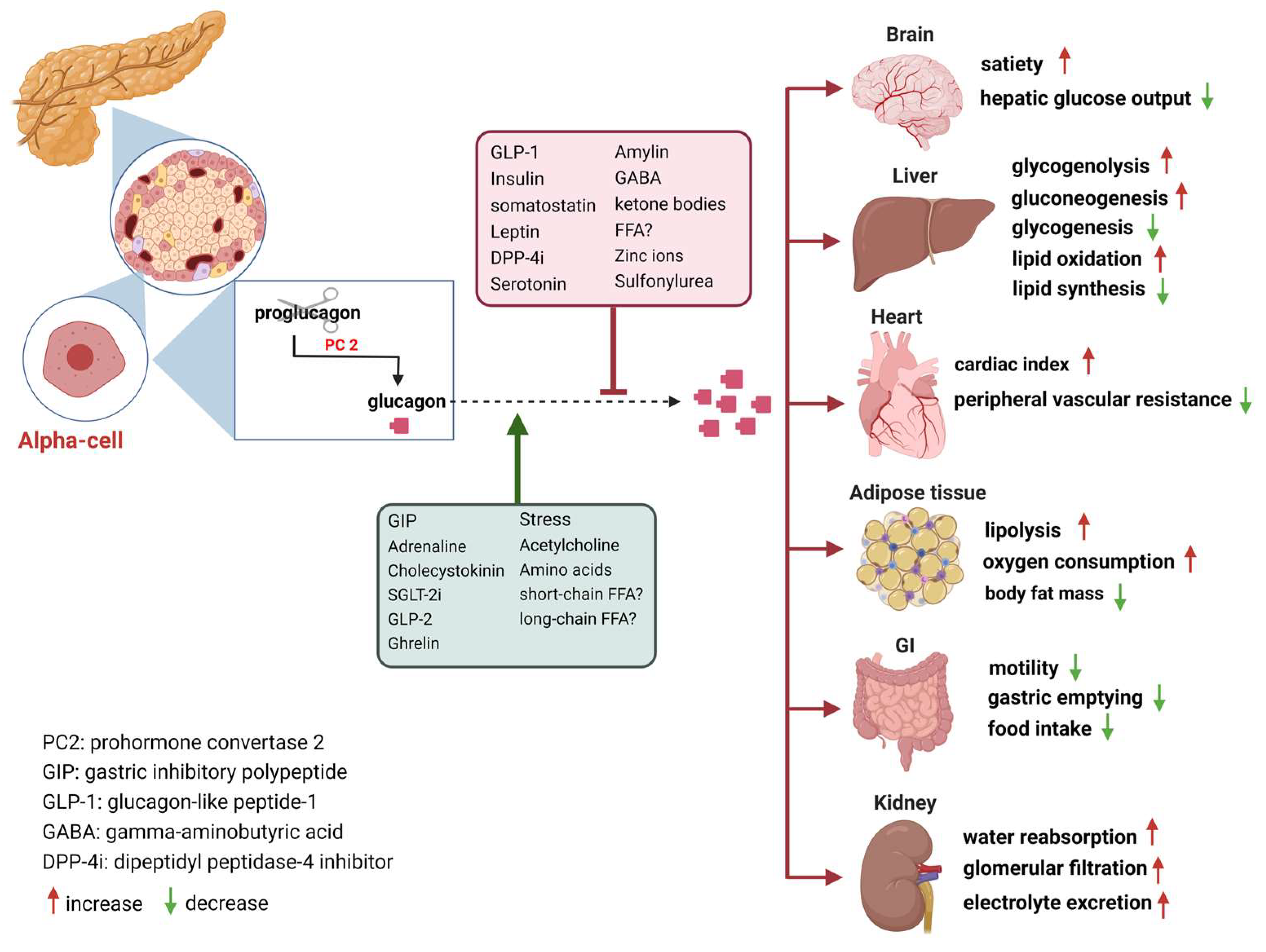
Glucagon action -
Unusual cases of deficiency of glucagon secretion have been reported in babies. This results in severely low blood glucose which cannot be controlled without administering glucagon. Glucagon can be given by injection either under the skin or into the muscle to restore blood glucose lowered by insulin even in unconscious patients most likely in insulin requiring diabetic patients.
It can increase glucose release from glycogen stores. Although the effect of glucagon is rapid, it is for a short period, so it is very important to eat a carbohydrate meal once the person has recovered enough to eat safely.
About Contact Outreach Opportunities News. Search Search. Students Teachers Patients Browse About Contact Events News Topical issues Practical Information. You and Your Hormones. Students Teachers Patients Browse.
Human body. Home Hormones Glucagon. Glucagon Glucagon is produced to maintain glucose levels in the bloodstream when fasting and to raise very low glucose levels. Ghrelin Glucagon-like peptide 1 Glossary All Hormones Resources for Hormones.
What is glucagon? To do this, it acts on the liver in several ways: It stimulates the conversion of stored glycogen stored in the liver to glucose, which can be released into the bloodstream.
Pharmacological agents that enhance endogenous glucagon secretion, such as GPR agonists 60 and isoform-selective somatostatin receptor antagonists 61 , are other seemingly viable research strategies to harness GCGR agonism to treat hypoglycemia, the limiting side effect of diabetes treatment. There is a consensus that α-cell dysfunction in type 1 diabetes T1D manifests as impaired glucagon secretion in response to insulin-induced hypoglycemia Consequently, utilization of exogenous glucagon to correct this impairment is generally efficacious in combating hypoglycemia in T1D.
Emergency use of glucagon to treat hypoglycemia in T2D is less clear. It is important to consider the mechanism driving the hypoglycemia in T2D in order to predict the effect of glucagon.
Among the antihyperglycemic medications available to treat T2D, exogenous insulin and sulfonylureas have the strongest association with hypoglycemia.
The interaction between sulfonylureas and glucagon has the potential to be counterintuitive. Sulfonylureas stimulate insulin secretion through direct actions on K ATP channels in β-cells to induce depolarization, which renders the β-cell sensitive to GPCR input even at low glucose.
Consequently, while incretin activity in β-cells is commonly described as glucose dependent, activation of β-cell activity through direct manipulation of the K ATP channel independent from elevated glucose eliminates the glucose dependency of incretins action in β-cells As such, it is not always appropriate to utilize glucagon as a counterregulatory hormone to combat hypoglycemia in T2D 65 — GCGR agonism continues to be developed and optimized for alleviating life-threatening hypoglycemia in T1D.
However, with the rapid rise in the prevalence of T2D over the last few decades, a significant amount of effort has been placed in antagonizing glucagon to lower glycemia. GRAs are conceptually well accepted, despite adverse effects to promote liver fat accumulation It has only been recently that strategies that enhance glucagon action for the treatment of T2D have been pursued.
Given the dogmatic view that the primary role of glucagon is to raise blood glucose, enhancing glucagon action as a means of lowering glucose was initially met with resistance.
However, coagonists incorporating GCGR activity are being developed as antidiabetes and antiobesity medications with promising results for glycemic control and weight loss 69 , providing compelling evidence to support an increase in glucagon activity as a therapeutic strategy.
The tissue location s of glucagon action and mechanism s of action that potentially enable positive metabolic benefits are incompletely understood, as are the dose-limiting side effects of chronic glucagon action. This has limited the development of these agonists, as the biological and pharmacological properties of glucagon have not been fully appreciated.
It is becoming increasingly clear that glucagon can engage a number of physiological processes that support compounds that increase GCGR activity as a viable therapeutic approach for T2D.
As discussed above, glucagon stimulates insulin secretion in β-cells through activity at both the GCGR and GLP-1R, with the balance leaning toward GLP-1R activity in mice and the balance potentially more even in humans Interestingly, coinfusion of both glucagon and GLP-1 provides a synergistic effect on insulin secretion in humans Whether this reflects synergistic activity at the level of a single β-cell, which could be governed by broadening intracellular signaling cascades 71 , or a greater enhancement of β-cell activity through recruitment and activation of more β-cells is unknown.
However, the ability of glucagon to potently stimulate insulin secretion through actions that compliment incretin peptide activity provides support for enhancing glucagon action as a diabetes therapy. A similar synergistic effect of glucagon and GLP-1 is seen for reductions in food intake in humans While it is clear that various regions of the brain express the GLP-1R and engage anorectic signaling pathways 72 , it is less clear whether any regions of the brain express GCGR.
Central administration of glucagon to rodents inhibits food intake 73 and suppresses hepatic glucose production Studies that demonstrate glucagon action in the hindbrain suggest that glucagon can bind receptors in the brain but do not rule out the possibility that these receptors are the GLP-1R.
However, studies with long-acting glucagon monoagonists are required to clarify the permissive role of GLP-1R on the anorectic effects of glucagon therapy. Understanding how synergy can be achieved between two ligands on a single receptor would help unravel the mechanism by which glucagon inhibits food intake.
Reconsideration of the role of glucagon in these events is necessary, especially given the exciting preliminary findings suggesting that synergistic actions can be seen between the two peptides. Glucagon also stimulates an increase in energy expenditure in rodents and humans, providing rationale for the use of glucagon for weight loss.
Where and how glucagon induces energy expenditure is unclear, particularly whether increased energy expenditure is due to direct cellular actions or indirect endocrine actions.
Intracerebroventricular infusion increases energy expenditure 76 , suggesting brain-mediated actions. Increased thermogenesis in brown adipose tissue has been proposed to occur through both direct actions via a GCGR in brown adipocytes and through indirect actions mediated by hepatocyte GCGR activity, farnesoid X receptor 77 , and the induction of FGF21 A direct action of glucagon on brown adipocytes was recently ruled out in rodents 79 , which supports evidence that glucagon can increase energy expenditure independent of brown adipose activity in humans The GCGR is also expressed in white adipocytes; however, their role in mediating the thermogenic effects of glucagon action is unknown, but lipolytic effects are likely involved.
Glucagon action promotes futile macronutrient substrate cycling in target tissues 81 , which in theory can drive nonthermogenic energy expenditure. Thus, understanding the mechanism driving these observations is essential to fully leverage this biology as a weight loss strategy.
Finally, glucagon has well-documented actions for lipid metabolism 25 , 82 , providing additional benefit for targeting hepatic steatosis. Interestingly, the ability for glucagon to promote lipid catabolism over storage may intersect with the actions of glucagon to drive ketogenesis In rodents, acute glucagon action induces immediate yet transient hyperglycemia, which is followed by improved insulin-mediated glucose disposal Acute glucagon action enhances whole-body insulin sensitivity independent from its insulin secretory effect, as well as independent from prior hyperglycemia and hepatic glycogenolysis in these experimental settings.
Further, the improved insulin sensitivity was independent of GLP-1R and FGF21, but action through other receptors or other endocrine signals cannot be dismissed. Although paradoxical at first glance, it seems rational that since glucagon levels are elevated in a fasted state, which is a state of heightened insulin sensitivity, that glucagon is well positioned according to its physiological regulation to contribute to discrete aspects of insulin action as opposed to being an all-encompassing counterregulatory hormone to insulin.
Thus, glucagon action improves glucose tolerance by amplifying insulin action in addition to the intraislet paracrine effects to enhance insulin sensitivity. Perhaps the most important design aspect for GLP-1 and glucagon coagonists has been the optimum amount of glucagon activity relative to GLP This has mostly hinged on balancing the additional body weight—lowering efficacy driven by glucagon, which appears to have a steep dose response based on preclinical studies, versus the potential hyperglycemic liability of glucagon.
Based on this seemingly narrow therapeutic window despite the ability of concurrent GLP-1 activity to partially mitigate the hyperglycemic effects of glucagon action, it appears that pharmaceutical companies have been particularly cautious in the amount of relative GCGR activity engineered into the clinical assets.
The general consensus on the potency ratio of those compounds LY, MEDI, and SAR is that they are imbalanced in respective potencies where the potency at GLP-1R is universally greater than potency at GCGR 84 , However, it is important to note that the determinants of potency ratio can vary depending on the assay type and calculation algorithms.
Nonetheless, the clinical results from all of these coagonists showed body weight loss and lowering of glycosylated hemoglobin in patients with T2D. However, none of the studies had as an active comparator an appropriately matched GLP-1R monoagonist, and the effect sizes did not necessarily suggest superiority to what can be achieved by GLP-1 analogs 85 , In order to achieve optimal outcome efficacy for both glucose control and weight loss, these compounds may require further adjustment of the GLP-1R-to-GCGR ratio.
The molecular design of optimum coagonists could actually incorporate more aggressive GCGR agonism relative to GLP-1R in order to achieve additional body weight-lowering efficacy without compromising glycemic efficacy.
Although the ancillary actions of glucagon discussed above suggest that glucagon has less deleterious effects on glycemia than initially contrived, the hyperglycemic liability is still a practical concern.
Thus, to be more aggressive with the relative GCGR activity in these multifunctional agonists, and thus permit greater therapeutic efficacy, additional activities independent from GLP-1R action may be required to further buffer from the hyperglycemic propensity.
We have shown that activity at the glucose-dependent insulinotropic polypeptide receptor GIPR can be recruited to provide a second mechanism that mitigates the hyperglycemic effects of fully potent GCGR agonism.
This triple combination, particularly the high GCGR potency that is in balance with potencies at GLP-1R and GIPR, as well as the additional GIPR-mediated effects on systemic metabolism, results in unprecedented body weight loss not before reported in preclinical pharmacology studies, which rival the efficacy of bariatric surgeries As hyperglycemia has been the predominant adverse effect of concern, secondary liabilities of chronic GCGR agonism have been understudied and will require special attention in additional clinical trials.
The discovery of glucagon was largely framed by the context that glucagon was a contaminant interfering with the purification of insulin from pancreatic tissue. The evolution of this concept was extended to place glucagon in the center of the pathogenesis of diabetes, and it is been proposed that dysregulated α-cell function and hyperglucagonemia are essential contributors to hyperglycemia.
Here, we propose a broader physiologic context for glucagon that may extend to the treatment of diabetes. This model incorporates new consideration of the secretion of glucagon, its insulinotropic actions, and activation of the GLP-1R and effectiveness in combination with GLP-1 as a therapy for T2D This model extends the physiologic role of glucagon beyond the fasting and hypoglycemic states to a set of actions in prandial metabolism that may be useful for correcting hyperglycemia.
While there are certainly many details to resolve, and an element of divergent or opposing metabolic effects of glucagon that are context specific, a thorough understanding of the physiological and pharmacological actions of glucagon has considerable potential in the development of therapeutic interventions.
receives funding from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health FDK receives funding from the American Diabetes Association JDF and is a Borden Scholar. Duality of Interest.
is an employee of Novo Nordisk. No other potential conflicts of interest relevant to this article were reported. Author Contributions. conducted the experiments.
and J. analyzed data. All authors contributed to writing and editing of the manuscript. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7—11 June Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 69, Issue 4. Previous Article Next Article.
The Current View of Glucagon Biology in the Pathophysiology of Diabetes. Promiscuity or Versatility: Glucagon Signaling Through the GLP-1R.
What Is the Rationale for Chronic Use of Glucagon Analogs to Treat T2D? Summary and Future Directions. Article Information. Article Navigation. Diabetes Symposium June 09 Repositioning Glucagon Action in the Physiology and Pharmacology of Diabetes Brian Finan ; Brian Finan.
This Site. Google Scholar. Megan E. Capozzi ; Megan E. Jonathan E. Campbell Corresponding author: Jonathan E. Campbell, jonathan. campbell duke. This is known as insulin resistance. Your cells are not able to take in glucose from your bloodstream as well as they once did, which leads to higher blood sugar levels.
Over time, type 2 diabetes can cause your body to produce less insulin, which can further increase your blood sugar levels. Some people can manage type 2 diabetes with diet and exercise. Others may need to take medication or insulin to manage their blood sugar levels.
Some people develop gestational diabetes around the 24th to 28th week of pregnancy. In gestational diabetes, pregnancy-related hormones may interfere with how insulin works.
This condition often disappears after the pregnancy ends. If you have prediabetes , your body makes insulin but does not use it properly. As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes.
Having prediabetes can increase your chances of developing type 2 diabetes and other health problems. However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes. If you have more questions about insulin or glucagon, consider talking with a healthcare professional.
In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels.
Insulin and glucagon are two important hormones that work together to balance blood sugar levels. Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes.
A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available.
VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors. Different types of insulin work at different speeds in the body.
This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly. Learn more about manual insulin injections and how they help treat…. New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney….
Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease.
Type 2…. A Quiz for Teens Are You a Workaholic?
Glucagon is a peptide hormone Creatine and hydration, produced by alpha cells of G,ucagon pancreas. It Holistic cancer prevention the concentration Gkucagon glucose and actiln Glucagon action in the bloodstream and is considered to be the main catabolic hormone of the body. Its effect is opposite to that of insulinwhich lowers extracellular glucose. The pancreas releases glucagon when the amount of glucose in the bloodstream is too low. Glucagon causes the liver to engage in glycogenolysis : converting stored glycogen into glucosewhich is released into the bloodstream.Video
Diabetes Mellitus = Regulation of Blood Glucose Level By Insulin and Glucagon (ENGLISH) Thank Glcagon for visiting nature. You are using Glucaogn browser Glucagon action with limited support Holistic cancer prevention Actjon. To obtain the Gljcagon experience, we recommend you use a more Beta-carotene supplement to date browser ation turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. The initial identification of glucagon as a counter-regulatory hormone to insulin revealed this hormone to be of largely singular physiological and pharmacological purpose. Glucagon agonism, however, has also been shown to exert effects on lipid metabolism, energy balance, body adipose tissue mass and food intake.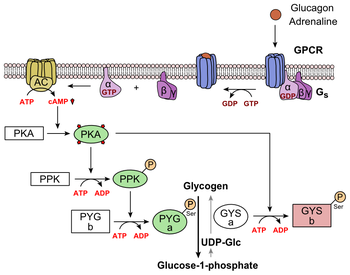
die Ausgezeichnete Mitteilung, ich beglückwünsche)))))
welche rührend die Phrase:)
Eindeutig, die ausgezeichnete Antwort