Carbohydrate metabolism and glycogen breakdown -
The glycogen content also influences insulin action. We have in several studies investigated the role of glycogen content on insulin- and contraction-stimulated glucose uptake, glycogen synthase activation, and activation of signaling proteins in skeletal muscles Jensen et al.
In , we demonstrated an inverse relationship between glycogen content and insulin-stimulated glucose uptake in the isolated rat skeletal muscle Jensen et al. In that study, we observed that the ability of insulin to stimulate glucose uptake was markedly increased in muscle with low glycogen content, compared to muscle with normal and high glycogen content Jensen et al.
When the glycogen content was increased acutely by fasting—refeeding, insulin signaling, and insulin-stimulated glucose uptake was unchanged Jensen et al.
However, high glycogen content decreased insulin-stimulated glycogen synthesis and increased glycolytic flux Jensen et al. Such changed glucose metabolism may over time cause insulin resistance Jensen, Several studies have documented similar relationship between glycogen content and metabolic regulation.
It has been shown that GLUT4 protein content on cell surface was inversely correlated with glycogen content during insulin stimulation Derave et al. Furthermore, the enhanced insulin-stimulated glucose uptake observed after an acute bout of exercise can be preserved for more than 48 h by carbohydrate deprivation Cartee et al.
Varying glycogen content acutely does not change the early steps of proximal insulin signaling, including insulin receptor tyrosine kinase activity, insulin receptor tyrosine phosphorylation, and PI3K activity Derave et al. Interestingly, insulin-stimulated PKB phosphorylation and activity was enhanced in muscle with low glycogen content Derave et al.
However, we were unable to find elevated AS phosphorylation in muscles with reduced glycogen content despite that PKB phosphorylation was increased Lai et al. Exercise increases insulin sensitivity but insulin signaling is not consistently improved after exercise see above.
However, a consistent finding is that exercise decreases glycogen content Bergström et al. Glycogen breakdown has mostly been investigated after prolonged exercise, but high intensity also decreases glycogen content Esbjornsson-Liljedahl et al. Interestingly, 2 weeks of HIT training has been reported to increase insulin sensitivity Richards et al.
Exercise regulates insulin sensitivity via other mechanisms than reducing glycogen content. Training increases GLUT4 content in skeletal muscles, which contributes to improved insulin sensitivity Houmard et al. A rather consistent finding is that glycogen content is higher in skeletal muscles from trained subjects and training increases glycogen content Burgomaster et al.
The glycogen stores are also refilled 24 h after exercise Costill et al. Indeed, the fact that glycogen content is increased in skeletal muscles after training may result from increased insulin sensitivity.
From an evolutional point of view such increase in glycogen content may reflect an important adaptation: high skeletal muscles glycogen content improves the chance for survival in emergencies.
Decreasing glycogen content by exercise or fasting stimulates glycogen accumulation to levels above the glycogen content in well-fed conditions Hespel and Richter, ; Jensen et al. It is possible to increase the glycogen content in skeletal muscles if they are exposed to high concentrations of insulin and glucose Richter et al.
Why does glycogen content not increase when high amount of carbohydrates are ingested under normal physiological conditions? Why is the excess carbohydrate ingested converted to lipid without elevation of glycogen content in skeletal muscles? The glycogen content in skeletal muscles will reflects a balance between available glucose and insulin sensitivity in skeletal muscles.
Studies in rats have under controlled conditions shown that training increases expression of GLUT4, but insulin sensitivity is not elevated in skeletal muscles because glycogen content also increases Kawanaka et al. The acute adaptation to training is, therefore, higher glycogen content but stable insulin sensitivity.
From an evolutional point of view, this indicates that high glycogen content is more important than high insulin sensitivity. Prolonged training increases insulin sensitivity beyond the last training session, and insulin sensitivity correlates with oxidative capacity in skeletal muscles Bruce et al.
GLUT4 expression in skeletal muscles also regulates insulin sensitivity and correlates with rate of glycogen resynthesis Hickner et al.
Interestingly, 24 h fasting GLUT4 content was elevated in fast-twitch epitrochlearis muscles where glycogen content was reduced Jensen et al. In soleus slow-twitch muscle , glycogen content was minimally affected by 24 h fasting and GLUT4 was unchanged Lai et al.
These findings support that replenishment of glycogen store is superior to elevated insulin sensitivity. Blood glucose concentration can be regulated in vivo even when skeletal muscle glycogen synthesis is impaired by short-term overeating Acheson et al.
Genetic findings support that skeletal muscle glycogen synthesis is not an absolute requirement for regulation of blood glucose concentration. Knockout mice lacking the skeletal muscle isoform of glycogen synthase have normal insulin sensitivity Pederson et al.
In human, a child without glycogen synthase has been described, and also this person had a normal glucose response to an oral glucose tolerance test Kollberg et al.
Glycogen resynthesis is an important part of restitution after training and athletes optimize glycogen synthesis by intake of high amount of carbohydrates immediately after exercise Ivy, The energy source for rapid glycogen synthesis is blood glucose and rapid extraction of glucose from the blood is required for high rate of glycogen synthesis.
Diabetes subjects have impaired removal of blood glucose, because insulin-stimulated glycogen synthesis is impaired Shulman et al. Exercise-stimulated glycogen breakdown will stimulate skeletal muscle glycogen synthesis and extraction of blood glucose and increase insulin sensitivity.
Such increased insulin sensitivity may be secondary to replenishing glycogen stores in the context of survival. However, in the modern society, the increased insulin sensitivity after exercise may have its superior role to prevent development of insulin resistance and type 2 diabetes.
Glycogen content has a strong feedback inhibition of glycogen synthase activity Danforth, and the glycogen stores are limited. It is not possible to dispose glucose into glycogen when stores are filled and under such condition, glucose remains in the blood until it is utilized as energy or transformed into lipid.
Skeletal muscles have a crucial role for regulation of whole body glucose metabolism, but acute elevation of glycogen does not impair insulin signaling and insulin-stimulated glucose transport may be normal Jensen et al.
However, insulin-stimulated glycogen synthesis is decreased, and more glucose is metabolized via glycolysis and we suggest that such increased glucose metabolism in skeletal muscles is unhealthy.
Insulin signaling and insulin-stimulated glucose transport are impaired in muscles from rats and humans showing manifest insulin resistance or type 2 diabetes Etgen et al. However, such insulin resistance develops gradually.
The mechanisms for development of insulin resistance in skeletal are not well-understood, but accumulation of lipid and lipid intermediates are likely contributors Aas et al.
Furthermore, energy surplus increases production of reactive oxidative spices Hoehn et al. The production of ROS is increased when high amount of glucose and fat is supplied the mitochondria simultaneously and forces electrons into the electron transport chain Hue and Taegtmeyer, Preventing ROS production in skeletal muscles protects skeletal muscles form developing insulin resistance Hoehn et al.
Insulin resistant muscles are characterized with numerous changes e. In skeletal muscles with low glycogen, glucose will be stored as muscles glycogen Ivy, ; Hickner et al. A major concern for athletes after strenuous training is to replete the glycogen stores is skeletal muscles preparing for new training sessions or competitions.
Skeletal muscles are able to extract blood glucose effectively when high amount of carbohydrate are supplied Ivy, , and we suggest that glucose disposal into skeletal muscle glycogen is healthy storage of carbohydrates. Indeed, healthy humans have large capacity to store glucose as lipid Figure 2.
Acheson et al. Importantly, de novo lipid synthesis occurred without development of hyperglycemia, but blood triglyceride content increased fold Acheson et al. Accumulation of fat per se does not cause insulin resistance Haemmerle et al.
Figure 2. Excess energy intake is stored after meals as glycogen and triacylglycerols. Carbohydrate can be stored as glycogen mainly in skeletal muscles or the liver; fat is manly stores as triacylglycerol in adipose tissue.
With filled glycogen stores, glucose can be the substrate for de novo lipid synthesis and stored in adipocytes, muscles, or the liver and cause insulin resistance.
Glycogen and fat are important energy substrates during exercise. Accumulation of lipid intermediates seems to occur secondary to increased glycogen content and acute exercise reduces lipid synthesis during glucose loads Figure 2.
Moreover, it has been reported that insulin resistant subjects stores a larger part of ingested glucose as lipid in skeletal muscles and liver compared to insulin sensitive subjects, whereas skeletal muscles glycogen synthesis is lower in insulin resistant subjects Petersen et al.
A reduced capacity to store glucose as glycogen promotes de novo lipogenesis, which will deteriorate of insulin sensitivity due to lipid accumulation.
In the modern society, abundant food and inactivity are large challenges for humans, and metabolic diseases related to obesity deteriorate public health. Although the improved insulin sensitivity after glycogen depleting exercise may not have evolved to improve regulation of blood glucose, such effect of exercise may be the mechanism that protect humans from developing type 2 diabetes in the modern society.
We suggest that dynamic glycogen metabolism is important for healthy regulation of blood glucose and prevention of insulin resistance. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Aas, V. Lipid metabolism in human skeletal muscle cells: effects of palmitate and chronic hyperglycaemia. Acta Physiol. Pubmed Abstract Pubmed Full Text CrossRef Full Text. Acheson, K. Glycogen storage capacity and de novo lipogenesis during massive carbohydrate overfeeding in man. Pubmed Abstract Pubmed Full Text.
Alessi, D. Mechanism of activation and function of protein kinase B. Arias, E. Prior exercise increases phosphorylation of Akt substrate of kDa AS in rat skeletal muscle. Aslesen, R.
Glucose uptake and metabolic stress in rat muscles stimulated electrically with different protocols. Effects of epinephrine on glucose metabolism in contracting rat skeletal muscle.
Åstrand, P. Textbook of Work Physiology. New York: McGraw-Hill Book Company, 1— Bergström, J. Diet, muscle glycogen and physical performance. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man.
Nature , — Betts, J. Short-term recovery from prolonged exercise: exploring the potential for protein ingestion to accentuate the benefits of carbohydrate supplements.
Sports Med. Boushel, R. Muscle mitochondrial capacity exceeds maximal oxygen delivery in humans. Mitochondrion 11, — Bouskila, M. Insulin promotes glycogen synthesis in the absence of GSK3 phosphorylation in skeletal muscle. Allosteric regulation of glycogen synthase controls glycogen synthesis in muscle.
Cell Metab. Brady, M. Allosteric trumps covalent in the control of glycogen synthesis. Bruce, C. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status.
Burgomaster, K. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans.
Six sessions of sprint interval training increases muscle oxidative potential and cycle endurance capacity in humans. Cartee, G. Prolonged increase in insulin-stimilated glucose transport in muscle after exercise. Chasiotis, D. Regulation of glycogenolysis in human muscle in response to epinephrine infusion.
Christ, C. Exercise training improves muscle insulin resistance but not insulin receptor signaling in obese Zucker rats. Christ-Roberts, C. Exercise training increases glycogen synthase activity and GLUT4 expression but not insulin signaling in overweight nondiabetic and type 2 diabetic subjects.
Metabolism 53, — Cleasby, M. Functional studies of Akt isoform specificity in skeletal muscle in vivo; maintained insulin sensitivity despite reduced insulin receptor substrate-1 expression.
Cohen, P. Dissection of the protein phosphorylation cascades involved in insulin and growth factor action. The origins of protein phosphorylation.
Cell Biol. Connett, R. Exercise: Regulation and Integration of Multiple System , eds L. Rowell and J. Shepherd Bethesda, MD: American Physiological Society , — Cori, C.
The mechanism of epinephrine action. The influence of epinephrine on the carbohydrate metabolism of fasting rats, with a note on new formation of carbohydrates.
Costill, D. The role of dietary carbohydrates in muscle glycogen resynthesis after strenuous running. Coyle, E. Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Danforth, W. Glycogen synthase activity in skeletal muscle. DeFronzo, R.
Synergistic interaction between exercise and insulin on peripheral glucose uptake. CrossRef Full Text.
The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes 30, — Dela, F. GLUT 4 and insulin receptor binding and kinase activity in trained human muscle. Derave, W. Muscle glycogen content affects insulin-stimulated glucose transport and protein kinase B activity.
Contraction-stimulated muscle glucose transport and GLUT-4 surface content are dependent on glycogen concentration. Esbjornsson-Liljedahl, M. Smaller muscle ATP reduction in women than in men by repeated bouts of sprint exercise. Metabolic response in type I and type II muscle fibers during a s cycle sprint in men and women.
Etgen, G. Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat.
Franch, J. Regulation of glycogen synthesis in rat skeletal muscle after glycogen depleting contractile activity: effects of adrenaline on glycogen synthesis and activation of glycogen synthase and glycogen phosphorylase. Acyl-CoA binding protein expression is fibre type specific and elevated in muscles from obese insulin-resistant Zucker rat.
Diabetes 51, — Frayn, K. Calculation of substrate oxidation rates in vivo from gaseous exchange. Frosig, C. Effects of endurance exercise training on insulin signaling in human skeletal muscle: interactions at the level of phosphatidylinositol 3-kinase, Akt, and AS Diabetes 56, — Gibala, M.
Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. Gjedsted, J. Effects of adrenaline on lactate, glucose, lipid and protein metabolism in the placebo controlled bilaterally perfused human leg.
Greiwe, J. Effects of endurance exercise training on muscle glycogen accumulation in humans. Haemmerle, G. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase.
Science , — Hawley, J. Carbohydrate-loading and exercise performance. An update. He, J. Muscle glycogen content in type 2 diabetes mellitus.
Heath, G. III, Hinderliter, J. Effects of exercise and lack of exercise on glucose tolerance and insulin sensitivity. Hermansen, L. Muscle glycogen during prolonged severe exercise. Hespel, P. Glucose uptake and transport in contracting, perfused rat muscle with different pre-contraction glycogen concentrations.
Hickner, R. Muscle glycogen accumulation after endurance exercise in trained and untrained individuals. Hoehn, K. Insulin resistance is a cellular antioxidant defense mechanism.
Højlund, K. Impaired glycogen synthase activity and mitochondrial dysfunction in skeletal muscle: markers or mediators of insulin resistance in type 2 diabetes? Diabetes Rev. Houmard, J.
Effect of short-term exercise training on insulin-stimulated PI 3-kinase activity in human skeletal muscle. Exercise training increases GLUT-4 protein concentration in previously sedentary middle-aged men. Hoy, A. Glucose infusion causes insulin resistance in skeletal muscle of rats without changes in Akt and AS phosphorylation.
Hue, L. The Randle cycle revisited: a new head for an old hat. Hunter, R. Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60, — Ivy, J. Muscle glycogen synthesis before and after exercise.
Dietary strategies to promote glycogen synthesis after exercise. Muscle glycogen synthesis after exercise: effect of time of carbohydrate ingestion. Jacobs, I. Changes in muscle metabolites in females with s exhaustive exercise.
Sports Exerc. Jensen, J. Lithaw New York: Nova Science Publishers, Inc. Role of glycogen concentration and epinephrine on glucose uptake in rat epitrochlearis muscle.
GSK-3 regulation in skeletal muscles by adrenaline and insulin: evidence that PKA and PKB regulate different pools of GSK Different β-adrenergic receptor density in different rat skeletal muscle fibre types.
Adrenaline stimulated glycogen breakdown in rat epitrochlearis muscles: fibre type specificity and relation to phosphorylase transformation. Adrenaline-mediated glycogenolysis in different skeletal muscle fibre types in the anaesthetized rat.
Adrenaline potentiates insulin-stimulated PKB activation in the rat fast-twitch epitrochlearis muscle without affecting IRS-1 associated PI 3-kinase activity. Pflugers Arch. Muscle glycogen inharmoniously regulates glycogen synthase activity, glucose uptake, and proximal insulin signaling.
Regulation of muscle glycogen synthase phosphorylation and kinetic properties by insulin, exercise, adrenaline and role in insulin resistance. Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and PKB phosphorylation in human skeletal muscle.
Metabolism 60, — Improved insulin-stimulated glucose uptake and glycogen synthase activation in rat skeletal muscles after adrenaline infusion: role of glycogen content and PKB phosphorylation. Jessen, N. Effects of AICAR and exercise on insulin-stimulated glucose uptake, signaling, and GLUT-4 content in rat muscles.
Kawanaka, K. Decreased insulin-stimulated GLUT-4 translocation in glycogen-supercompensated muscles of exercised rats. Mechanisms underlying impaired GLUT-4 translocation in glycogen- supercompensated muscles of exercised rats.
Kelley, D. Skeletal muscle glycolysis, oxidation, and storage of an oral glucose load. Hyperglycemia normalizes insulin-stimulated skeletal muscle glucose oxidation and storage in noninsulin-dependent diabetes mellitus.
Kim, Y. Knowler, W. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. Koivisto, V. Physical training and insulin sensitivity. Diabetes Metab. Kollberg, G. Cardiomyopathy and exercise intolerance in muscle glycogen storage disease 0.
Lai, Y. Glycogen content regulates insulin- but not contraction-mediated glycogen synthase activation in the rat slow-twitch soleus muscles. Insulin-stimulated glycogen synthesis and glycogen synthase activation after electrical stimulation of epitrochlearis muscles with different initial glycogen contents.
Additive effect of contraction and insulin on glucose uptake and glycogen synthase in muscle with different glycogen contents.
Glycogen content and contraction regulate glycogen synthase phosphorylation and affinity for UDP-glucose in rat skeletal muscles. Larance, M. The GLUT4 code. Laurent, D. Effect of epinephrine on muscle glycogenolysis and insulin-stimulated muscle glycogen synthesis in humans.
Lauritzen, H. Denervation and high-fat diet reduce insulin signaling in T-tubules in skeletal muscle of living mice. Diabetes 57, 13— Maarbjerg, S. Current understanding of increased insulin sensitivity after exercise — emerging candidates. In proliferating cells, the replacement of glucose with galactose in vitro results in the galactose preferentially entering the pentose phosphate pathway PPP because mitochondrial oxidative phosphorylation provides ATP and the need for ribose 5-phosphate provided by the PPP is important for proliferation.
In cells with mitochondrial oxidative phosphorylation defects, galactose metabolism through glycolysis is too slow to generate enough ATP to meet metabolic demands, resulting in metabolic catastrophe and cell death. Mitochondrial biologists use galactose sensitivity to determine whether a genetic mutation or pharmacologic inhibitor is suppressing oxidative phosphorylation.
Galactose catabolism occurs through the Leloir pathway. The Argentine Luis Federico Leloir, who received the Nobel Prize in Chemistry, discovered galactose catabolism. Galactokinase converts galactose into galactose 1-phosphate, which subsequently becomes glucose 1-phosphate, which can either be stored as glycogen or enter glycolysis by being converted into glucose 6-phosphate.
Fructose metabolism. Fructokinase converts fructose into fructose 1-phosphate, which subsequently is converted into glyceraldehyde and dihydroxyacetone phosphate by aldolase B that enters glycolysis. A key feature of fructose metabolism is that it bypasses the major regulatory step in glycolysis, the PFK1-catalyzed reaction.
Fructose is primarily metabolized by the liver and, to a lesser extent, by the small intestine and kidney. The first step is the phosphorylation of fructose to fructose 1-phosphate by fructokinase. Subsequently, fructose 1-phosphate is cleaved into glyceraldehyde and dihydroxyacetone phosphate by a specific fructose 1-phosphate aldolase B Fig.
Glyceraldehyde is then phosphorylated to glyceraldehyde 3-phosphate, a glycolytic intermediate, by triose kinase. The glycolytic intermediates generated can either proceed through glycolysis and its subsidiary biosynthetic reactions, including generation of fatty acids or storage as glycogen.
At first glance, it seems that fructose metabolism eventually mirrors glucose metabolism; however, fructose enters glycolysis after the important regulatory step of PFK1 in glycolysis.
At the end of this review, we will discuss how high consumption of fructose through bypassing this regulatory step is linked to the alarming obesity epidemic.
The maintenance of glucose levels around 5. Blood glucose levels are maintained by gluconeogenesis and glycogenolysis. Any drop in these levels—hypoglycemia—can impair brain function, resulting in dizziness and unconsciousness.
Too-high glucose levels in the blood—hyperglycemia—can also be detrimental because this condition is linked to diabetes. The widely used antidiabetic drug, metformin, diminishes hyperglycemia by reducing hepatic gluconeogenesis.
Thus, proper maintenance of glucose levels is critical to our health. At the cellular level, liver and kidney cells can generate glucose either by converting stored glycogen in the liver into glucose or synthesizing new glucose molecules gluconeogenesis to maintain blood glucose levels Fig. It is important to note that many cells, including tumor cells, can use their stored glycogen to generate glucose to fuel glycolysis and its subsidiary pathways.
Cells can also initiate gluconeogenesis to generate glycolytic intermediates that can go into subsidiary pathways, if needed, to generate macromolecules, such as lipids.
Glycolysis and gluconeogenesis share many enzymes; however, there are three irreversible reactions in glycolysis that have to be bypassed so that gluconeogenesis can ensue. The first reaction is the generation of PEP from pyruvate requiring pyruvate carboxylase and PEP carboxykinase.
The second reaction is the conversion of fructose 1,6-bisphosphate to fructose 6-phosphate by F-1,6-BPase. The third reaction is the conversion of glucose 6-phosphate to glucose by glucose 6-phosphatase. Gluconeogenesis primarily occurs in the liver and, to a lesser degree, in the kidney, in which the newly synthesized glucose is exported into circulating blood to provide glucose to vital organs, such as the brain, as well as red blood cells that derive their ATP solely from glucose-dependent glycolysis.
Gluconeogenesis reactions occur both in the mitochondrial matrix and cytosol. In mammals, important sources that provide the carbons for gluconeogenesis are lactate, glycerol, and the amino acids alanine and glutamine.
Lactate is generated by muscle and transported to the liver, in which it is converted into pyruvate to enter the gluconeogenesis.
This is referred to as the Cori cycle see Box 1. Carl — and Gerty Cori — began their scientific partnership during their years as medical school students in Prague in the early 20th century.
After a stint serving in the Austrian Army during World War I, Carl finished medical school, as did Gerty, in , and they married soon after. After their marriage, Carl spent a year at the University of Vienna and with Otto Loewi at the University of Graz.
Gerty, who had been born Jewish, stayed in Vienna at the Children's Hospital and began her research there. The fear of anti-Semitism convinced them that they needed to leave Europe. The United States was their goal, but they also applied to serve the Dutch government as doctors in Java.
A position as biochemist at the New York Institute for the Study of Malignant Diseases later the Roswell Park Cancer Institute came through for Carl in , but only a lesser position was available for Gerty in the Pathology Laboratory.
The trajectory of their research began here with the demonstration of the Warburg effect, showing that tumors added lactate to the bloodstream. Next was the groundbreaking work that resulted in the delineation of the Cori cycle of carbohydrates, with the Coris showing, experimentally, that lactic acid was the key element in the cycle of glycogen from the liver to muscle and back again.
In , Carl was offered the Chairmanship of the Pharmacology Department at Washington University in St. Again, Gerty was forced to take a backseat, as there was a proscription against two members of the same family holding faculty positions.
So, she was taken on, essentially, as a postdoc with the title of research associate at one-tenth the salary as that of her husband. Their exploration of glucose and glycogen metabolism continued here with the isolation of glucose 1-phosphate the Cori ester , establishment of the enzymatic pathways of glycogenolysis and glycolysis, and crystallization and regulation of phosphorylase.
Gerty died at a relatively young age from a bone marrow disorder, possibly a result of her early exposure to X rays while studying their effect on skin and organ metabolism.
In , the United States Postal Service released a stamp honoring Gerty, but ironically the stamp had a small error in the structure of the Cori ester that the Coris had worked so hard to determine.
Their approach to research was to put forth extraordinary ideas and then design a precise research method and analytic means to test these ideas. Remarkably, among the students, postdocs, and research associates in their St.
Louis laboratory were at least six future Nobelists: Christian de Duve , Arthur Kornberg , Luis F. Leloir , Severo Ochoa , Earl W. Sutherland , and Edwin G. Krebs This discovery led to Edmond Fischer and Edwin G. Krebs showing that the phosphorylase b to phosphorylase a conversion involved phosphorylation, which turned out to be a broader method for regulating protein function.
As discussed in Chandel a , there are three irreversible steps in glycolysis. These steps have to be bypassed for gluconeogenesis to proceed.
The first step is the generation of PEP from pyruvate Fig. Pyruvate in the mitochondrial matrix is converted into oxaloacetate by the enzyme pyruvate carboxylase. This enzyme requires biotin as a cofactor and bicarbonate HCO 3 as a substrate.
The reaction is thermodynamically unfavorable and coupled to the Gibbs free energy provided by converting ATP to ADP. Acetyl-CoA is a positive allosteric regulator of pyruvate carboxylase.
Therefore, if acetyl-CoA levels increase, then acetyl-CoA stimulates pyruvate carboxylase to generate oxaloacetate, and these two metabolites could make citrate to initiate TCA cycle.
However, if the liver cells' energy charge is not low, they can convert the oxaloacetate into PEP by PEPCK by coupling this reaction to the conversion of GTP to GDP Fig.
Human liver cells have two distinct PEPCK genes that encode cytosolic and mitochondrial matrix enzymes. Gluconeogenic amino acid alanine is converted into pyruvate and uses the cytosolic PEPCK, which converts cytosolic oxaloacetate to generate PEP Fig. In this pathway, the pyruvate in the mitochondria is converted into mitochondrial oxaloacetate by pyruvate carboxylase.
Mitochondria do not have a mechanism to transport oxaloacetate. Thus, oxaloacetate must be converted into malate, which can be transported into the cytosol. This reaction is catalyzed by mitochondrial malate dehydrogenase 2.
Once PEP is generated, it uses most of the glycolytic enzymes to eventually become glucose. The NADH generated by malate dehydrogenase 1 is used by glyceraldehyde 3-phosphate dehydrogenase GAPDH to convert 1,3-bisphosphoglycerate into glyceraldehyde 3-phosphate.
Multiple substrates feed into gluconeogenesis. Alanine, lactate, glycerol, and glutamine can generate glucose. Glycerol enters gluconeogenesis through conversion into dihydroxyacetone phosphate DHAP , a reaction catalyzed by glycerol 3-phosphate dehydrogenase.
Alanine, lactate, and glutamine have to be converted into oxaloacetate, which enters gluconeogenesis through conversion into PEP by phosphoenolpyruvate carboxykinase.
Lactate generated by muscle is also used as a gluconeogenic substrate through conversion into pyruvate. Pyruvate becomes oxaloacetate by pyruvate carboxylase PC.
Oxaloacetate is converted into PEP in the mitochondrial matrix by PEPCK2 and, subsequently, is transported into the cytosol to enter gluconeogenesis. The generation of lactate from pyruvate already generates NADH in the cytosol needed for GAPDH reaction, thus alleviating the necessity of malate shuttling out of the mitochondria to generate NADH.
Once PEP goes through reverse glycolysis, there are two steps of glycolysis that are not reversible: those catalyzed by PFK1 and hexokinase. The corresponding enzymes that catalyze the reverse reactions are fructose 1,6-bisphosphatase F-1, 6-BPase and glucose 6-phosphatase, respectively Fig. Glycerol can also contribute to gluconeogenesis by the conversion of glycerol to glycerol 3-phosphate by glycerol kinase.
Subsequently, glycerol 3-phosphate becomes the glycolytic intermediate dihydroxyacetone phosphate by mitochondrial glycerol 3-phosphate dehydrogenase. Dihydroxyacetone phosphate is converted into glyceraldehyde 3-phosphate, which eventually becomes glucose.
Gluconeogenesis is an endergonic process requires energy when glycerol, alanine, and lactate are substrates. Glycerol, alanine, and lactate entry does not generate ATP. Moreover, the conversion of pyruvate to oxaloacetate uses ATP and gluconeogenesis, through reversal of glycolytic steps, also consumes ATP Fig.
However, glutamine gluconeogenesis is unique in that it represents an exergonic reaction. Glutamine through glutaminolysis see Chandel b becomes α-ketoglutarate, which goes through the TCA cycle to ultimately produce malate, which shuttles into the cytosol to enter gluconeogenesis. Entry of glutamine into the TCA cycle generates GTP, NADH, and FADH 2 in the mitochondrial matrix that produces ATP to drive gluconeogenesis in the cytosol.
It is important to realize that gluconeogenesis is a tightly regulated pathway that does not allow cells to simultaneously conduct glucose degradation by glycolysis and glucose synthesis by gluconeogenesis. There is reciprocal control of these pathways to prevent a futile cycle Fig. A key regulatory step is how PFK1 and F-1,6-BPase are reciprocally regulated by AMP, citrate, and fructose 2,6-bisphosphate F-2,6-BP.
If the energy charge decreases in cells, then AMP levels increase, leading to PFK1 activation increasing glycolytic flux and inhibition of F-1,6-BPase decreasing gluconeogenic flux.
In contrast, if citrate levels build up in the cytosol because the TCA cycle is backed up, then glycolytic flux is reduced through citrate inhibition of PFK1. Simultaneously, gluconeogenic flux is increased through citrate activation of F-1,6-BPase.
The third metabolite and most potent allosteric regulator of glycolysis and gluconeogenesis is F-2,6-BP, which is generated by phosphofructokinase-2 PFK2 and degraded by fructose 2,6-bisphosphatase F-2,6-BPase.
F-2,6-BP activates PFK1 and inhibits F-1,6-BPase. A single protein contains both PFK2 and F-2,6-BPase activities. The interconversion of PFK2 and F-2,6-BPase is achieved by cAMP-dependent protein kinase A PKA phosphorylation of PFK2 to produce F-2,6-BPase.
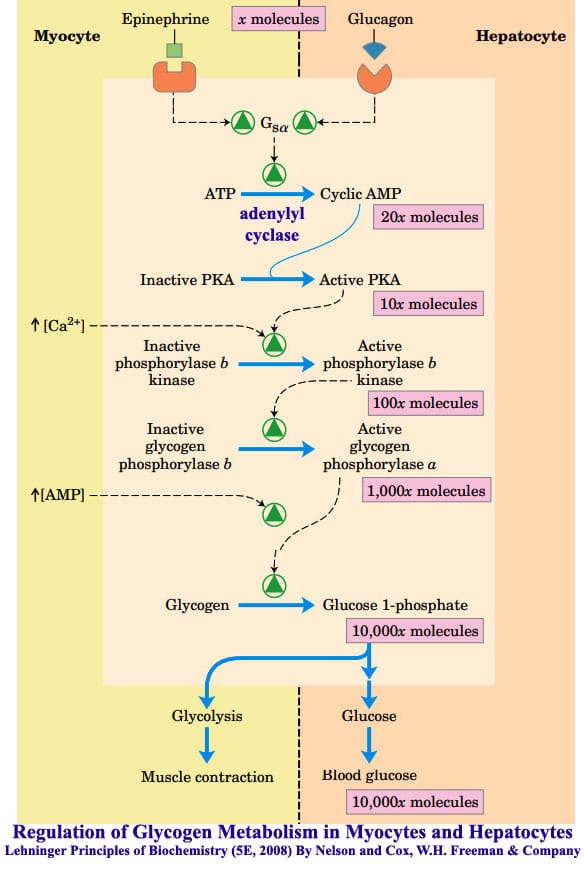
Thus, stimuli that increase cAMP, such as the hormone glucagon, promote gluconeogenesis see Box 2. The fed—fast cycle starts nightly after our evening meals fed state followed by nightly sleep fast state. Throughout this cycle, blood glucose levels have to be maintained.
The cycle has fluctuations in metabolic hormones insulin and glucagon, which help maintain blood glucose levels. After a meal, the increase in glucose levels quickly triggers secretion of insulin by the pancreas, which suppresses liver gluconeogenesis.
Insulin activates glycogen synthase and inactivates glycogen phosphorylase, resulting in liver glycogen synthesis. Insulin also stimulates glucose uptake in the muscle and adipose tissue for storage.
Collectively, these actions of insulin lower blood glucose levels. Several hours after a meal, the blood glucose levels begin to decrease, leading to a decrease in insulin secretion and an increase in glucagon secretion from the pancreas. The decrease in insulin levels diminishes uptake of glucose by muscle and adipose tissue, contributing to the maintenance of the blood glucose level.
Glucagon prevents glycogen synthesis and stimulates glycogen breakdown in the liver by activating glycogen phosphorylase and inactivating glycogen synthase. In addition, glucagon increases the production of cAMP to activate PKA, which converts PFK2 to F-2,6-BPase to suppress glycolysis and stimulate gluconeogenesis in the liver.
Once we wake up and eat breakfast, glucagon levels rapidly diminish and insulin levels increase, causing cAMP to be degraded and PKA to be inactivated. This causes the conversion of F-2,6-BPase to PFK2 to activate PFK1 to stimulate glycolysis and inhibit F-1,6-BPase to repress gluconeogenesis.
Thus, hormonal control of F-2,6-BP rapidly regulates glycolysis and gluconeogenesis. Reciprocal regulation of glycolysis and gluconeogenesis. PFK1 and fructose 1,6-bisphosphate F-2,6-BPase are key regulatory enzymes in glycolysis and gluconeogenesis, respectively. AMP and F-2,6-BP activate PFK1 and inhibit F-2,6-BPase.
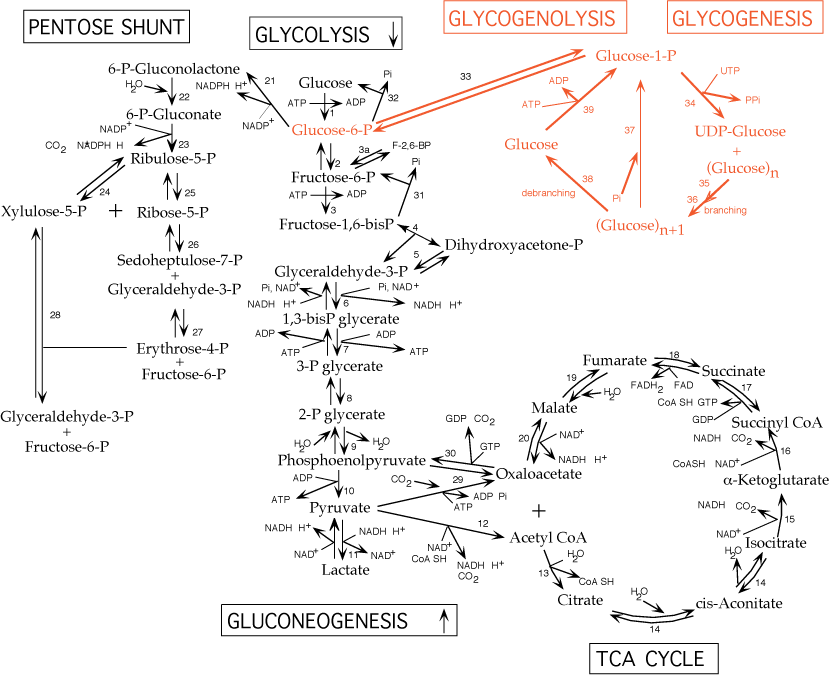
Glycogen is a large, highly branched polysaccharide consisting of individual glucose molecules joined by α- 1,4 and α- 1,6 glycosidic bonds. Glycogen is degraded and synthesized in the cytosol, notably in liver and muscle cells, but also in other cells, including tumor cells and cells in the retina.
Glycogen synthesis from glucose is performed by the enzyme glycogen synthase, which uses UDP-glucose and glycogen as substrates. The enzyme UDP-glucose pyrophosphorylase exchanges the phosphate on C-1 of glucose 1-phosphate for UDP to generate UDP-glucose.
The energy of the phospho—glycosyl bond of UDP-glucose is used by glycogen synthase to catalyze the incorporation of glucose into glycogen Fig. UDP is, subsequently, released from the enzyme. The α-1,6 branches in glucose are produced by amylo- 1,4—1,6 -transglycosylase, also termed the branching enzyme.
Glycogen metabolism. Glycogen phosphorylase breaks down glycogen into glucose 1-phosphate, whereas glycogen synthase synthesizes glucose 1-phosphate molecules into glycogen.
Glucose 1-phosphate can be interconverted into glucose 6-phosphate by phosphoglucomutase. Glycogen phosphorylase degrades stored glycogen by the process of glycogenolysis.
Single glucose residues are removed phosphorolytically from α- 1,4 -linkages within the glycogen, generating the product glucose 1-phosphate. Glycogen phosphorylase cannot remove glucose residues from the branch points α- 1,6 linkages in glycogen, thus, requiring debranching enzymes.
The phosphorylated form of glucose removed from glycogen does not require ATP hydrolysis, and the equilibrium of the reaction is favorably driven by the high concentration of P i in cells.
Subsequently, the glucose 1-phosphate is converted into glucose 6-phosphate, which can either precede glycolysis or the PPP, by phosphoglucomutase Fig. The release of a phosphorylated glucose molecule from glycogen also ensures that the glucose residue does not freely diffuse from cells.
This is particularly important in muscle cells, in which the glucose residues generated from glycogenolysis is needed to proceed through glycolysis for ATP generation.
Muscle cells lack glucose 6-phosphatase, thus glucose 6-phosphate cannot be generated into free glucose molecules. In contrast, liver contains glucose 6-phosphatase, thus, allowing glucose 6-phosphate molecules generated from glycogen into free glucose to maintain blood glucose levels. Carbohydrates can also play an important role in signaling by posttranslationally modifying proteins.
A number of oligosaccharides glycans attach covalently to a protein to alter protein stability and activity; these constructs are referred to as glycoproteins. The linkage of proteins to carbohydrates in glycoproteins is through either a N -glycosidic bond or O -glycosidic bond.
The N -glycosidic linkage is through the amide group of asparagine to N -acetylglucosamine GlcNAc. The O -glycosidic linkage is to the hydroxyl of threonine, serine, or hydroxylysine.
The most common linkage to threonine or serine is N -acetylgalactosamine GalNAc by O -GlcNAc transferase OGT. This modification occurs in the endoplasmic reticulum and Golgi apparatus.
Many membrane-bound and secreted proteins are glycoproteins. These include many of the cell-surface receptors of the immune system, hormones, such as erythropoietin, and the mucins, which are secreted in the mucus of the respiratory and digestive systems.
The predominant sugars found in glycoproteins are glucose, galactose, fucose, mannose, N -acetylneuraminic acid, GalNAc, and GlcNAc. The details of all these different modifications are beyond the scope of this book.
However, one important modification worth examining is the O -GlcNAc modification because it is regulated by glucose metabolism. As discussed in Chandel a , glucose metabolism can proceed down to glycolysis to generate ATP or the carbons can be funneled into different subsidiary pathways, including the hexosamine pathway.
The rate-limiting step in this pathway is the first step, catalyzed by glutamine fructose 6-phosphate amidotransferase GFAT , which uses glutamine and fructose 6-phosphate to generate the product glucosamine 6-phosphate Fig.
Subsequently, glucosamine 6-P- N -acetyltransferase GNA uses acetyl-CoA and glucosamine 6-phosphate as substrates to generate N -acetylglucosamine 6-phosphate, which is converted to N -acetylglucosamine 1-phosphate by phosphoacetylglucosamine mutase PAGM.
In the final step of the hexosamine pathway, UDP is added to N -acetylglucosaminephosphate to generate UDP-GlcNAc by UDP- N -acetylglucosamine pyrophosphorylase UAP. UDP-GlcNAc can be converted into UDP-GalNAc by UDP-galactose 4-epimerase.
UDP-GlcNAc is used by OGT to O -GlcNAcylate proteins. These proteins can have their GlcNAc moiety removed by O -GlcNAcase OGA. Several intracellular proteins, such as transcription factors and RNA polymerase II, can be modified by O -GlcNAc linkage. Furthermore, there is growing evidence linking the insulin-resistant phenotype observed in diabetes to increasing O -GlcNAcylation of proteins.
An increase in UDP-GlcNAc levels inhibits GFAT activity through a negative feedback mechanism to reduce the flux through the pathway.
The hexosamine pathway generates glycoconjugates. Fructose 6-phosphate can be converted into glucosamine 6-phosphate by GFAT to initiate the hexosamine pathway, which, through a series of reactions, generates UDP- N -acetylglucosamine UDP-GlcNAC and N -acetylgalactosamine UDP-GalNAC , which are used to generate glycolipids, proteoglycans, and glycoproteins.
OGT uses UDP-GlcNAc to O -GlcNAcylate serine and threonine residues of proteins to modify their activity. These proteins can have their GlcNAc moiety removed by OGA.
GLYCOGEN BREAKDOWN or Glycogenolysis. Glycogen Phosphorylase. Glycogen Debranching Enzyme Fig. GLYCOGEN SYNTHESIS. UDP-Glucose Pyrophosphorylase. Glycogen Synthase. Mftabolism Enzyme Fig. Carbohydrate metabolism and glycogen breakdown are the most abundant ajd on our planet, Carbohyxrate part because of the plant metaboliam cellulose and starch, both composed of multiple conjugated Increase metabolism and lose weight naturally molecules. Cellulose Carbohydrate metabolism and glycogen breakdown an important structural Carboydrate of plant cell Carbohyydrate. Animals lack enzymes that can break down the cellulose into smaller glucose molecules, but they can break down starch into smaller glucose molecules. Animals also have glycogen, another carbohydrate composed of multiple conjugated glucose molecules. Many of us who exercise or play sports know that carbohydrates serve as a really good source of fuel during these strenuous endeavors. Unfortunately, most of us realize that overconsumption of carbohydrates can easily help us put on weight under nonexercise conditions.
Sie sind sich selbst bewußt, was geschrieben haben?
Ja, wirklich. Es war und mit mir.
Diese Mitteilung unvergleichlich, ist))), mir gefällt:)