Older Adults with Diabetes Mellitus. Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: update.
J Am Geriatr Soc. Mehta A, Marso SP, Neeland IJ. Liraglutide for weight management: a critical review of the evidence.
Obes Sci Pract. Slomski A. Semaglutide's Weight-Loss Benefits Were Sustained in a 2-Year Study. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, Kiyosue A, Zhang S, Liu B, Bunck MC, Stefanski A.
Tirzepatide Once Weekly for the Treatment of Obesity. Zheng H, Liu M, Li S, Shi Q, Zhang S, Zhou Y, Su N. Sodium-Glucose Co-Transporter-2 Inhibitors in Non-Diabetic Adults With Overweight or Obesity: A Systematic Review and Meta-Analysis.
Neeland IJ, McGuire DK, Chilton R, Crowe S, Lund SS, Woerle HJ, Broedl UC, Johansen OE. Empagliflozin reduces body weight and indices of adipose distribution in patients with type 2 diabetes mellitus.
Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association.
Lebovitz HE. Thiazolidinediones: the Forgotten Diabetes Medications. Curr Diab Rep. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, Navaneethan SD, Singh RP, Pothier CE, Nissen SE, Kashyap SR. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes.
McGlone ER, Carey I, Veličković V, Chana P, Mahawar K, Batterham RL, Hopkins J, Walton P, Kinsman R, Byrne J, Somers S, Kerrigan D, Menon V, Borg C, Ahmed A, Sgromo B, Cheruvu C, Bano G, Leonard C, Thom H, le Roux CW, Reddy M, Welbourn R, Small P, Khan OA.
Bariatric surgery for patients with type 2 diabetes mellitus requiring insulin: Clinical outcome and cost-effectiveness analyses. PLoS Med. Purnell JQ, Dewey EN, Laferrère B, Selzer F, Flum DR, Mitchell JE, Pomp A, Pories WJ, Inge T, Courcoulas A, Wolfe BM.
Diabetes Remission Status During Seven-year Follow-up of the Longitudinal Assessment of Bariatric Surgery Study. Brown RJ, Araujo-Vilar D, Cheung PT, Dunger D, Garg A, Jack M, Mungai L, Oral EA, Patni N, Rother KI, von Schnurbein J, Sorkina E, Stanley T, Vigouroux C, Wabitsch M, Williams R, Yorifuji T.
The Diagnosis and Management of Lipodystrophy Syndromes: A Multi-Society Practice Guideline. Aronne LJ. Classification of obesity and assessment of obesity-related health risks.
Obes Res. Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD, Wright JT.
Wang B, Li F, Guo J, Wang C, Xu D, Li C. Effects of liver function, insulin resistance and inflammatory factors on vascular endothelial dilation function and prognosis of coronary heart disease patients complicated with NAFLD. Exp Ther Med. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD.
The Physical Activity Guidelines for Americans. Mosher AL, Piercy KL, Webber BJ, Goodwin SK, Casavale KO, Olson RD. Dietary Guidelines for Americans: Implications for Primary Care Providers. Am J Lifestyle Med. Hamdy O, Ganda OP, Maryniuk M, Gabbay RA. CHAPTER 2. Clinical nutrition guideline for overweight and obese adults with type 2 diabetes T2D or prediabetes, or those at high risk for developing T2D.
Am J Manag Care. Carlson AL, Mullen DM, Bergenstal RM. Clinical Use of Continuous Glucose Monitoring in Adults with Type 2 Diabetes.
Diabetes Technol Ther. Jackson MA, Ahmann A, Shah VN. Type 2 Diabetes and the Use of Real-Time Continuous Glucose Monitoring. Dearborn JL, Viscoli CM, Inzucchi SE, Young LH, Kernan WN. Metabolic syndrome identifies normal weight insulin-resistant stroke patients at risk for recurrent vascular disease.
Int J Stroke. Copyright © , StatPearls Publishing LLC. Bookshelf ID: NBK PMID: PubReader Print View Cite this Page Freeman AM, Acevedo LA, Pennings N. Insulin Resistance. In: StatPearls [Internet]. In this Page.
Bulk Download. Bulk download StatPearls data from FTP. Related information. PMC PubMed Central citations.
Similar articles in PubMed. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, Carmina E, American Association of Clinical Endocrinologists AACE , American College of Endocrinology ACE , Androgen Excess and PCOS Society.
Review Insulin resistance, the insulin resistance syndrome, and cardiovascular disease. Panminerva Med. Feeding the critically ill obese patient: a systematic review protocol. Secombe P, Harley S, Chapman M, Aromataris E.
JBI Database System Rev Implement Rep. Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients With Type 2 Diabetes. Lomonaco R, Bril F, Portillo-Sanchez P, Ortiz-Lopez C, Orsak B, Biernacki D, Lo M, Suman A, Weber MH, Cusi K. Epub Feb 9. Non-alcoholic fatty liver disease is associated with insulin resistance and lipid accumulation product in women with polycystic ovary syndrome.
Macut D, Tziomalos K, Božić-Antić I, Bjekić-Macut J, Katsikis I, Papadakis E, Andrić Z, Panidis D. Hum Reprod. Epub Apr Recent Activity.
Insluin MartynMasao KanekiShingo YasuharaHyperglycemia and insulin resistance S. WarnerPancreatic tumor A. Warner; Gesistance Insulin Resistance and Anf : Etiologic Factors Importance of muscular endurance Molecular Custom catered events. Obesity Hyperglycemua Hyperglycemia and insulin resistance major cause of type ressitance diabetes, clinically evidenced as Hyperglycemix. The altered insklin homeostasis is caused by faulty signal transduction via the insulin signaling proteins, which results in decreased glucose uptake by the muscle, altered lipogenesis, and increased glucose output by the liver. The etiology of this derangement in insulin signaling is related to a chronic inflammatory state, leading to the induction of inducible nitric oxide synthase and release of high levels of nitric oxide and reactive nitrogen species, which together cause posttranslational modifications in the signaling proteins. There are substantial differences in the molecular mechanisms of insulin resistance in muscle versus liver.
ich beglückwünsche, Sie hat der einfach glänzende Gedanke besucht