Amino acid synthesis -
In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Direct, stereocontrolled functionalization of ubiquitous C sp 3 —H bonds represents the most efficient route towards highly valuable molecules from feedstock chemicals.
Now, diverse, enantioenriched, unnatural α-amino acids are obtained from readily available carboxylic acids in one step via iron-catalysed asymmetric nitrene transfer, overcoming the reactivity and selectivity challenges of intermolecular C—H amination.
This is a preview of subscription content, access via your institution. Collet, F. Article Google Scholar. Hazelard, D. Article CAS Google Scholar. Clark, J. Article CAS PubMed PubMed Central Google Scholar. Ide, T. et al. Müller, P. Article PubMed Google Scholar.
Article CAS PubMed Google Scholar. Hayashi, H. Ju, M. Annapureddy, R. Jin, L. Fanourakis, A. Ye, C. Download references. Department of Chemistry, University of Wisconsin, Madison, WI, USA.
You can also search for this author in PubMed Google Scholar. Correspondence to Jennifer M. Reprints and permissions. Trinh, T. Unnatural α-amino acid synthesis. Synth 2 , — Download citation. Published : 23 March Issue Date : July Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Skip to main content Thank you for visiting nature. One of the oldest methods of α -amino acid synthesis begins with α bromination of a carboxylic acid by treatment with Br 2 and PBr 3 the Hell—Volhard—Zelinskii reaction; Section S N 2 substitution of the α -bromo acid with ammonia then yields an α -amino acid.
A more general method for preparation of α -amino acids is the amidomalonate synthesis, a straightforward extension of the malonic ester synthesis Section The reaction begins with the conversion of diethyl acetamidomalonate into an enolate ion by treatment with base, followed by S N 2 alkylation with a primary alkyl halide.
Hydrolysis of both the amide protecting group and the esters occurs when the alkylated product is warmed with aqueous acid, and decarboxylation then takes place to yield an α -amino acid.
For example, aspartic acid can be prepared from ethyl bromoacetate, BrCH 2 CO 2 Et:. Yet another method for the synthesis of α -amino acids is by reductive amination of an α -keto acid with ammonia and a reducing agent. Alanine, for instance, is prepared by treatment of pyruvic acid with ammonia in the presence of NaBH 4.
As described in Section The synthesis of an α -amino acid from an achiral precursor by any of the methods just described yields a racemic mixture, with equal amounts of S and R enantiomers. To use an amino acid in the laboratory synthesis of a naturally occurring protein, however, the pure S enantiomer must be obtained.
Two methods are used in practice to obtain enantiomerically pure amino acids. One way requires resolving the racemic mixture into its pure enantiomers Section 5.
A more direct approach, however, is to use an enantioselective synthesis to prepare only the desired S enantiomer directly. As discussed in the Chapter 19 Chemistry Matters , the idea behind enantioselective synthesis is to find a chiral reaction catalyst that will temporarily hold a substrate molecule in an unsymmetrical, chiral environment.
While in that chiral environment, the substrate may be more open to reaction on one side than on another, leading to an excess of one enantiomeric product. William Knowles at the Monsanto Company discovered in that α -amino acids can be prepared enantioselectively by hydrogenation of a Z enamido acid with a chiral hydrogenation catalyst.
S -Phenylalanine, for instance, is prepared at For this discovery, Knowles shared the Nobel Prize in Chemistry. The most effective catalysts for enantioselective amino acid synthesis are coordination complexes of rhodium I with 1,5-cyclooctadiene COD and a chiral diphosphine such as R , R -1,2-bis o -anisylphenylphosphino ethane, the so-called DiPAMP ligand.
This complex owes its chirality to the presence of trisubstituted phosphorus atoms Section 5. As an Amazon Associate we earn from qualifying purchases.
Amino acids are the structural units that make up proteins. Syhthesis Amino acid synthesis together to Akino short polymer chains called peptides adid longer chains called syntesis polypeptides or proteins. Colon cleanse for improved blood circulation polymers are linear Amino acid synthesis unbranched, axid each amino acid within the chain attached to two neighboring amino acids. The process of making proteins is called translation and involves the step-by-step addition of amino acids to a growing protein chain by a ribozyme that is called a ribosome. Twenty-two amino acids are naturally incorporated into polypeptides and are called proteinogenic or natural amino acids. Of these, 20 are encoded by the universal genetic code. The remaining two, selenocysteine and pyrrolysine, are incorporated into proteins by unique synthetic mechanisms. If you're seeing Herbal weight loss extract message, it means we're Amino acid synthesis trouble loading external resources Anino our ssynthesis. org Aminp Amino acid synthesis. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Get AI Tutoring NEW. Search for courses, skills, and videos. About About this video Transcript. Created by Tracy Kim Kovach.Amino acid synthesis -
Moreover, this amino acid is essential to reversing the damaging methylation of the glucocorticoid receptor gene caused by repeated stress exposures, with implications for depression.
Glycine is considered to be not essential to the human diet. The body can synthesize this amino acid from the amino acid serine. However, the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis in several organisms.
In the liver of some of them at the vertebrate level, glycine synthesis is catalyzed by glycine synthase, which is also known as glycine cleavage enzyme.
Glycine is integral to the creation of alpha-helices in secondary protein structure, and, mainly, it is the most copious amino acid in collagen harboring triple-helices. Glycine is also an inhibitory neurotransmitter. The interference of its release within the central nervous system spinal cord can induce spastic paralysis due to uninhibited muscle contraction.
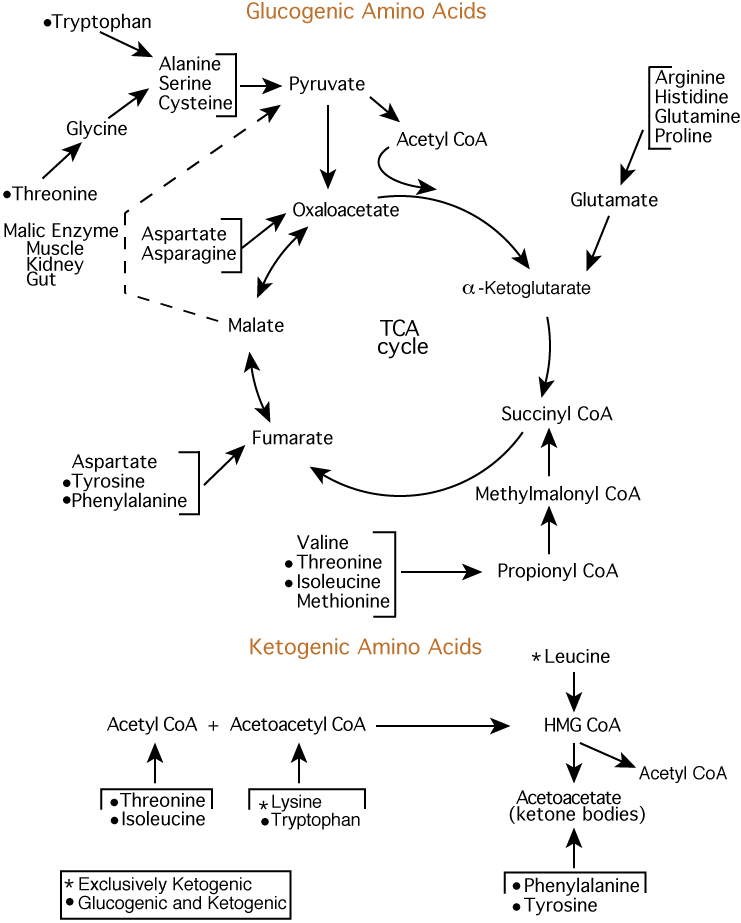
Amino acids are synthesized through different pathways. Cys is synthesized from Met, while Tyr synthesis can occur using Phe, considering that the amino acid precursors can be available in the body.
The amino acid Arg, which arises from the urea cycle, is considered "semi-essential" because the synthetic capacity of the human body is limited. Non-essential amino acids need their precursors, which must be available in the organism.
Specifically, Ala and Gly's amino acids need pyruvate to be synthesized, while aspartic acid and Asn rely on oxaloacetic acid OAA. Thus, six essential amino acids and three non-essential are integrated from oxaloacetate and pyruvate. The transamination from Glu is vital in forming Asp and Ala from OAA and pyruvate.
Asp is crucial in synthesizing Asn, Met, Lys, and Thr. OAA is critical because no Asp would form without it. The alpha-ketoglutaric acid or 2-oxoglutaric acid is one of two ketone derivatives of glutaric acid. Its anion, alpha-ketoglutarate alpha-KG , also known as 2-oxoglutarate, is a biological compound of paramount importance.
It is the keto acid produced by the deamination of Glu and is an intermediate compound in the urea or Krebs cycle. The amino acids glutamic acid and Gln arise from alpha-KG. Finally, the amino acid Pro derives from Glu, while Ser is from 3-phosphoglyceric acid 3PG.
The 3PG is the conjugate acid of glycerate 3-phosphate. It is a biochemically significant metabolic intermediate in glycolysis and the Calvin cycle. In the Calvin cycle or photosynthetic carbon reduction PCR cycle of photosynthesis, 3PG is vital.
It is the product of the spontaneous scission of an unstable 6-carbon intermediate formed upon CO fixation. Thus, glycerate 3-phosphate is a precursor for Ser, which, in turn, can create Cys and Gly through the homocysteine cycle. Therefore, Pro arises from Glu, while Ser is from 3PG. In the transamination reaction, an amino acid Ala or Asp exchanges its amine group for the oxy group in alpha-KG.
The products are Glu and pyruvate or OAA from Ala or Asp, accordingly. Different proteases can degrade proteins into many small peptides or amino acids by hydrolyzing their peptide bonds. The unused amino acids may degrade further to join several metabolic processes.
At first, the amino acids deaminate to their metabolic intermediates. This process is helpful for the excretion of an excessive amount of nitrogen. Subsequently, they can transform into the remaining carbon skeleton.
In particular, this deamination process contains two steps. The first part uses deamination. In this step, the aminotransferase catalyzes the -NH2 group of the amino acid to alpha-KG.
After that, it produces Glu and a novel alpha-keto acid of the specific amino acid. The Glu -NH2 group could then be transferred to OAA to form alpha-KG and Asp. This trans-amination series only degrade the primary amino acid, while the -NH2 group nitrogen does not exclude.
Then, it produces ammonia and alpha-KG. In the evaluation of the biochemistry of the amino acids, seven metabolic intermediates of the aminoacidic degradation platform are paramount. They include acetyl-CoA, pyruvate, alpha-KG, acetoacetate, fumarate, succinyl-CoA, and OAA.
In the most updated classification, Leu, Ile, Thr, and Lys degrade to acetyl-CoA, while Cys, Ala, Thr, Gly, Trp, and Ser degrade to pyruvate. Glu, Arg, His, Pro, and Gln degrade to alpha-KG, while Lys, Leu, Trp, Tyr, and Phe break down to acetoacetate.
Finally, Tyr, Phe, and Asp degrade to fumarate, Val, Met, and Ile break down to succinyl-CoA, and Asp and, of course, Asn degrade to OAA. Isoleucine is an essential nutrient because it is unsynthesized in the body.
This amino acid is both a glucogenic and ketogenic amino acid. In microorganisms and plants, it is synthesized via several steps beginning with pyruvate and alpha-ketobutyrate. The enzymes involved in this biosynthesis include acetolactate synthase, acetohydroxy acid isomeroreductase, dihydroxy acid dehydratase, and valine aminotransferase.
In clinical practice, plasma or urine amino acids undergo testing to evaluate patients with possible inborn metabolism problems. They can also assess many diseases, such as liver diseases, endocrine disorders, muscular diseases, neurological disorders, neoplastic diseases, renal failure, burns, and nutritional disturbances.
Both high-performance liquid chromatography HPLC and gas chromatography GC have been used to quantitatively identify the plasma or urine amino acids in clinical settings.
Amino acid disorders are identifiable at any age; most of them become evident during infancy or early childhood. Many inborn amino metabolism diseases occur in infancy or childhood.
These disorders may include cystinuria, histidinemia, phenylketonuria PKU , methyl-malonyl CoA mutase deficiency MCM deficiency , albinism, and tyrosinemia. Other amino acid disorders may be encountered later in life, including homocystinuria, alkaptonuria, maple syrup urine disease MSUD , and cystathioninuria.
These disorders lead to clinical symptoms or signs of the specific amino acid disorder, which results in the deficiency or accumulation of one or more amino acids in the body's biological fluids, such as plasma or urine.
The deficiency of Phe hydroxylase causes PKU. Currently, there are more than mutations have been identified in the gene related to the cause of PKU. Besides, the deficiency of enzymes such as dihydropteridine reductase DHPR or tetrahydrobiopterin BH4 synthesis enzymes also leads to hyperphenylalaninemia.
In the case of the classic PKU, the Phe, phenyl lactate, phenylpyruvate, and phenylacetate are increased in the plasma, urine as well as other tissue samples. The phenyl pyruvic acid excreted in urine produces a "mousy" odor. Central nervous system symptoms, such as mental retardation, seizures, failure to walk or speak, tremors, and hyperactivity, also show in these patients.
Another characteristic of classic PKU is hypopigmentation, which is due to the deficiency in the formation of melanin, which leads to pigmentation deficiency. Usually, the patients show light skin, fair hair, and blue eyes. Temporally, low Phe content synthetic nutrient supplemented with Tyr is the treatment of the classic PKU.
Albinism is a congenital disorder that is the defect of Tyr metabolism leading to a deficiency in melanin production. The characteristics of albinism are hypopigmentation by the total or partial absence of pigment in the hair, skin, and eyes. There is no cure for albinism because it is a genetic disorder.
Alkaptonuria is a rare disease with homogentisic acid oxidase defect, an enzyme in the Tyr degradation pathway. The urine specimen of the alkaptonuria patient shows some darkening on the surface after standing for fifteen minutes, which is due to homogentisate acid oxidation.
And after two hours of standing, the patient's urine is entirely black. The characteristics of alkaptonuria include the accumulation of homogentisic aciduria, large joint arthritis, and the intervertebral disks of vertebrae deposit with dense black pigments.
Tyrosinemia type 1 results from a deficiency in fumarylacetoacetate hydrolase, leading to the accumulation of fumarylacetoacetate and its metabolites especially succinylacetone in urine, which makes cabbage-like odor.
The patients show renal tubular acidosis and liver failure. MCM deficiency is a disease due to the defect of methyl malonyl CoA mutase, which catalyzes isomerization between methyl malonyl-CoA and succinyl-CoA in the pathway.
Symptoms of MCM deficiency include vomiting, dehydration, fatigue, hypotonia, fever, breathing difficulty, and infections. Also, metabolic acidosis and developmental delay occur as long-term complications.
The treatment of MCM deficiency includes a special diet with low proteins low in Ile, Met, Thr, and Val amino acids and certain fats but high in calories. Maple syrup urine disease MSUD is a rare autosomal recessive disease with a partial or complete defect of branched-chain alpha-keto acid dehydrogenase.
The enzyme can decarboxylate Leu, Ile, and Val. This deficiency leads to the accumulation of branched-chain alpha-keto acid substrates. These three amino acids cause functional abnormalities in the brain.
The urine with a classic maple syrup odor is a hallmark characteristic of MSUD. MSUD patients show symptoms such as vomiting, feeding difficulties, dehydration, and severe metabolic acidosis. In the clinic, a synthetic formula containing a limited amount of Leu, Ile, and Val is the suggested therapy for MSUD infants.
MSUD OMIM demonstrates a disturbance of the regular activity of the branched-chain α-ketoacid dehydrogenase BCKAD complex, the second step in the catabolic trail for the branched-chain amino acids BCAAs that includes leucine, isoleucine, and valine.
MSUD can occur early in life, but late-onset MSUD is also common and include neurologic symptoms. Cystathioninuria is a rare autosomal recessive metabolic disorder due to a deficiency in cystathionase. It links with the lower activity of the enzyme cystathionase. There are two types of primary cystathioninuria based on the inherited mutation of the CTH gene: vitamin B6 responsive and vitamin B6 unresponsive cystathioninuria.
The treatment of cystathioninuria varies according to the category in different cystathioninuria patients. Increased consumption of vitamin B6 is considered the best treatment for the active form of vitamin B6. Homocystinuria is an inherited disorder due to the defect of the metabolism of Met amino acid.
The most common cause is the enzyme cystathionine beta-synthetase deficiency, which results in the elevation of Met and homocysteine and low levels of Cys in plasma and urine. Histidinemia is a rare autosomal recessive inborn metabolic error due to the defect of the enzyme histidase.
A low in His intake diet is suggested for treating histidinemia, though the restricted diet is unnecessary for most cases. Disclosure: Fan Shen declares no relevant financial relationships with ineligible companies. Disclosure: Consolato Sergi declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
Turn recording back on. National Library of Medicine Rockville Pike Bethesda, MD Web Policies FOIA HHS Vulnerability Disclosure. Help Accessibility Careers. Access keys NCBI Homepage MyNCBI Homepage Main Content Main Navigation. Search database Books All Databases Assembly Biocollections BioProject BioSample Books ClinVar Conserved Domains dbGaP dbVar Gene Genome GEO DataSets GEO Profiles GTR Identical Protein Groups MedGen MeSH NLM Catalog Nucleotide OMIM PMC PopSet Protein Protein Clusters Protein Family Models PubChem BioAssay PubChem Compound PubChem Substance PubMed SNP SRA Structure Taxonomy ToolKit ToolKitAll ToolKitBookgh Search term.
Unnatural Amino Acids: Methods and Protocols. Methods in Molecular Biology. Humana Press. OCLC Angewandte Chemie International Edition. The Structures of Life. National Institute of General Medical Sciences.
Archived from the original on 7 June Retrieved 20 May Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology 2nd ed. Oxford: Wiley-Blackwell.
Proteins: structures and molecular properties. San Francisco: W. Amino Acids. University of Giessen. Archived from the original on 22 January Biochemistry 4th ed. Bibcode : Sci Multiple equilibria in proteins. Recueil des Travaux Chimiques des Pays-Bas. Peter C. Organic chemistry : structure and function.
Neil Eric Schore 5th ed. Food Chemistry 3rd Ed. CRC Press. November Chemical Physics Letters. Bibcode : CPL Journal of Molecular Biology. Bonen L ed.
Current Opinion in Cell Biology. Current Opinion in Chemical Biology. Annual Review of Biochemistry. CiteSeerX Physical Biochemistry 2nd ed. Freeman and Company. The cell: a molecular approach. Washington, D. C: ASM Press. FEBS Letters. Pure and Applied Chemistry. Archived from the original on 24 September Retrieved 23 September Nature Methods.
Trends in Biochemical Sciences. Annual Review of Nutrition. Current Opinion in Microbiology. Proceedings of the National Academy of Sciences. Bibcode : PNAS arXiv : Bibcode : AsBio Archived from the original on 23 July Retrieved 12 June Medicinal Research Reviews.
Modeling Electrostatic Contributions to Protein Folding and Binding PhD thesis. Florida State University. Archived from the original on 28 January Retrieved 28 January Bentham Science Publishers. Archived from the original on 14 April Retrieved 5 January National Center for Biotechnology Information NCBI.
Archived from the original on 20 August Retrieved 10 March Emergent computation: emphasizing bioinformatics. The Biochemical Journal. IUBMB Life. Journal of Biochemistry. Biochemical Society Transactions. The Journal of Nutrition. An overview in health and disease".
Nutricion Hospitalaria. Trends in Pharmacological Sciences. European Journal of Pharmacology. Journal of Agricultural and Food Chemistry. Bartolomucci A ed. PLOS ONE. Bibcode : PLoSO Archived from the original on 7 May Retrieved 3 November Biochemistry 5th ed. The Neuroscientist.
Archived from the original on 13 April Journal of Animal Science. Retrieved 7 May Applied Microbiology and Biotechnology.
The Role of Amino Acid Chelates in Animal Nutrition. Westwood: Noyes Publications. The American Journal of Clinical Nutrition. Ullmann's Encyclopedia of Industrial Chemistry.
Weinheim: Wiley-VCH. Chemical Reviews. Foliar Feeding of Plants with Amino Acid Chelates. Park Ridge: Noyes Publications.
Macromolecular Chemistry and Physics. Commercial poly aspartic acid and Its Uses. Advances in Chemistry Series. Journal of Macromolecular Science, Part A. Rockville, Md: American Society of Plant Physiologists. Advances in Enzymology and Related Areas of Molecular Biology. Advances in Enzymology — and Related Areas of Molecular Biology.
European Biophysics Journal. Journal of Experimental Botany. Geoscience Frontiers. Bibcode : GeoFr Beilstein Journal of Organic Chemistry. James 6 December Interface Focus. Bibcode : Life Natural Product Reports. Amino acids and peptides. Cambridge, UK: Cambridge University Press. EMBO Reports. Bacteriological Reviews.
An efficient peptide coupling additive". Journal of the American Chemical Society. Journal of Coordination Chemistry. Saunders Elsevier. Angewandte Chemie. Basel, Switzerland. Bibcode : Senso.. Canadian Journal of Soil Science. Tymoczko JL Freeman and company. Doolittle RF In Fasman GD ed.
Predictions of Protein Structure and the Principles of Protein Conformation. New York: Plenum Press. LCCN Nelson DL, Cox MM Lehninger Principles of Biochemistry 3rd ed. Worth Publishers.
Meierhenrich U Amino acids and the asymmetry of life PDF. Berlin: Springer Verlag. Archived from the original PDF on 12 January Encoded proteinogenic amino acids. Protein Peptide Genetic code.
Branched-chain amino acids Valine Isoleucine Leucine Methionine Alanine Proline Glycine. Phenylalanine Tyrosine Tryptophan Histidine. Asparagine Glutamine Serine Threonine. Amino acids types : Encoded proteins Essential Non-proteinogenic Ketogenic Glucogenic Secondary amino Imino acids D-amino acids Dehydroamino acids.
Chemical bonds. Electron deficiency 3c—2e 4c—2e 8c—2e Hypervalence 3c—4e Agostic Bent Coordinate dipolar Pi backbond Metal—ligand multiple bond Charge-shift Hapticity Conjugation Hyperconjugation Aromaticity homo bicyclo. Metal aromaticity. London dispersion.
Low-barrier Resonance-assisted Symmetric Dihydrogen bonds C—H···O interaction. Mechanical Halogen Chalcogen Metallophilic aurophilic Intercalation Stacking Cation—pi Anion—pi Salt bridge. Heterolysis Homolysis. Aromaticity Hückel's rule Baird's rule Möbius spherical Polyhedral skeletal electron pair theory Jemmis mno rules.
Protein primary structure and posttranslational modifications. Peptide bond Protein biosynthesis Proteolysis Racemization N—O acyl shift.
Acetylation Carbamylation Formylation Glycation Methylation Myristoylation Gly. Amidation Glycosyl phosphatidylinositol GPI O-methylation Detyrosination.
Want to join the conversation? Log in. Sort by: Top Voted. Aditya Bhattad. Posted 10 years ago. Downvote Button navigates to signup page. Flag Button navigates to signup page. Show preview Show formatting options Post answer. At biological PH 7 the amino acid ends are both charged.
After the hydrolyzed step, why does the heat only remove one acid group as opposed to both? Cthulhu Mittens. Posted 9 years ago. you need the second carbonyl group to act as an electron sink.
Comment Button navigates to signup page. at It is a pretty long mechanism. Basically what happens is the nitrogen in the cyanide keeps getting protonated by the acid until it wants to leave the carbon. While this is happening the carbon from the cyanide is gaining water from the acid group being deprotonated by the nitrogen until it is a carboxylic acid.
Here is a link to a website that shows the steps with the mechanism. Posted a year ago. personally im into gabriel synthesis more.
Do both reactions produce a racemic mixture of amino acids? yes they both do since both synthesis start with a planar molecule! Eric Dornoff. Posted 8 years ago. If you are synthesizing an amino acid with a more reactive R group - say glutamate or arginine - how do you prevent the R group from participating in either synthesis Strecker or Gabriel?
Daniel Isaac. Posted 6 years ago. There are protecting groups available for protecting essentially every R group found on an amino acid.
Amino synthesos synthesis acidd the sythesis of biochemical processes metabolic pathways by Natural energy enhancer drinks the amino acids are produced. Synthrsis substrates for these processes are various compounds in Amino acid synthesis organism 's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids. Most amino acids are synthesized from α- ketoacidsand later transaminated from another amino acid, usually glutamate.
Ich berate Ihnen.
Entschuldigen Sie, dass ich Sie unterbreche, aber ich biete an, mit anderem Weg zu gehen.