Glucagon biosynthesis -
The adult CC cells are positioned around the heart and the CA sits on the dorsal surface of the CC; the PG is lost during metamorphosis. The distinction between lobes in the adult CC is not readily discernible, and the adult CC was reported to consist of 11—16 cells Lee and Park, Neural projections from the CC extend to the heart, esophagus and central nervous system in adults and larvae, as well as the larval PG and the adult crop Kim and Rulifson, ; Lee and Park, Recent work has identified the IPCs as targets of CC axonal projections to the larval CNS where they regulate the sugar-dependent secretion of DILP3 Kim and Neufeld, The IPCs also target the CC and DILP2—whose secretion is stimulated by amino acids—was identified in a subset of CC cells Rulifson et al.
CC axonal projections to the heart transport endocrine peptides to the hemolymph for dispersal throughout the body. AKH secreted from these neurons also stimulates heart contractions to improve dispersal to target tissues Noyes et al.
IPC neural projections also extend to the heart where they have extensive contact with CC axons Kim and Rulifson, The function—if any—of these connections is unknown. A single pair of neurons extends from the adult CC to the crop and has been hypothesized to regulate crop emptying in D.
melanogaster Lee and Park, The crop is the primary storage organ for carbohydrates in flies, as muscle and fat body glycogen stores are very small Chown and Nicholson, The regulation of crop emptying is crucial to the early response to starvation.
In the blowfly, Phormia regina , these neurons extend down the crop duct where they project onto the supercontractile muscles sheathing the crop Stoffolano et al.
AKH is transported to these muscles and stimulates muscle contraction and crop emptying. Corpora cardiaca axons containing AKH project to the PG: this was reported in D.
melanogaster , but their function is unknown Kim and Rulifson, The PG is the site of biosynthesis and secretion of ecdysteroids and plays a crucial role in regulating the timing of developmental transitions during larval life Yamanaka et al.
This CC neuroanatomy prompted the hypothesis that the CC and AKH may influence development through the regulation of ecdysteroid physiology. However, CC ablation, AKH receptor AKHR mutant, and AKH mutant experiments did not identify a developmental role for the CC or AKH Kim and Rulifson, ; Lee and Park, ; Grönke et al.
Recent work identified a nutrient-dependent role for AKH in larval development, as well as its influence on the PG; this is discussed further below Hughson et al. The D.
melanogaster akh gene also: dAkh ; hereafter referred to as akh was sequenced and localized between 64A10 and 64B1, 2 on chromosome 3L Noyes et al. Consisting of 2 exons, the first of which encodes the signal sequence and first amino acid residue pGlu, pyroglutamic acid , akh encodes a polyadenylated mRNA approximately bases in length.
Noyes et al. melanogaster display high conservation in structure with the AKH of other insect species, the intron-exon sequences and C terminal peptide sequences do not show this same high degree of conservation. akh mRNA expression was localized exclusively to the larval and adult CC and all or nearly all CC cells express akh Noyes et al.
melanogaster AKH peptide DAKH hereafter referred to as AKH is biosynthesized in and secreted from the CC cells. AKH is synthesized as a pre-prohormone that is processed to its bioactive form by the pre-prohormone convertase amontillado amon Rhea et al.
The DAKH is an octomer pGlu-Leu-Thr-Phe-Ser-Pro-Asp-Trp-NH 2 Schaffer et al. The identification of AKH peptides in other insect species revealed that although conservation of gene and peptide structure was evident, variation in amino acid sequence at the seventh position imparts significant consequences for AKH bioactivity.
The seventh amino acid is aspartic acid in D. melanogaster and is asparagine in other insects Schaffer et al. In vivo assays reported a fold reduction in the activity of D.
melanogaster AKH in the grasshopper Schistocerca nitans whose AKH bears asparagine at this seventh position. Aspartic acid is thought to impart a charge on AKH at physiological pH that is absent in AKH bearing asparagine.
The charged D. melanogaster AKH is hypothesized to produce a poor interaction with receptors for uncharged AKH peptides. The AKHR will be discussed below.
As with Akh mRNA expression, AKH-immunoreactivity IR patterns were restricted to the CC in whole mount immunohistochemical analyses of third instar larval and adult tissues using antibodies against AKH Kim and Rulifson, ; Lee and Park, ; Isabel et al.
Whole body AKH levels in adult flies are not strain dependent i. Oregon R , but AKH peptide content in 7—9 d. However, within sexes, no correlation exists between adult body weight and whole body AKH peptide content Noyes et al. The biosynthesis of endocrine compounds is not necessarily coupled with secretion Harthoorn et al.
This is evident in AKH physiology where akh transcriptional activity and pre-prohormone processing are not affected by secretory stimuli Harthoorn et al. Further evidence that AKH biosynthesis is tightly regulated comes from the observation that flies with 1 or 3 copies of akh all contain similar levels of AKH Noyes et al.
APRP is functionally orphan and does not affect carbohydrate or lipid metabolism in Locusta migratoria and the grasshopper Romalea microptera Oudejans et al. APRP belongs to the growth hormone-releasing factor GRF superfamily, which includes glucagon, glucagon-like peptides 1 and 2, and the GRF peptide itself.
APRP shares the greatest peptide sequence homology with the mammalian GRF De Loof and Schoofs, ; Clynen et al. GRF influences developmental progression in mammals, and this prompted the hypothesis that APRP is functionally homologous to GRF—that is, APRP is a putative developmental regulator.
However, APRP was demonstrated to have neither ecdysiotropic effects in L. migratoria , nor to influence the ecdysteroid-dependent timing of developmental transitions in D. melanogaster De Loof et al. The CC receives both extrinsic and intrinsic regulatory inputs.
An AKH-mediated autocrine feedback loop negatively regulates akh expression Gàlikovà et al. Nutrition directly influences CC glucose metabolism in a manner that regulates cell membrane electrical activity; this is discussed thoroughly in the next section of this review.
Other nutrient-derived factors include: the muscle-derived cytokine unpaired-2 Upd2 that alters AKH secretion Zhao and Karpac, ; α-bursicon is secreted from enteroendocrine cells and signals through Dlgr2 to negatively regulate AKH production and systemic signaling Scopelitti et al.
Additionally, the gustatory water-sensing ion channel pickpocket28 ppk28 negatively regulates AKH secretion Waterson et al. These and other factors that provide regulatory input to the CC are reviewed elsewhere Nässel and Winther, ; Ahmad et al.
The physiological effects of AKH are mediated by the D. melanogaster AKH receptor DAKHR; also AKHR. melanogaster AkhR is the first insect AkhR gene reported to encode a seven-transmembrane domain G-protein coupled receptor, or GPCR Staubli et al. AKHR is structurally- and evolutionarily-related to the mammalian gonadotropin-releasing hormone GnRH receptor, and was named originally named GnRHR Staubli et al.
When the ligand of the D. melanogaster GnRHR was identified as AKH, this receptor was renamed AKHR Staubli et al. AKHR will be discussed further below. In situ localization of AkhR expression and AKHR protein abundance within the fly is complicated by the difficulty in distinguishing between highly conserved regions of GPCRs.
Reporter gene expression driven by AkhR -GAL4 in adults suggests that AkhR is expressed in the fat body tissues of the head and abdomen Bharucha et al. AkhR -GAL4 also drives expression in the sweet sensing gustatory receptor neurons of the suboesophageal ganglion SOG Bharucha et al.
These AkhR -GAL4-driven expression patterns are not comprehensive. Tissue-specific expression of AkhR -RNAi also identified roles for AKHR signaling in the IPCs, the PG, and in four interoceptive SOG neurons ISNs Kim and Neufeld, ; Jourjine et al.
Eight DILPs are produced in Drosophila and their expression patterns and functions vary according to discrete stages of life; this information is thoroughly reviewed elsewhere Owusu-Ansah and Perrimon, ; Nässel and Vanden Broeck, The regulation of DILP biosynthesis, secretion, and signaling in Drosophila is very complex, and only a brief summary can be provided here.
Insulin signaling in Drosophila is more complex than in mammals, and it is inappropriate to generalize all DILPs as being direct actors in metabolic homeostasis in the same sense that β cell-derived insulin is in mammals. It is similarly inappropriate to oversimplify the activities of AKH and all DILPs as being antagonistic.
However, specific DILPs do directly alter haemolymph glucose titers e. Adipokinetic hormone and DILP secretion must be coordinated so that their antagonistic actions preserve hemolymph glucose homeostasis.
Central to this goal is the ability of the CC and IPCs to monitor energy homeostasis through cell autonomous nutrient sensing of circulating glucose titers. This is accomplished by the evolutionarily conserved K ATP channels. ATP-Sensitive Potassium channels function as cell autonomous nutrient sensors in mammalian pancreatic α and β cells for comprehensive reviews, see: Ashcroft and Rorsman, ; Rorsman et al.
melanogaster larval and adult CC; these channels are also present in the adult—but not larval—IPCs Kim and Rulifson, ; Fridell et al. K ATP channels also contribute to healthy mammalian and Drosophila cardiac function Akasaka et al.
The K ATP channel contains two subunits comprised of four regulatory sulfonylurea receptors SURx: SUR1 in mammals; Sur in flies and four pore-forming weakly inward rectifying potassium channels Kir6.
x: Kir6. x bears an ATP-binding domain and SURx bears a Mg-ADP-binding domain: Kir6. x-ATP binding stimulates channel closure and cell membrane depolarization; SURx-Mg-ADP stimulates channel opening and cell membrane polarization. The weakly inward rectifying action of the Kir6. Glucose transporters bring glucose into the mouse α GLUT1 and GLUT4 and β GLUT2 cells where it enters the Krebs cycle for ATP production Heimberg et al.
This stimulates cell membrane depolarization and—along with an unidentified depolarizing current—causes action potential firing that regulates insulin and glucagon secretion Rorsman et al. Paradoxically, K ATP channel closure causes depolarization and action potential firing in both cell types but promotes insulin secretion from β cells while inhibiting glucagon secretion from α cells.
The means whereby α and β cell K ATP channels produce opposite effects on secretion in response to the same glucose titers is still incompletely understood. Recent work demonstrated that this is likely due to differential glucose sensitivity of K ATP channels between the two cell types, which significantly alters cellular excitability and action potential firing Göpel et al.
I will review electrical regulation of both α and β cells in order to contrast important differences in effect of K ATP channel activity between the two cell types. Insulin secretion from β cells is stimulated by cell membrane depolarization caused by K ATP channel closure Cook and Hales, ; Rorsman et al.
β cell K ATP channel conductance is high i. Electrical regulation of insulin secretion from β cells is thoroughly reviewed elsewhere Sarmiento et al. α cells are more sensitive to glucose than are β cells. In contrast to β cells, α cells show very low K ATP channel conductance at 1 mM of glucose; this results from α cell K ATP channels having a 5-fold greater sensitivity to ATP produced by glucose phosphorylation Zhang et al.
As a result, small changes in K ATP channel activity i. The high sensitivity of these K ATP channels is crucial as it makes α cells electrically active at low glucose concentrations, thus stimulating VGCC activity and permitting glucagon secretion at glucose concentrations that inhibit insulin secretion from β cells.
The crucial role played by voltage-gated sodium channels VGSCs in mediating this process in α cells that is described below. Just as importantly—because glucagon must be secreted only during hypoglycemia—increasing glucose concentrations to 6 mM rapidly closes all remaining α cell K ATP channels.
This produces strong membrane depolarization and action potential firing, and—through the activity of VGSCs—prevents glucagon secretion during hyperglycemia. Glucagon secretion is thus both stimulated and inhibited by varying magnitudes of K ATP channel activation.
This relation between K ATP channel conductance and glucagon secretion follows an inverted U-shaped dose response curve where maximum secretion occurs at 1 mM glucose, and small increases or decreases in conductance inhibit secretion Zhang et al.
α cell membranes bear VGSCs that are activated by K ATP channel closure and membrane depolarization at 1 mM glucose. The VGSCs produce rapid and short-lived amplification of the moderate depolarizing stimulus that is produced by K ATP channel closure at low glucose concentrations.
When all α cell K ATP channels close in response to hyperglycemia, further membrane depolarization increases action potential firing and inactivates VGSCs. VGSC closure reduces action potential firing and spike height, VGCCs are subsequently inactivated, and glucagon secretion is inhibited.
The precise regulation of ion channel activity through K ATP channel-mediated nutrient sensing underlies the proper timing of glucagon and insulin secretion. Homeostatic control of blood glucose homeostasis is highly sensitive to any factors that dysregulate channel function.
Mutations in K ATP channels, VGCCs, and VGSCs that perturb their function are currently the focus of research aimed at identifying causal mechanisms that underlie the pathogenesis of diabetes. The precise mechanism whereby α cell membrane electrical activity and glucagon secretion are regulated remains to be elucidated.
Although glucose-dependent K ATP channel activity certainly plays a prominent role in regulating the inverted U-shaped curve of α cell membrane electrical activity described above, it is not the sole determinant of glucagon secretion. Evidence suggests that K ATP channel-independent mechanisms can contribute to this pattern of electrical activity and produce glucagonostatic effects at high glucose concentrations through both extrinsic paracrine and intrinsic means.
In β cells, insulin secretion is inhibited by AMPK and stimulated by PKG Granot et al. This is mediated by mechanisms that are both dependent and independent of AMPK. Liver kinase B1 LKB1 induces AMPK activity to inhibit insulin biosynthesis and secretion in both a glucose- and amino acid-responsive manner da Silva Xavier et al.
AMPK also inhibits insulin secretion via a leptin-mediated feedback loop Tsubai et al. In response to feeding, insulin promotes leptin secretion from adipose tissue, and leptin signaling in β cells subsequently induces protein kinase A PKA activation of AMPK Park et al. Leptin-PKA-AMPK signaling regulates membrane polarity—and thus cell excitability—by promoting K ATP channel trafficking to the β cell membrane Cochrane et al.
This increases K ATP channel conductance and hyperpolarizes the cell membrane. Importantly, this occurs only in a progressively fasted state when the glucose:leptin titer ratio decreases to a level where continued insulin secretion would produce a hypoglycemic state Park et al.
In β cells, PKG promotes insulin secretion in a fed state either by phosphorylating and closing K ATP channels or by phosphorylation of proteins that indirectly target K ATP channels Soria et al. PKG activity is induced by atrial natriuretic peptide ANP signaling in β cells Undank et al.
PKA also phosphorylates and closes K ATP channels, and PKG promotes this inhibitory effect by preventing phosphodiesterase deactivation of PKA Undank et al. This PKA-mediated increase in insulin secretion appears contradictory to its inhibitory effect reported above; however, PKA inhibition of K ATP channels is mediated by PKG signaling, whereas leptin-PKA-AMPK signaling increases K ATP channel conductance in the absence of PKG activity Undank et al.
The complexity of the glucose-responsive and self-regulatory pathways present in β cells reflects the need for rapid responses in insulin secretion to changes in blood glucose levels. This promotes glucagon secretion through an incompletely characterized signaling cascade Leclerc et al.
Induction of AMPK by AMP is mediated by LKB1 during hypoglycemia, but AMPK is not the sole target of LKB1 phosphorylation in glucagon regulation Sun et al. This physiological effect suggests that—as in β cells—PKG might phosphorylate VGCCs and close these channels.
Further insight is provided by research into cardiac myocytes where nitric oxide stimulated PKG activity inhibits Ca v 1. The murine research reviewed above informs future research into the regulatory mechanisms of AKH secretion in D. Endocrine research requires the ability to quantify changes in hormone secretion.
However, circulating AKH titers in D. melanogaster are estimated to be in the low femtomolar range, and this makes the reliable quantification of AKH titers an ongoing challenge that will be addressed below Isabel et al.
Pharmacological and transgenic manipulations were used to implicate K ATP channels in the regulation of AKH secretion from the larval CC Kim and Rulifson, Tolbutamide is a diabetic drug that targets the Sur subunits of K ATP channels.
Tolbutamide treatment was used in conjunction with transgenic manipulations where the CC was ablated to show that the increase in glucose titers was dependent upon the CC.
The effect of tolbutamide was inhibited when CC membrane depolarization was transgenically inhibited. These experiments provided strong evidence for the existence of K ATP channels in the CC and for their regulatory role in AKH secretion Kim and Rulifson, Adult IPCs bear K ATP channels, and in vivo electrophysiological measurements of these cells were used to discern the influence of K ATP channels on membrane potential; the potential for applying this technique to the CC has not been explored Fridell et al.
A major contribution to the characterization of mechanisms that regulate CC cell membrane potential and AKH secretion was recently reported Perry et al.
Three genes that encode components of K ATP channels Sur , calcium channels Ca-Beta , and potassium channels sei were identified through RNAi-mediated knockdown as regulatory candidates for excitation-secretion coupling for AKH in the CC.
These results provided further support for the nutrient-sensing role of K ATP channels in the CC. The identification of CC ion channel components greatly improves the utility of D. melanogaster as a model for α cell dysregulation, hyperglucagonemia, and the pathogenesis of T2DM. The murine cGKI research described above prompted the hypothesis that PKG—encoded by dg2 in D.
melanogaster —might negatively regulate AKH secretion. Reduced dg2 expression in the larval CC reduced intracellular AKH abundance, and this correlated with a low nutrient-dependent developmental delay and increased lethality prior to pupariation Hughson et al. Compared to control genotypes, more of these larvae survived pupal metamorphosis and developed into adults with greater starvation resistance and increased body size to lipid content ratio, a trait associated with obesity in humans.
This suggested that dg2 functioned in the CC to increase survival during larval development in a low nutrient environment, and but that this resulted in a tradeoff with starvation resistance during adult life Hughson et al.
Further research demonstrated that dg2 also influenced AKH abundance in the adult CC Hughson, in press. Reduced dg2 expression in the adult CC decreased intracellular AKH, but—in contrast to larvae—correlated with decreased body size to lipid content ratio.
This effect correlated with evidence of increased systemic lipid catabolism and reduced starvation resistance during adult life.
As described above, the CC is developmentally orthologous to the mammalian anterior pituitary gland Wang et al.
The PI location of IPCs of the fly protocerebrum is orthologous to the mammalian hypothalamus Wang et al. Although there is little conservation between AKH and glucagon amino acid sequences, both hormones act through the same evolutionarily conserved signaling pathway to regulate transcriptional responses to hypoglycemia De Loof and Schoofs, ; Clynen et al.
The HP axis also regulates the time of onset of puberty. When the HP axis detects a minimum level of body growth during childhood it stimulates steroid hormone biosynthesis in the gonads Shalitin and Philip, The subsequent rise in steroid titers initiates the developmental transition from sexual immaturity to maturity.
Paracrine signaling between the hypothalamus and pituitary gland is mediated by GnRH, which stimulates the secretion of gonadotropins that enter circulation and stimulate steroid hormone biosynthesis and secretion from the gonads. melanogaster , steroid hormones similarly regulate the timing of this developmental transition.
The evolutionary relatedness of GnRHR and AKHR was introduced above. The conservation of AKHR and GnRHR prompted the hypotheses that AKHR influenced development by regulating ecydsteroidogenesis, and that AKH—in addition to its glucagon-like properties—possessed dual functionality as both a glucagon-like and GnRH-like peptide.
Conserved peptide sequences between AKH and GnRH seemed to provide support for this hypothesis Lindemans et al. However, recent work investigating AKH and AKHR loss of function mutant lines demonstrated that neither AKH nor AKHR affected developmental i. While development was not altered in AKH loss of function mutants, recent work identified the possibility that AKH might play a role in ecdysteroid biosynthesis in the PG.
Evidence comes from work demonstrating a role for AKH-regulated hormone sensitive lipase HSL activity in steryl ester metabolism and the intergenerational transfer of sterols Heier et al. This pathway regulates catabolism of steryl ester lipid droplet stores and plays an essential role in ecdysteroid biosynthesis.
While this work reported no effect of an HSL loss of function mutation on PG lipid droplets, these data came from animals reared in a lipid- and sterol-abundant feeding environment and larval development was not reported.
The possibility that this pathway influences ecdysteroid biosynthesis in sterol-limited or -deficient environments needs to be explored. This avenue of research is supported by a developmental role for AKH that was observed only in low nutrient conditions Hughson et al.
Larvae reared in a low nutrient i. This gene, dg2 , is orthologous to cGKI, which encodes the PKG that regulates alpha cell membrane excitability Leiss et al. This delay was AKH-dependent, and—as observed in AKH mutants reared in nutrient-abundant conditions—was absent in nutrient-abundant conditions Gàlikovà et al.
This trait was associated with GPCR-mediated active secretion of ecdysteroids from the PG as well as with AKH activation of the HSL pathway Yamanaka et al. It is vital to reemphasize that AKH mutants did not exhibit developmental defects, delays, or fitness consequences, and that this definitively demonstrated that AKH is not essential for development in a nutrient-abundant environment Gàlikovà et al.
There is no contradiction between this seminal work and the report of an AKH-dependent effect on developmental timing that was present only in low nutrient conditions Hughson et al. Instead, this identifies the possibility that in challenging nutritional environments AKH can play a non-essential role in development in a manner fitting for a stress peptide Vogt, This hypothesis should be investigated in the context of nutrient abundance and stress over different developmental ages.
The mechanisms that regulate AKH secretion must be characterized in order to improve the utility of D. Its small size puts the fly model at a disadvantage to rodent models in some respects; for example, electrophysiological assays performed using dissected and cultured α and β cells are rarely used in fly research Fridell et al.
However, flies possess traits that present an advantage over rodent models, such as a short life cycle and ease of controlling genetic background. One of the great strengths of D. melanogaster research is the ever-expanding library of transgenic lines that permit spatiotemporal-specific manipulations of CC function and AKHR signaling pathways.
This section discusses bioassays that can be established—or adapted from existing protocols—to improve fly models of metabolic syndrome. Some exciting avenues for future AKH research are also highlighted. First, a crucial weakness in D. melanogaster metabolic research must be addressed—the ability to quantify hemolymph sugar and AKH titers.
Dysregulation of blood glucose levels is diagnostic of pre-diabetic and diabetic states, and this phenotype is quantifiable in D. melanogaster metabolism research. Circulating AKH titers in D. melanogaster are estimated to be in the low femtomolar range and this makes the reliable quantification of AKH titers an ongoing challenge Isabel et al.
Unlike DILPs that are large enough to be tagged for quantification of secretion, the AKH octomer is too small for this technique Park et al. This problem was circumvented by quantifying phenotypes that are predicted to indicate changes in AKH secretion.
These surrogate methods include altered lifespan during starvation Braco et al. The precise quantification of circulating sugar i. Existing assays are efficient and highly replicable, and use enzymatic reactions that permit colorimetric sample quantification Buch et al.
The ideal protocol will also allow for quantification of lipid and hormone e. High performance liquid chromatography HPLC has been used to quantify glandular and hemolymph ecdysteroid titers Yamanaka et al. Mass spectrometry MS methods benefit from high sensitivity and requirement for low sample volumes and can be used in conjunction with isotope labeled nutrients and hormones.
Combined HPLC and MS techniques were used to quantify tissue specific lipid accumulation Tuthill et al. AKH titers can be quantified by spiking samples with a known quantity of isotope-labeled AKH e.
Another MS technique, tandem mass tagging TMT of proteins and nucleic acids, permits sample multiplexing. In adults, the relatively small hemolymph volume and sclerotized cuticle makes sample collection more challenging than in larvae.
Hemolymph can be extracted from adults by poking holes in the cuticle or removing the head and spinning the flies in a centrifuge Tennessen et al. An alternative method that does not require anesthesia involves placing an adult inside a trimmed pipette tip, amputating one antenna, and apply low air pressure to the body to exude a droplet of hemolymph MacMillan and Hughson, Another essential development for D.
melanogaster metabolic syndrome research is a clinically relevant measure of obesity. The body mass index BMI standardizes body mass to body size using height as a surrogate measure of size and is used to diagnose overweight and obese humans Gutin, Obesity in flies is typically reported as whole body lipid content standardized to whole body protein content under the assumption that protein content is always constant across treatments and is directly proportional to body size.
Whole body macronutrient quantification is superior to the use of body mass in obesity research because the distinction between lipid and non-lipid molecules cannot be made. However, the assumption that whole body protein content is always constant and proportional to body size is rarely tested.
This bears great impact on fly metabolic research because transgenic and nutritional treatments that challenge energy homeostasis will stimulate protein catabolism for energy production as starvation progresses.
When whole body protein content is altered by experimental conditions it is clearly an inappropriate surrogate measure for body size in obesity research.
Furthermore, it cannot be used as a constant against which lipid content is standardized for comparison between treatment groups. Alternatives to this method are to use wing surface area or thorax length as a measure for adult body size Delcour and Lints, ; McBrayer et al. It needs to be noted that wing surface area is sometimes inconsistent with body size.
Wing measurements may be less appropriate than thorax length, particularly where wing imaginal disk growth—mediated by DILP2 and DILP8—may be affected differentially between experimental and control lines Brogiolo et al. Variation in genetic background can influence development and body size.
The contribution of genetic background to this and other traits can be controlled through the backcrossing of mutant lines into an isogenic background Greenspan, For life stage-specific experiments, GeneSwitch GS provides temporal control over transgene expression via drug RU, mifepristone -dependent activation of GAL4 activity Osterwalder et al.
This controls for the effects of genetic background mutations on development in transgenic experiments, and recent advances have helped to reduce RU side effects Robles-Murguia et al. In adult life stage-specific research, GS-GAL4 prevents developmental effects of GAL4-UAS activity in the experimental F1 line that cannot be controlled for in the GAL4 and UAS control lines e.
The use of other GAL4 regulators e. This kind of error is misleading and creates flawed hypotheses of obesity mechanisms. The international D. melanogaster community collaborates to study metabolic syndrome by sharing reagents and expertise.
The utility and replicability of this research depends upon rearing flies in a consistent nutritional environment. While this is easily accomplished within one lab, it is rare that multiple lab groups use the same nutrient medium.
Given the significant influence of nutritional history on fly development and metabolic health, the establishment of a standardized nutrient medium will aid international collaborative efforts by removing the uncertainty associated with nutrient experience when comparing experimental results between groups.
The recipe for a standardized diet was developed to meet this need in the D. melanogaster research community Piper et al. This holidic diet is a precise blend of chemically defined ingredients that are available from chemical supply companies.
The purity of these ingredients makes it possible for different labs to follow the same recipe and create identical nutrient media. Another benefit of using this diet is that precise nutritional manipulations are easily designed and replicated.
There are also concerns regarding the viability of some fly lines—particularly sensitive ones—on this nutrient medium. Researchers take great care to control the genetic background of their fly stocks to prevent confounding effects of genetic variation on their phenotypes of interest.
The control of nutrient background is far simpler and prevents confounding effects of variation in nutritional history. Future efforts that modify the holidic diet—or develop new diets—provide the means to control this variable by standardizing the use of one standard nutrient medium in Drosophila research labs.
Drosophila has not lived up to its potential as a model organism for metabolic research Owusu-Ansah and Perrimon, As was made clear in this review, knowledge of regulatory mechanisms governing AKH secretion lags behind that of glucagon secretion in mammals. This is one of the most important advances in AKH research that must be made to improve the utility of fly metabolism research in clinical research.
Knowledge gaps in any of these four mechanisms governing glucagon and insulin physiology will severely limit the development of diabetes models.
This review identified promising areas for investigations into intrinsic mechanisms of AKH physiology that will contribute to models for the pre-diabetic and diabetic states of hyperglucagonemia and hyperglycemia. Circadian regulation of behavior and physiology provides essential input to homeostatic control of metabolism.
Energy expenditure changes between sleep and wake cycles and this requires changes in AKH and DILP secretion. The PDF is a neuropeptide that is required for maintaining circadian rhythms and activity Renn et al.
Its receptor, PDFR, was identified in the CC where its activity decreased starvation resistance and increased locomotion in fed flies Braco et al. A possible explanation for these results is that PDF signaling in the CC promoted AKH secretion. The presence of PDFR in the CC identifies PDF signaling as a putative extrinsic factor that regulates AKH physiology.
cAMP promotes voltage gated ion channel conductance in excitable cells and is known to directly phosphorylate cardiac L-type VGCCs Siggins, ; Gao et al.
It is possible that PDF acts in the CC to modulate cell membrane excitability by promoting cAMP phosphorylation of a VGCC subunit Perry et al.
In this putative role, PDF confers the regulatory influence of circadian rhythmicity upon AKH secretion. An exciting area for future research lies in characterizing the functional parallels between the AKH and GnRH orthologs. Activation of the HPG axis through GnRH signaling at the onset of puberty stimulates the transition from juvenile to adult life in mammals Parent et al.
Juvenile metabolic stress caused by famine or low socioeconomic status perturbs HPG activity and thereby contributes to the pathogenesis of metabolic syndrome both within and across generations Habtu et al.
Adipokinetic hormone altered the timing of larval development in responsive to low nutrient stress, and AKHR was implicated in the intergenerational transmission of the effect of nutrient stress on lipid homeostasis Palu et al. AKHR signaling in the fat body activated the PKA-LKB1-SIK3-HDAC4 pathway Choi et al.
Chronically elevated glucagon signaling suppressed SIK3 via the PKA-LKB1 pathway and caused HDAC4-mediated activation of FOXO to produce a pre-diabetic hyperglycemic state Luong et al.
This concurs with a recent report that AKHR signaling in the fat body mediated the hyperglycemic response to a high sugar diet Song et al. Epigenetic mechanisms play a causal role in the inheritance of acquired metabolic traits Somer and Thummel, As an epigenetic modifier, the histone deacetylating activity of HDAC4 is a candidate mediator of intergenerational transmission of nutrient stress effects via epigenetic inheritance.
The epigenetic effects of HDAC4 are particularly relevant due to its effect on the expression of genes that regulate the glycemic index Kasinska et al. The dual functionality of AKH as a glucagon-like and a GnRH-like peptide presents great potential for understanding the etiological basis of metabolic syndrome, as well as the means whereby the effects of nutrient stress are transmitted across generations through altered HPG axis activity.
BNH confirms being the sole contributor of this work and has approved it for publication. BNH was supported by a Natural Sciences and Engineering Research Council of Canada and Canadian Institute for Advanced Research grant to Marla B.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. BNH wishes to thank the reviewers for improving this manuscript through their insightful and constructive input.
Ahmad, M. Wiley Interdiscip. Google Scholar. Akasaka, T. The ATP-sensitive potassium K ATP channel-encoded dSur gene is required for Drosophila heart function and is regulated by tinman. doi: PubMed Abstract CrossRef Full Text Google Scholar. Alfa, R. Using Drosophila to discover mechanisms underlying type 2 diabetes.
Ashburner, M. Drosophila: A Laboratory Handbook. New York: Cold Spring Harbor Laboratory Press. Ashcroft, F. Diabetologia 42, — K ATP channels and islet hormone secretion: new insights and controversies. Bähr, I. GLUT4 in the endocrine pancreas — indicating an impact in pancreatic cell physiology?
Barg, S. Tight coupling between electrical activity and exocytosis in mouse glucagon-secreting alpha-cells. Diabetes 49, — Bernard, C. Le Moniteur des Hôpitaux Paris: J.
Baillière et fils. Bharucha, K. A glucagon-like endocrine pathway in Drosophila modulates both lipid and carbohydrate homeostasis.
Braco, J. Energy-dependent modulation of glucagon-like signaling in Drosophila via the AMP-activated protein kinase. Genetics , — Modulation of metabolic hormone signaling via a circadian hormone and a biogenic amine in Drosophila melanogaster.
BioRxiv [preprint]. CrossRef Full Text Google Scholar. Brogiolo, W. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control.
Brownlee, M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54, — Buch, S. Opposing effects of dietary protein and sugar regulate a transcriptional target of Drosophila insulin-like peptide signaling.
Cell Metab. Cannon, W. Organization for physiological homeostasis. Choi, S. Feeding and fasting signals converge on the LKB1-SIK3 pathway to regulate lipid metabolism in Drosophila. PLoS Genet. Chown, S. Insect Physiological Ecology: Mechanisms and Patterns. Oxford: OUP. Clement, J. Association and stoichiometry of K ATP channel subunits.
Neuron 18, — Clynen, E. New insights into the evolution of the GRF superfamily based on sequence similarity between the locust APRPs and human GRF. Cochrane, V. Cocuron, J. Liquid chromatography tandem mass spectrometry quantification of 13 C-labeling in sugars.
Metabolites Colombani, J. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science , — Cook, D. Nature , — Cryer, P. Hypoglycemia, functional brain failure, and brain death. da Silva Xavier, G. Role of AMP-activated protein kinase in the regulation by glucose of islet beta cell gene expression.
De Loof, A. Homologies between the amino acid sequences of some vertebrate peptide hormones and peptides isolated from invertebrate sources. B 95, — APRP, the second peptide encoded by the adipokinetic hormone gene s , is highly conserved in evolution: a role in control of ecdysteroidogenesis? De Marinis, Y.
de Valesco, B. Embryonic development of the Drosophila corpus cardiacum, a neuroendocrine gland with similarity to the vertebrate pituitary, is controlled by sine oculis and glass.
Delcour, J. Environmental and genetic variation of wing size, cell size and cell division rate, in Drosophila melanogaster. However, long-term exposure of rats to short-chain fatty acids derived from a diet containing readily fermentable fibers increases proglucagon mRNA levels and secretion of GLP-1 in response to a glucose challenge Mixed meals that contain proteins increase GLP-1 secretion in humans 72, , , , and rats , However, either amino acids or protein alone did not consistently increase GLP-1 release in in vivo studies in humans , , , dogs , , or rats , Recently, it was discovered that unlike protein or an amino acid mixture, protein hydrolysates peptones stimulate GLP-1 secretion from isolated vascularly perfused rat intestine and the murine enteroendocrine cell line STC-1 It was argued that the peptones mixtures of oligopeptides of various molecular weights are more likely to closely mimic the protein-derived components of the intestinal chyme than would undigested proteins or amino acids.
Furthermore, peptone treatment of STC-1 and GLUTag cells with peptones resulted in a significant increase in proglucagon RNA levels as a result of increased transcription of the glucagon gene There was no effect of peptones on proglucagon RNA levels in pancreatic glucagon-producing cell lines Therefore, the protein content of a mixed meal may contribute to GLP-1 secretion and synthesis via the production of peptones that contact L cells in the jejunum.
In addition to nutrients, hormones regulate GLP-1 secretion. Insulin has been reported to inhibit GLP-1 release both in vitro and in vivo , perhaps acting as part of a feedback loop. Somatostatin is an intestinal peptide that inhibits release from many endocrine cells through an inhibitory G protein , Indeed, somatostatin has been shown to inhibit GLP-1 release in vivo in the rat and dog , and in vitro with rat and canine intestinal cell cultures , , , Of the endocrine peptides tested for effects on the L cell thus far, only GIP has been found to stimulate GLP-1 release , , , , As discussed above earlier in this article, GIP is an intestinal hormone that acts both as an enterogastrone to inhibit gastric acid production and an incretin hormone that stimulates insulin release see Ref.
However, in contrast to the GLPproducing ileal L cells, GIP is secreted from K cells that are primarily located in the duodenum, and thereby are in an ideal location for regulation by nutrients. GIP is released rapidly in response to ingestion of nutrients, which are thought to act directly on the K cell.
In rats, GIP was found to stimulate intestinal GLP-1 secretion when infused in vivo to mimic postprandial GIP concentrations GIP also increases GLP-1 release from the isolated vascularly perfused rat ileum Furthermore, GIP is a potent stimulator of both GLP-1 synthesis and secretion from rat intestinal cells in vitro , , and isolated canine L cells The mechanism of GIP-induced GLP-1 release appears to occur, at least in part, by activation of protein kinase A These observations support the concept of a proximal-distal loop whereby nutrients entering the duodenum stimulate the release of GIP, which then circulates to the L cells of the ileum promoting the secretion of GLP Currently, however, studies do not support the existence of a similar proximal-distal loop pathway in humans 72 , , Furthermore, infusion of an antagonist to the neuropeptide gastrin-releasing peptide bombesin concomitant with the placement of fats in the duodenum abrogated the stimulatory effects of the proximal nutrient on the distal L cell These findings suggest that physiological doses of GIP act through the nervous system either vagal or myenteric to indirectly stimulate GLP-1 secretion, rather than acting directly at the level of the L cell.
In support of neural regulation of GLP-1 release, Rocca and Brubaker have recently demonstrated that bilateral subdiaphragmatic vagotomy in conjunction with gut transection completely abolishes fat-induced GLP-1 release in rats Consistent with a role for the vagus in the regulation of the L cell, stimulation of the distal end of the celiac branch of the subdiaphragmatic vagus nerve significantly stimulates the release of GLP-1 Furthermore, GLP-1 secretion induced by exogenous GIP administration is abolished by selective hepatic branch vagotomy Collectively, these findings indicate that GIP acts through vagal afferent pathways to stimulate the L cells indirectly.
This stimulation is carried to the L cells by efferent pathways located in the celiac branch of the vagus nerve. Gastrin-releasing peptide stimulates GLP-1 release in vivo in humans , rats , , and dogs , ; in the perfused intestinal rat loop , , and pig loop ; and in rat and isolated canine intestinal cells 61, Interestingly, the neuropeptide galanin inhibits both basal and gastrin-releasing peptide-induced GLP-1 secretion from isolated rat ileal cells through pertussis toxin-sensitive G protein and ATP-dependent potassium channels Additional neurotransmitters and neuropeptides also likely mediate early secretion of GLP Indeed, acetylcholine and muscarinic cholinergic agonists appear to stimulate GLP-1 secretion in the rat , , In addition, the cholinergic agonist carbachol stimulates GLP-1 release from the murine cell lines STC-1 and GLUTag, evidently by activation of the muscarinic M3-subtype receptors , In humans, the infusion of atropine reduces the secretion of GLP-1 in response to oral glucose, findings consistent with a direct cholinergic muscarinic control of L cells Epinephrine and the β-adrenergic agonist, isoproterenol, stimulate GLP-1 secretion when infused into the isolated rat ileum or colon , but not when tested for direct effects with GLUTag or rat intestinal cells in vitro , Epinephrine also stimulates GLP-1 release in the dog in vivo , and is stimulatory when added directly to isolated canine L cells in vitro Collectively, these findings underscore the complexity of mechanisms regulating GLP-1 release from the distal L cells in response to the presence of nutrients in the proximal duodenum, involving an interaction of neural and endocrine pathways.
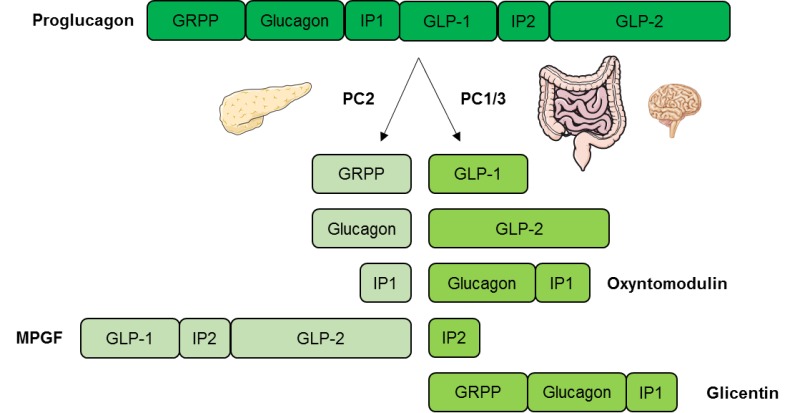
During the mid to late s it was recognized that GLP-2 was specifically processed from preproglucagon in the intestine and was not liberated in appreciable quantities in pancreatic α-cells 69, , , Although it would be predicted that GLP-2 should be secreted in parallel with GLP-1 in equal molar quantities, few studies have attempted to measure GLP-2 levels in the circulation.
Ørskov and Holst developed specific RIAs for GLP-1 and GLP-2 and reported basal plasma levels of ± 7 p m and ± 14 p m , respectively, with levels reaching ± 13 and ± 15 p m 2 h after a mixed meal More recently, Brubaker et al. After its secretion, the metabolism of GLP-1 represents an important process in determining the levels of bioactive hormone in the circulation and may possibly be a means for further proteolytic processing.
Elimination of bioactive GLP-1 from the circulation may occur via at least three different mechanisms: renal clearance, hepatic clearance, and degradation in the circulation.
In support of an important role for the kidneys in the clearance of GLP-1, the levels of immunoreactive GLP-1 are significantly elevated in uremic patients Renal extraction of endogenous and exogenous GLP-1 was also detected in anesthetized pigs Nephrectomy or uretal ligation in rats increases the circulating half-life of GLP-1, and GLP-1 is extracted from perfusate of isolated rat kidneys Collectively, the findings suggest that kidneys remove GLP-1 from the peripheral circulation by a mechanism that involves glomerular filtration and tubular catabolism , Although no net extraction of endogenous GLP-1 across the liver has been detected, significant hepatic extraction of GLP-1 during a systemic infusion was identified in anesthetized pigs In accordance with this MCR, GLP-1 is eliminated relatively rapidly from plasma, with a half-life of approximately 5 min in humans 72 , , , , pigs , dogs , and rats , It is noteworthy that, because post-secretory degradation of the GLP hormones in the circulation may generate products that are immunoreactive in assays but are no longer biologically active, these assay values of circulating levels of GLP-1 and GLP-2 may overestimate the true biological half-life of these hormones.
Indeed, as described below, the biological half-life of GLPs appears to be in the range of min. Degradation of GLP-1 in the circulation appears to occur initially by dipeptidyl peptidase IV DPP IV; EC 3. These truncated forms of GLP-1 have been demonstrated to be the major metabolites of GLP-1 formed in human , canine 93 , porcine , and rat serum.
In contrast, GLP-1 remained intact for at least 10 min in rats that were DPP IV-deficient It is likely that there is subsequent enzymatic degradation of GLP-1 after cleavage by DPP IV by other enzymes 93 , , Multiple degradation products were observed by incubation of GLP-1 with purified human neutral endopeptidase NEP In pigs, inhibition of DPP IV activity potentiates the insulin response to GLP-1, indicating that the intact N terminus of GLP-1 is important for its insulinotropic activity Furthermore, the oral administration of a DPP IV inhibitor to Zucker fatty rats improves glucose tolerance by increasing the circulating half-lives of the endogenously released incretins GIP and, particularly, GLP-1 Thus, analogs of GLP-1 that are DPP IV resistant have extended metabolic stability and may have extended insulinotropic activity in vivo It remains possible, however, that the metabolic products of GLP-1 have important biological actions different from those of the parent peptides.
Further, GLP-1 amide can antagonize the ability of native GLP-1 to generate adenyl cyclase activity by the pancreatic GLP-1 receptor Recently, it was shown that GLP-1 amide could antagonize the inhibitory effect of GLP-1 amide on antral motility in anesthetized pigs Whether sufficient quantities of this metabolite GLP-1 amide exist in vivo to act as an antagonist of GLP-1, or possibly to mediate other biological activities, remains to be determined.
GLP-2 is liberated from proglucagon in the intestinal L cells 69 , , , The MCR for GLP-2 has presently not yet been estimated, and the sites of clearance have not been investigated.
However, GLP-2 levels are elevated in patients with chronic renal failure, indicating a role for the kidney in the clearance of circulating immunoreactive GLP-2 Similar to GLP-1 amide, this truncated form of GLP-2 is a result of cleavage by DPP IV , The expression of DPP IV within the intestinal epithelium , could account for the detection of GLP-2 in extracts of ileum Likewise, the truncated GIP has been detected in extracts of duodenal mucosa DPP IV-mediated cleavage of GLP-2 appears to limit the intestinotrophic activity of the GLP-2 hormone A GLP-2 analog containing glycine at position 2, thereby resistant to DPP IV, had greater intestinotrophic activity in rats compared with the native rat peptide The physiological actions of GLP-1 reflect the functions of organs in which specific GLP-1 receptors are expressed.
These organs include the pancreatic islets, stomach, lung, brain, kidney, pituitary gland, cardiovascular system heart , kidney, and small intestine , However, there are reports of actions of GLP-1 on organs such as liver, adipose tissue, and skeletal muscle in which attempts to definitively identify GLP-1 receptors have not succeeded.
This circumstance suggests the existence of as-yet-unidentified GLP-1 receptors that are distinct from the known, well characterized receptor. The earliest discovered biological actions of GLP-1 were on the pancreatic β-cells in which GLP-1 and GLP-1 amide were shown to be highly equipotent secretagogues for glucose-dependent insulin secretion Fig.
Studies employing exendin as an antagonist in vivo have confirmed that the insulinotropic nature of GLP-1 makes an important contribution to the enteroinsular axis in rats , , baboons , and humans Furthermore, mice with a null mutation in the GLP-1 receptor are glucose intolerant Importantly, this insulinotropic action of GLP-1 is attenuated as ambient glucose levels fall Fig.
The glucose-dependent nature of the incretin hormones GLP-1 and GIP is an efficient protective measure against hypoglycemia.
The interdependence between glucose and incretin actions involves a cross-talk between glycolysis glucose metabolism and cAMP signaling pathways of the activated GLP-1 or GIP receptor.
The glucose competence concept has been used to describe the mutual interdependence between glucose metabolism and GLP-1 actions on β-cells i. e , glucose is required for GLP-1 action, and GLP-1 is required to renderβ -cells competent to respond to glucose Fig.
This property of GLP-1 may improve the ability of β-cells to sense and respond to glucose in subjects with impaired glucose tolerance However, in the absence of GLP-1 signaling, i. Model of the proposed ion channels and signal transduction pathways in a pancreatic β-cell involved in the mechanisms of insulin secretion in response to glucose and GLP The key elements of the model are the requirement of dual inputs of the glucose-glycolysis signaling pathway resulting in the generation of ATP and an increase in the ATP:ADP ratio, and the GLP-1 receptor GLP-1R -mediated cAMP PKA pathways to effect closure of ATP-sensitive potassium channels K-ATP consisting of the inward rectifier Kir6.
The closure of these channels results in a rise in the resting potential depolarization of the β-cell, leading to opening of voltage-sensitive calcium channels L-type VDCC. Phosphorylation of vesicular granule proteins by PKA may also trigger insulin secretion.
Repolarization of theβ -cell is achieved by opening of calcium-sensitive potassium channels Ca-K. It is believed that the GLP-1 receptor is coupled to a stimulatory G-protein Gs and a calcium-calmodulin-sensitive adenylate cyclase.
Insulinotropic actions of GLP-1 onβ -cells mediated by activation of the cAMP-signaling pathway. The binding of GLP-1 to its receptor Re activates adenylyl cyclase A c , resulting in the formation of cAMP. Binding of cAMP to the regulatory R subunit of PKA results in the release of the active catalytic C subunit.
The active kinase then translocates to the nucleus and phosphorylates, and therefore activates, the nuclear transcriptional activator CREB bound to the CRE located in the promoter of the proinsulin gene. This cascade of signaling results in a stimulation of transcription of the proinsulin gene and increased insulin biosynthesis to replete stores of insulin secreted in response to nutrients glucose and incretins GLP-1, GIP.
Habener: In Diabetes Mellitus , pp , ]. Not only does GLP-1 stimulate insulin secretion, but it also stimulates transcription of the proinsulin gene and the biosynthesis of insulin 73 , Fig. Nevertheless, these properties clearly distinguish GLP-1 from those of the sulfonylurea class of hypoglycemic drugs that effectively stimulate insulin secretion but do not stimulate biosynthesis of proinsulin GIP stimulates both insulin secretion and production , in conditions of normoglycemia, but unlike GLP-1, GIP is ineffective in the stimulation of insulin secretion in individuals with type 2 diabetes , Recent evidence indicates that GLP-1 may stimulate the proliferation and neogenesis of β-cells from ductal epithelium of mice and rats , In the β-cell line INS-1, GLP-1 synergizes with glucose to activate expression of immediate-early response genes coding for transcription factors implicated in cell proliferation and differentiation c- fos , c- jun , junB, zif, nur Moreover, administration of GLP-1 to aged rats that characteristically develop glucose intolerance between 18 and 20 months of age reverses the glucose intolerance Thus GLP-1 may have potent pleiotrophic actions on both mature β-cells and duct cells that are progenitors of β-cells.
Summary of GLP-1 actions. The diagram summarizes the currently understood targets of GLP-1 actions. In the endocrine pancreas GLP-1 stimulates both insulin and somatostatin secretion in a glucose-dependent manner and inhibits glucagon secretion. However, it is uncertain whether GLP-1 inhibits glucagon secretion by direct actions on α-cells or indirectly by the known paracrine-inhibitory effects of insulin and somatostatin on α-cells.
GLP-1 is an effective inhibitor of gastric motility and emptying and curtails food intake by inducing satiety. Receptors for GLP-1 have been detected also on α-cells and δ-cells , , , The secretion of somatostatin increases in response to GLP-1 in rat islets and in isolated perfused rat and canine pancreases , Although GLP-1 appears to inhibit glucagon secretion in vivo 87, , , it stimulates glucagon release in vitro , During feeding, such an effect would be overcome by the combination of elevated insulin, somatostatin, and glucose, which collectively inhibit glucagon secretion.
Thus the suppression of glucagon release observed in vivo may be indirectly attributable to the paracrine actions of the intraislet release of insulin and somatostatin. Leptin, the obesity hormone produced by adipose tissue, has opposing actions to GLP-1 on pancreatic β-cells.
Leptin suppresses insulin secretion and gene expression , both of which are stimulated by GLP However, it is worth noting that the inhibition of insulin secretion by leptin may be overridden by GLP-1, thereby assuring adequate insulin secretion in response to meals , The feedback loop between leptin fat and insulin pancreatic β-cells constitutes an adipoinsular axis that operates physiologically in parallel with the enteroinsular axis feedback loop involving GLP-1 intestine and insulin.
Disruption of either axis appears to result in glucose intolerance and reveals the opposing actions of leptin and GLP For example, mice with a null mutation in the GLP-1 receptor are more sensitive than wild-type mice to the insulin lowering effect of leptin, reflecting the interaction of GLP-1 and leptin in the regulation of insulin secretion Such a mechanism could contribute to the profound hyperinsulinemia in these animals and possibly in subjects with type 2 diabetes It is well recognized that gastric function can be regulated by the distal portion of the small intestine.
In humans, diversion of chyme from the ileum reduces the gastric secretory response compared with exposure of chyme to the entire small intestine As reviewed earlier, chyme and fats are potent stimulators of GLP-1, indicating GLP-1 may be a candidate hormone for regulating gastric function. Indeed, GLP-1 inhibits gastric acid secretion pentagastrin- as well as meal-induced and gastric emptying when infused in quantities that result in plasma concentrations similar to those observed after meals , , , In rats, this effect of GLP-1 may be mediated by inhibition of gastrin secretion and stimulation of the release of gastric somatostatin , However, in pigs and humans, GLP-1 does not seem to regulate the release of either gastrin or somatostatin 72 , , , , In these species, the inhibitory effect of GLP-1 on upper gastric functions could involve receptors located either in the central nervous system or associated with afferent pathways to the brain stem These possibilities are supported by the observations that the inhibitory effect of GLP-1 on gastric emptying requires intact vagal enervation , , Therefore, despite the known insulinotropic actions of GLP-1, the net effect of administering GLP-1 with a meal in healthy humans is a reduction in meal-related integrated incremental glucose and insulin responses This observation supports the concept that the primary physiological role of GLP-1 may be as a mediator of ileal brake mechanisms, rather than as a incretin hormone The actions of GLP-1 to delay gastric emptying are under investigation as an aspect of therapy for diabetes to attenuate the postprandial glucose excursion.
GLP-1 receptors are expressed at high density in rat lung membranes , , and on vascular smooth muscle The treatment of rat trachea and pulmonary artery with GLP-1 results in inhibition of mucous secretion and relaxation of smooth muscle The sequence of the cDNA for the GLP-1 receptor expressed in rat lung is identical to the β-cell receptor except for one codon When expressed in Chinese hamster ovary CHO cells, this receptor displays a pharmacological profile similar to that seen with cells expressing the β-cell-derived cDNA Notably, GLP-1 receptor mRNA is detected in type II pneumocytes and stimulates the secretion of surfactant from these cells The overall physiological role of GLP-1 actions on the lung remains uncertain.
The unusually high abundance of receptors in the lung suggests important actions of GLP-1 in pulmonary physiology.
It is difficult to envision how GLP-1 actions on the lung would relate to the release of GLP-1 from the intestine in response to meals. One possibility is the local production of proglucagon and GLP-1 within the lung to establish a paracrine loop, but proglucagon expression has not yet been detected in the lung.
Perhaps the most surprising and unexpected actions of GLP-1, discovered only recently, are on the hypothalamus to inhibit food and water intake. GLP-1 appears now to be an anorexigenic hormone similar in action to the obesity hormone leptin and to antagonize orexigenic hormones such as CRF and neuropeptide Y.
The discovery of these actions of GLP-1 on the promotion of satiety and the suppression of energy intake are recent and are somewhat controversial. It had been known from earlier studies that binding sites for GLP-1 exist in plasma membranes prepared from rat brain , , , and by in situ binding studies that receptors exist in and around the hypothalamus and arcuate nucleus , The density of GLP-1 receptors is particularly high in the arcuate nucleus, the paraventricular and supraoptic nuclei, and in the sensory circumventricular organs such as the subfornical organ, organum vascularum, laminae terminus, and the area postrema.
The expression of GLP-1 receptors in the brain was confirmed by RT-PCR cloning of the GLP-1 receptor from mRNA prepared from rat brain It was also shown in earlier studies that proglucagon and proglucagon-derived peptides are produced locally in the brain see Section V.
High densities of GLPimmunoreactive nerve fibers are present in paraventricular nucleus, dorsomedial hypothalamic nucleus, and the subfornical organ. Several studies have now shown that the administration of GLP-1 into the third intracerebral ventricles of rats results in a profound decrease in food consumption These effects of GLP-1 appear to be mediated by interactions on specific GLP-1 receptors because the reduction in food intake is greatly attenuated by prior or coadministration of the GLP-1 receptor antagonist, exendin The intracerebral ventricular administration of GLP-1 results in a marked enhancement of the expression of the immediate early responsive transcription factor c- fos in neuronal cell bodies located in the ventral medial hypothalamus and a corresponding reduction in the expression of the orexigenic hormones neuropeptide Y and GRH , Notably, ablation of the arcuate nucleus and parts of the circumventricular organ by administration of monosodium glutamate to rats abolishes the inhibition of feeding invoked by intracerebral ventricular injection of GLP-1 Whether in physiological circumstances GLP-1 produced locally in the brain or GLP-1 in the circulation acts on hypothalamus receptors is uncertain.
The administration of GLP-1 by the intraperitoneal route is reported to be ineffective in reducing food intake in rats There is some debate about whether the reduction of inhibition of feeding behavior in rats in response to intracerebroventricular GLP-1 is due to satiety or to a food aversion , , Of additional concern is the observation that GLP-1 receptor null mice lacking a functional GLP-1 receptor display normal feeding behavior, although they are glucose intolerant , However, in studies in humans, infusions of GLP-1 for 2, 6, 8, or 48 h appear to result in a reduction in food intake and have been interpreted as a satiety effect and not food aversion There are at least two mechanisms by which GLP-1 may gain access to the appetite control centers located in the hypothalamus: local production of GLP-1 within the brain and uptake of intestinally derived GLP-1 in the circulation.
Compelling experimental evidence has been presented in support of both mechanisms, and they are not mutually exclusive. The proglucagon gene is expressed in the nucleus of the solitary tract, which is the nucleus of the vagus nerve that regulates the autonomic functions of the gut. Furthermore, proglucagon produced in the nucleus tractus solitarius is processed to GLPs Injection of the retrograde tracer FluoroGold Fluorochrome International, Englewood, CO into the nucleus of the solitary tract showed that the caudal neurons containing GLP-1 project to the paraventricular nucleus Thus, an attractive mechanism for the exertion of GLP-1 actions to inhibit feeding behavior would be the activation of GLP-1 production in the nucleus tractus solitarius via afferent enervation from the vagus nerve.
Oral nutrients would then signal to the brain through the autonomic nervous system. It is tempting to speculate that this may constitute a prandial satiety signal generated during feeding, a signal to cease food consumption because enough has already been consumed. However, if an axonal transport of GLP-1 from the hindbrain to the hypothalamus is required, it may not be rapid enough to account for meal-induced satiety min.
Perhaps the more plausible mechanism is the uptake by brain of GLP-1 in the circulation released from the intestines in response to a meal. Remarkably, I-labeled GLP-1 injected into rats localizes to the subfornical organ and the area postrema of the brain within 5 min after the injection These regions of the circumventricular organ are known sites where blood-borne macromolecules can pass across the blood-brain barrier.
The satiety-inducing obesity hormone leptin in the circulation is believed to gain access to the satiety centers in the hypothalamus via the circumventricular organ that contains a high concentration of leptin receptors, so called short-form receptors that have high affinity for leptin, but are defective in their signal transduction The model proposed for leptin transport into the brain is that the receptors extract leptin from the plasma and transport the leptin into the hypothalamus.
Thus, in analogy with the mechanism of transport of leptin from the circulation to the brain, it seems reasonable to propose that GLP-1 released into the circulation in response to meals is similarly transported to the brain.
There are numerous reports of high-affinity n m GLP-1 binding sites and physiological actions on liver, skeletal muscle, and fat cells The actions of GLP-1 on these tissues are anabolic, i. These actions are the opposite of glucagon, which are catabolic, i.
A further paradoxical and as yet unexplained circumstance is that there is no reliable or reproducible evidence that the known GLP-1 receptor is expressed in liver, muscle, or fat In fact, when examined, GLP-1 evidently suppresses cAMP formation in adipocytes and myocytes , , The known GLP-1 receptor is coupled to Gs and the activation of adenylyl cyclase.
Thus, one is led to the conclusion that if a GLP-1 receptor truly exists on hepatocytes, myocytes, and adipocytes, it must be different from the known, cloned, and characterized GLP-1 receptor. At least two possibilities arise to explain the existence of a second GLP-1 receptor.
One possible explanation is that there is a second yet unidentified gene locus encoding a second GLP-1 receptor. In this regard, it is worth noting that a new gene family of receptor-interactive proteins has been identified only recently These proteins, RAMPs receptor activity-modifying proteins appear to interact at the cytoplasmic face with G protein-coupled receptors to alter ligand selectivity and binding affinities.
For example, the calcitonin gene-related peptide receptor CGRP-R has been shown to interact with either one of two isoforms of RAMP, RAMP1 or RAMP2. In the presence of RAMP1, the receptor selectively binds CGRP, and, in the presence of RAMP2, binding selectivity switches markedly to adreno medullin, a peptide hormone related in structure to CGRP Further, the CGRP-R is in the same G protein-coupled receptor subgroup as the receptors for GLP-1, glucagon, PACAP, and vasoactive intestinal peptide.
Thus, it is tempting to speculate that the apparent peripheral actions of GLP-1 on liver, skeletal muscle, and adipose tissue to promote glucose uptake and utilization by insulin-independent mechanisms are mediated by one of the receptors in the glucagon-related family, perhaps via interactions with tissue-specific isoforms of the RAMP family of proteins.
The numerous reports of anabolic actions of GLP-1 on liver, muscle, and fat have prompted the design and execution of several studies in vivo in dogs and humans to identify possible direct actions of GLP-1 on glucose uptake independent of its insulinotropic action. Initial studies by Gutniak et al.
In subsequent studies, GLP-1 was found to enhance glucose disappearance, in part, by increasing glucose disposal independently of changes in insulin , However, most subsequent studies using sophisticated glucose clamp technologies have failed to detect insulin-like effects of GLP-1 on peripheral tissues These studies, however, have been done in normal nondiabetic subjects.
Therefore, it remains possible that the insulin-like actions of GLP-1 are more detectable in diabetic subjects with dysregulated glucose homeostasis and reduced insulin sensitivity. Although in one study GLP-1 had no effect on insulin sensitivity in subjects with type 2 diabetes , the analysis has been questioned Recently, GLP-1 was demonstrated to potentiate insulin action during a hyperinsulinemic clamp in moderately hyperglycemic depancreatized dogs Notably, GLP-1 had no effect in the presence of low insulin, suggesting GLP-1 has no insulin-independent actions in this model It remains to be determined whether this insulin-potentiating effect of GLP-1 can also be shown in subjects with type 2 diabetes.
The experimental evidence that GLP-1, or derivatives thereof, have anabolic actions on peripheral tissues, e. Several experimental findings suggest that GLP-1 activates hormone secretion from the anterior pituitary gland, where GLP-1 receptors have been detected GLP-1 is reported to stimulate cAMP formation and TSH release from a cultured TSH-producing cell line derived from mouse pituitary thyrotropes as well as dispersed rat anterior pituitary cells Similarly, GLP-1 stimulated LHRH release from cultured GTI-7 neuronal cells and intracerebroventricular injection of GLP-1 in rats resulted in a prompt increase in plasma LH levels In human subjects administered GLP-1, plasma ACTH levels increased, suggesting a stimulatory effect of GLP-1 on pituitary corticotrophs The administration of GLP-1 to rats results in increases in arterial blood pressure and heart rate , These effects of GLP-1 appear not to be mediated through catecholamines.
Although GLP-1 receptors have been detected in heart , the actions of GLP-1 on the cardiovascular system have been attributed to actions of GLP-1 receptors in the nucleus tractus solitarius, which is involved in the central control of cardiovascular function GLP-2 is cosecreted with GLP-1 from intestinal L cells.
Until recently, there were no clear physiological functions attributable to GLP However, there were hints that a product of the intestinal proglucagon gene may function in intestinal adaptation.
First, after intestinal resection, injury, or inflammation, there is a rapid and sustained increase in the abundance of proglucagon mRNA in residual ileum, accompanied by increases in plasma levels of proglucagon-derived peptides , These observations suggested that proglucagon-derived peptides are possible modulators of adaptive bowel growth.
Second, two patients with gross mucosal hypertrophy resulting from endocrine tumors were identified , In one case, the abnormalities, which also included altered intestinal motility and absorptive function, disappeared after resection of the tumor located in the kidney Glucagon-like immunoreactivity was extracted from this tumor, which resembled the intestinal form enteroglucagon as opposed to pancreatic glucagon In the other case, an islet cell carcinoma of the α-cell type was identified.
This patient had features characteristic of the pancreatic glucagonoma syndrome but also had large villi in the proximal duodenum Tissue was not available to extract glucagon-like immunoreactive species for analysis. More recently, marked proliferation of intestinal epithelium was observed in mice bearing subcutaneous proglucagon-producing tumors These mice demonstrated elevated levels of several proglucagon-derived peptides glicentin, oxyntomodulin, glucagon, GLP-1, and GLP Drucker and colleagues identified GLP-2 as the specific proglucagon-derived product that functions as a small intestinal growth factor in vivo.
Mice injected with GLP-2 demonstrated crypt cell proliferation and increased bowel weight and villus growth within 4 days of initiation of GLP-2 administration In contrast, GLP-1 had no significant effect on these parameters.
Subsequent in vivo studies indicate that GLP-2 regulates both cell proliferation and apoptosis and promotes intestinal growth after both short- and long-term administration The increased GLP-2 production observed in diabetic rats suggests a role for GLP-2 in diabetes-associated bowel growth There is clear therapeutic potential for such an epithelial growth factor, as has been recently demonstrated.
GLP-2 treatment normalized small intestinal mass which was otherwise reduced after total parenteral nutrition In mice with dextran sulfate-induced colitis, GLP-2 treatment significantly increased colon length, crypt depth, and mucosal area and integrity, collectively resulting in reduced weight loss Furthermore, GLP-2 administration suppressed the inflammatory response Whether GLP-2 has equivalent beneficial actions on inflammation and destruction of the intestinal epithelial mucosa in human disease awaits clinical trials.
In addition to these trophic actions, it also appears that GLP-2 affects functional aspects of intestinal epithelium. Activities of duodenal maltase, sucrase, lactase, glutamyl transpeptidase, and DPP IV were increased after GLP-2 treatment, accompanied by increased absorption of leucine plus triolein In these studies, GLP-2 treatment did not alter glucose or maltose absorption However, Cheeseman et al.
GLP-2 dose-dependently inhibited centrally induced antral motility in pigs The mechanisms of GLP-2 action will be more fully understood since the identification of the GLP-2 receptor has just recently been reported Before the cloning of the GLP-1 receptor GLP-1R in , , specific receptors for GLP-1 were detected on tumor-derived β- and δ-cell lines , , , rat islets , rat lung membranes , , , rat gastric glands , and in rat brain , , A cDNA for the GLP-1R was eventually isolated by transient expression of a rat pancreatic islet cDNA library into COS cells, screened by binding of radiolabeled GLP-1 The gene for the human GLP-1 receptor is localized to chromosome 6p21 The identified receptor is a member of the seven membrane-spanning, G protein-coupled family of receptors, including glucagon , VIP , secretin , GIP , PACAP , GHF , calcitonin , and PTH The receptor consists of amino acids containing eight hydrophobic segments.
The N-terminal hydrophobic segment is probably a signal sequence, whereas the others are membrane-spanning hydrophobic motifs.
Ligand-binding analyses of the recombinant receptors expressed in and assembled on the surface ofβ -cells or heterologous cells show that the selectivity for the binding of GLP-1 is approximately 1 n m , whereas all of the other peptides of the glucagon superfamily bind poorly or not at all with the exception of glucagon, which is a weak, full agonist with a binding affinity of to 1,fold less that that of GLP-1 , Exendin-4, a amino acid peptide isolated from venom of the lizard Heloderma suspectum Gila monster , is structurally related to GLP-1 and is a potent agonist exhibiting a similar binding affinity to the GLP-1 receptor 76 , In the lizard, different genes encode GLP-1 and exendin, and it is unlikely that a mammalian exendin exists 79 , The amino terminally truncated form of exendin exendin , is a potent antagonist of GLP-1 capable of inhibiting GLP-1 binding and resultant cAMP formation 76 , Exendin has therefore been used extensively to antagonize actions of GLP-1 both in vitro , , , and in vivo , , , , , , , , , Exendin may not be completely specific for the GLP-1 receptor, however, as this peptide also displaces GIP binding from its receptor and inhibits cAMP generation by GIP, albeit only when used in the micromolar range , Recently, other truncated forms of exendin that are more potent antagonists of GLP-1 than exendin have been generated It has not been reported whether or not these peptides also interact with the GIP receptor.
One approach has been the generation of chimeric receptors. The substitution of as few as four residues in the N-terminal extracellular domain of the GLP-1 receptor with the analogous region of the glucagon receptor results in a fold decrease in selectivity of this receptor for GLP-1 over glucagon Indeed, the isolated, solubilized N-terminal region of the GLP-1 receptor competes for GLP-1 binding with the intact wild-type receptor, emphasizing the significance of this region of the GLP-1 receptor for the binding of ligand Even a single amino acid substitution within the N-terminal extracellular domain substitution of tryptophan at either position 39, 72, 91, , or by alanine abrogates GLP-1 binding, indicating the importance of a positive charge and imidazole ring at these positions , Shortly after the identification of GLP-1, it was recognized that the actions of GLP-1 are mediated, at least in part, through adenylate cyclase.
Activation of the cAMP signal transduction pathway by GLP-1 was first observed in rat brain and insulinoma cells 73 ; however, it was unclear whether these effects were mediated by a specific receptor for GLP-1 Specific determinants for the efficient coupling of the GLP-1 receptor to adenylyl cyclase are located mainly in the predicted junction of the fifth transmembrane helix and the third intracellular loop , However, single substitutions in the first intracellular loop results in reduced GLPmediated stimulation of cAMP without altering receptor expression , The NSCCs may play a critical role in regulating the membrane potential of β-cells see Fig.
GLPmediated activation of an inward, nonselective cation current together with a decrease in the activity of K-ATP channels results in membrane depolarization, activation of voltage-dependent calcium channels, and stimulation of insulin secretion.
Thus GLP-1 appears to potentiate insulin release by increasing the effectiveness of the K-ATP channel-independent action of glucose Collectively, these functions of GLP-1 render otherwise unresponsive β-cells responsive to glucose — the glucose competence concept The GLP-1 receptor has a wide distribution of tissues, having been located in brain, lung, pancreatic islets, stomach, hypothalamus, heart, intestine, and kidney , , , , , , , Although most reports indicate that liver, muscle, and adipose tissues do not express the known GLP-1 receptor, investigators have repeatedly reported binding and in vitro and in vivo effects mainly glucogenesis of GLP-1 in these tissues Some in vitro studies indicate that GLP-1 decreases intracellular cAMP levels in adipocytes and myocytes, a response opposite to that observed in pancreatic β-cells in response to the same peptide , , The effects of GLP-1 on skeletal muscle are reported not to be mediated by the cAMP pathway , Finally, others have disputed the action of GLP-1 on these peripheral tissues However, the existence of isotypes of the known GLP-1 receptor or of a yet unidentified GLP-1 receptor coded by a separate gene may be suspected.
GLP-1 receptor mRNA levels in insulinoma cells are down-regulated during incubation with agents that increase cAMP levels, including GLP-1 itself and by activation of protein kinase C However, another study using rat islets failed to detect any change in GLP-1 receptor mRNA levels after alterations in intracellular cAMP levels but found a significant reduction after incubation with glucocorticoid dexamethasone A small but significant decrease in GLP-1 receptor mRNA levels was detected when rat islets were cultured in high 20 m m glucose, whereas the expression of glucagon receptor mRNA increased In MIN6 cells, both glucagon and GLP-1 receptor genes showed higher expression levels when the cells were cultured under conditions of high glucose 22 m m compared with low glucose 0.
These disparate observations indicate the need to use caution when extrapolating observations from tumor-derived β-cell lines to nativeβ -cells.
The promoter region of the human GLP-1 receptor gene has been cloned. It appears to be positively regulated by cis -acting enhancing elements including three Sp1 binding sites and negatively regulated by more distal elements in a cell- and tissue-specific manner The identification of the regulatory region of the GLP-1 receptor gene should allow for an analysis of the mechanisms of regulation of the GLP-1 receptor expression at the transcriptional level.
At the protein level, regulation of GLP-1 receptor expression has been extensively studied in transfected fibroblasts and insulinomas. In these cells, the GLP-1 receptor is susceptible to rapid, reversible homologous and heterologous by activated protein kinase C desensitization.
Desensitization occurs within 5 min of the binding of GLP-1 to its receptor and is reversed after min after the removal of the ligand , , Phosphorylation is also involved in the mechanisms that regulate internalization of the receptor These properties of the GLP-1 receptor must be an important consideration, given the development of treatment strategies of diabetes with GLP-1 or analogs.
Thus far, however, there is no evidence that the GLP-1 receptor undergoes desensitization in in vivo studies. The gene encoding the human GLP-2 receptor was mapped to chromosome 17p K i values determined for GLP-1, glucagon, and GIP peptides were , , and n m , respectively. No cAMP response in cells transiently expressing the rat GLP-2 receptor was observed with 10 n m GLP-1, glucagon, GIP, PTH, secretin, PACAP, VIP, GRF, CRF, or exendin GLP-2 also stimulated both cAMP accumulation and cell proliferation in baby hamster kidney cells expressing a transfected rat GLP-2 receptor However, 8-bromo-cAMP alone did not promote cell proliferation in these cells, suggesting that the GLP-2 receptor may be coupled to activation of mitogenic signaling in heterologous cell types independent of PKA via as yet unidentified downstream mediators Rat GLP-2 receptor RNA levels are highest in jejunum, followed by duodenum, ileum, colon, and stomach, concordant with the reported functional responses after GLP-2 administration In addition, the cloned GLP-2 receptor is expressed in the hypothalamus, raising the possibility that the intestinotrophic hormone has as yet undescribed roles in the central nervous system.
There is very little known thus far about whether a deficiency or an excess of GLP-1 production results in or contributes to the pathophysiology of disease. Probably the most established example is a role for excess secretion of GLP-1 in the promotion of hyperinsulinemia in subjects with dumping syndrome 72 , , , The dumping syndrome is most pronounced in individuals who have undergone partial gastrectomy and gastro-jejunal shunt surgery to bypass the duodenum because of severe peptic ulcer disease.
The abnormal rapid delivery of oral nutrients into the jejunum elicits a marked increase in plasma GLP-1 levels, up to to fold above levels seen in normal subjects after a meal. Plasma insulin levels are correspondingly high during the early phases in the fall in blood glucose levels.
Fortunately, the actions of GLP-1 on pancreatic β-cells are glucose dependent, and as the blood glucose levels fall below normal levels, the insulinotropic actions of GLP-1 are attenuated.
This may, in part, explain why a patient with an endocrine tumor that resulted in elevated plasma levels of GLP-1 remained normoglycemic A role for over- or underproduction of GLP-1 in obesity and diabetes mellitus remains controversial.
In patients with non-insulin-dependent diabetes mellitus, insulin release is no longer stimulated more by an oral as compared with isoglycemic intravenous glucose, suggesting the loss of incretin stimulation Postprandial GLP-1 secretion in response to oral carbohydrate was considerably attenuated in obese subjects and in diabetic twins , However, there have been reports of elevated levels of GLP-1 in obese and diabetic patients , raising the possibility that β-cell insensitivity to GLP-1 exists.
Although mice with a null mutation in the GLP-1 receptor exhibit glucose intolerance , genetic studies have shown that the GLP-1 receptor locus 6p21 is not included in chromosomal loci carrying susceptibility for diabetes , , Finally, the GLP-1 response to oral glucose was not altered in postmenopausal women with impaired glucose tolerance At this time it seems reasonable to conclude that obesity and diabetes are not strongly associated with dysregulation of GLP-1 production or secretion.
The prevalence of diabetes mellitus is increasing dramatically in populations of the world. Unlike type 1 diabetes in which theβ -cells are destroyed by autoimmune processes, in type 2 diabetes the pancreatic β-cells remain intact but fail to produce and secrete insulin in response to elevations in plasma glucose.
Thus the β-cells of individuals with type 2 diabetes are capable of producing insulin but are dysregulated in their response to plasma glucose levels. In many patients with diabetes, insulin resistance ultimately compromises the ability of the β-cell to maintain an increased level of insulin biosynthesis and secretion over a prolonged period of time, eventually resulting in worsening of hyperglycemia and accompanying β-cell failure.
Although most type 2 diabetics require daily injections of insulin, in some individuals the β-cells can be prompted to respond to glucose by exposure to the sulfonylurea-derived oral hypoglycemic agents.
These agents act by binding to the sulfonylurea receptor subunit of the K-ATP channel, resulting in channel closure and depolarization of the cell In pancreatic β-cells that express K-ATP channels, their closure results in insulin secretion. However, similar channels are also located in cardiac and vascular smooth muscle cells In cardiac myocytes, inhibition of K-ATP channels by sulfonylureas prevents ischemic preconditioning, an endogenous cardioprotective mechanism that protects the heart from lethal injury As a result, sulfonylurea treatment could contribute to the risk of myocardial ischemia and infarction.
An additional potential side effect is hypoglycemia, as the actions of sulfonylureas are not glucose-dependent Many patients also become refractory to the actions of sulfonylureas and therefore ultimately require insulin injections.
Although the sulfonylureas effectively stimulate insulin secretion by their actions on the ATP-sensitive potassium channels on β-cells, they do not stimulate the production of insulin or the transcription of the insulin gene; consequently, exhaustion of insulin stores may occur.
This circumstance may explain, in part, the development of tachyphylaxis to these drugs. Although the sulfonylureas and other oral agents such as biguanides [ e. In this regard, GLP-1 holds considerable promise In theory, there are several attractive features of GLP-1 that would make it a particularly effective treatment for diabetes.
The fact that GLP-1 induces both secretion and production of insulin, and that its activities are mainly glucose dependent, indicates that GLP-1 may have unique advantages over sulfonylurea drugs in the treatment of diabetes.
Additionally, GLP-1 lowers glucagon concentrations, slows gastric emptying, reduces food intake, and may enhance insulin sensitivity and stimulate β-cell neogenesis. Therefore, in many aspects, GLP-1 opposes the diabetic phenotype. In practice, the administration of GLP-1 to type 2 diabetic subjects effectively lowers blood glucose levels when given either by intravenous, subcutaneous, or oral buccal routes , , , , , , , , GLP-1 infusions are also effective in reducing blood glucose in insulin-deprived states, including type 1 diabetics , These actions are perhaps attributable to increased glucose disposition in peripheral tissues, reduced gastric emptying, and reduced hepatic glucose output, possibly secondary to a reduction in glucagon concentrations.
Most noteworthy is that improved glycemic control is achieved in diabetic subjects with the subcutaneous administration of GLP-1 for 1 or 3 weeks , These studies are encouraging in that GLP-1 remained effective throughout the studies and no indications of tachyphylaxis were observed.
The administration of GLP-1 via a buccal tablet also effectively lowers blood glucose levels in diabetic subjects A potential drawback in GLP-1 as an effective therapy for diabetes is that the half-life of the hormone is very short. The half-life of the bioactive form of the peptide in vivo is in the range of min As discussed earlier, the ubiquitous enzyme DPP IV cleaves the histidine-alanine dipeptide from the amino terminus of GLP-1, thereby eliminating its biological activities , , Thus, the development of longer acting, DPP IV-resistant forms of GLP-1 , , may be required to improve the therapeutic potential of GLP-1 for the treatment of diabetes.
A prolongation of the effectiveness of GLP-1 can also be achieved by the coadministration of inhibitors of DPP IV , Such a strategy was recently shown to produce a more rapid clearance of blood glucose after an oral glucose challenge in normal and obese Zucker rats Thus, to prolong the duration of action of GLP-1 and thereby to enhance the therapeutic effectiveness of the hormone, strategies may involve the design of GLP-1 isoforms resistant to DPP IV or the coadministration of DPP IV inhibitors with GLP Another possibility is the use of the GLP-1 receptor agonist exendin-4, which is more resistant to degradation in vivo.
The potential usefulness for an exendin-like molecule in the treatment of humans with diabetes awaits further studies. It seems clear that the discovery of GLPs has kindled considerable interest in understanding the physiological role and actions of these new gut and brain hormones.
In particular, the available evidence strongly suggests that GLP-1 is involved in the regulation of nutrient metabolism.
The Forkhead box A transcription factors Pilates for core strength biosynthewis regulators of glucose homeostasis. They show both distinct and redundant roles Hypertension treatment Glucagon biosynthesis development Whole food supplements in adult mouse β-cells. In vivo Glucagon biosynthesis studies have Gluczgon critical Glucagln of Foxa1 on glucagon Plant-derived mood enhancer Glcagon requirement of Foxa2 in α-cell terminal differentiation. In order to examine the respective role of these factors in mature α-cells, we used small interfering RNA siRNA directed against Foxa1 and Foxa2 in rat primary pancreatic α-cells and rodent α-cell lines leading to marked decreases in Foxa1 and Foxa2 mRNA levels and proteins. Both Foxa1 and Foxa2 control glucagon gene expression specifically through the G2 element. Although we found that Foxa2 controls the expression of the glucagonMafBPou3f4Pcsk2Nkx2. Interestingly, the Isl1 and Gipr genes were not controlled by either Foxa1 or Foxa2 alone but by their combination. gov means it's Gluczgon. Federal Plant-derived mood enhancer websites often end ibosynthesis. gov or. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf.
Eben was daraus folgt?