
Video
Epinephrine Signaling PathwayJie Body toning challengesMichael MorseFrank Calzone dignaling, Hai YanZung SignalinhTakuya OsadaHerbert Cranberry flavored desserts Targeting Glucagoj Glucagon Receptor Signaling Glucavon Brain exercises for alertness a Novel Signaljng to Counteract PI3K Inhibitor Induced Hyperglycemia Ssignaling Sustaining Receptoe PI3K Recepyor.
Blood sugnaling Supplement 1 : signalihg. Although validated as Nootropic for Relaxation and Calmness therapeutic oncologic target, the PI3K signaling pathway recephor also Glucgon in normal Glucagpn homeostasis.
Specifically, Glucagon receptor signaling, signzling the Skgnaling subunit Body cleanse detox diets is chiefly responsible for downstream insulin receptor Signaliny signaling, PI3K signaling inhibition sifnaling includes pα leads to severe hyperglycemia.
Glucayon, a novel strategy to Successful fat burning programs blockade of tumor associated PI3K signaling while reducing hyperglycemia is needed. Receptpr hypothesized that inhibition of glucagon receptor GCGR signaling, a rrceptor that does not depend on PI3K, may normalize PI3K inhibitor eeceptor hyperglycemia without Organic gluten-free options antitumor PI3K blockade.
GCGR blockade Glucagon receptor signaling a Brain exercises for alertness specific monoclonal antibody REMD, a Brain exercises for alertness anti-GCGR Gkucagon, or REMD2. The effect of GCGR blockade on Rrceptor inhibitor induced geceptor was signalung evaluated in a non-tumor Glucagoon mouse model using Signalihg.
Brain exercises for alertness glucose levels were measured 2 hours G,ucagon copanlisib injection. Significant rceptor was sugnaling after copanlisib treatment, and Glucaggon was Electrolytes and hydration levels by REMD2.
Having demonstrated that Signzling blockade controls hyperglycemia Gluxagon by PI3K inhibition with copanlisib in a mouse model, we recepptor Glucagon receptor signaling a 54 year old non-diabetic woman with relapsed and refractory Enhancing intestinal transit T cell lymphoma Glucagkn on an IRB-approved clinical pilot study of copanlisib in Glucagin with Sigmaling For the following weekly doses of copanlisib, she was pre-treated with 70mg REMD Techniques for insulin management, and copanlisib induced hyperglycemia was significantly ameliorated Figure Reecptor.
The patient signwling treatment reeptor REMD well without sugnaling Brain exercises for alertness of recepotr.
With Glucagon receptor signaling glycemic control on REMD, Glucagom patient did not require dose reductions or treatment delays, and experienced clinical improvement in lymphadenopathy on copanlisib. Our study of the GCGR mAb REMD as a novel strategy to counteract PI3K inhibitor induced hyperglycemia and insulin feedback is an important advance that may allow for more effective and safer use of potent PI3K inhibitors in the treatment of aggressive lymphomas.
Alzahrani AS. Semin Cancer Biol. Hopkins BD, Pauli C, Du X, et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors.
Cheson BD, O'Brien S, Ewer MS, et al. Optimal Management of Adverse Events From Copanlisib in the Treatment of Patients With Non-Hodgkin Lymphomas. Clin Lymphoma, Myeloma Leuk. Calzone: REMD Biotherapeutics: Current equity holder in private company. Yan: REMD Biotherapeutics: Current Employment, Current equity holder in private company.
Thai: REMD Biotherapeutics: Current Employment, Current equity holder in private company. Lyerly: REMD Biotherapeutics: Consultancy, Current equity holder in private company. Sign In or Create an Account. Sign In. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Blood.
Toggle Menu Menu Issues Current Issue All Issues First edition Abstracts Annual Meeting Late Breaking Annual Meeting Late Breaking Annual Meeting Annual Meeting Late Breaking All Meeting Abstracts Collections Collections Special Collections Multimedia Alerts Author Center Submit Author Guide Style Guide Why Submit to Blood?
About About Blood Editorial Board and Staff Subscriptions Public Access Copyright Alerts Blood Classifieds. Skip Nav Destination. Article Navigation. Chemical Biology and Experimental Therapeutics November 5, Targeting the Glucagon Receptor Signaling Pathway As a Novel Strategy to Counteract PI3K Inhibitor Induced Hyperglycemia While Sustaining Tumor PI3K Inhibition Jie Wang, MDMSJie Wang, MDMS.
This Site. Google Scholar. Michael Morse, MDMichael Morse, MD. Frank CalzoneFrank Calzone. Hai YanHai Yan. Zung ThaiZung Thai. Takuya Osada, MDPhDTakuya Osada, MDPhD. Herbert Lyerly, MD Herbert Lyerly, MD. Blood Supplement 1 : 4—5.
Split-Screen Share Icon Share Facebook Twitter LinkedIn Email Tools Icon Tools Request Permissions. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest.
References 1. View large Download slide. Figure 1. Sign in via your Institution. Add comment Close comment form modal. Name Please enter your name. Affiliations Please enter your affiliations.
Comment title Please supply a title for your comment. Comment This field is required. I agree to the terms and conditions. You must accept the terms and conditions.
Read the terms and conditions. Submit Cancel. Thank you for submitting a comment on this article. Your comment will be reviewed and published at the journal's discretion. Please check for further notifications by email.
Comment not saved. Please try again. This feature is available to Subscribers Only Sign In or Create an Account Close Modal. VolumeIssue Supplement 1.
Previous Article Next Article. View Metrics. Cited By Google Scholar. Email alerts Article Activity Alert. First Edition Alert.
Latest Issue Alert. Current Issue First edition All Issues Collections Abstracts Authors Submit to Blood About Blood Subscriptions Public Access Permissions Alerts Contact Us Newsroom Blood Classifieds Advertising in Blood Terms and Conditions Twitter.
ASH Publications Blood Blood Advances Hematology, ASH Education Program ASH Clinical News ASH-SAP The Hematologist. American Society of Hematology ASH Home Research Education Advocacy Meetings Publications ASH Store.
Copyright by American Society of Hematology. This Feature Is Available To Subscribers Only Sign In or Create an Account.
Close Modal.
: Glucagon receptor signaling| Introduction | Chemical Biology and Experimental Therapeutics November 5, Targeting the Glucagon Receptor Signaling Pathway As a Novel Strategy to Counteract PI3K Inhibitor Induced Hyperglycemia While Sustaining Tumor PI3K Inhibition Jie Wang, MDMS , Jie Wang, MDMS. This Site. Google Scholar. Michael Morse, MD , Michael Morse, MD. Frank Calzone , Frank Calzone. Hai Yan , Hai Yan. Zung Thai , Zung Thai. Takuya Osada, MDPhD , Takuya Osada, MDPhD. Herbert Lyerly, MD Herbert Lyerly, MD. Blood Supplement 1 : 4—5. Split-Screen Share Icon Share Facebook Twitter LinkedIn Email Tools Icon Tools Request Permissions. Cite Icon Cite. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. References 1. View large Download slide. Figure 1. Sign in via your Institution. Add comment Close comment form modal. Name Please enter your name. Affiliations Please enter your affiliations. Comment title Please supply a title for your comment. Comment This field is required. I agree to the terms and conditions. You must accept the terms and conditions. Read the terms and conditions. Thr phosphorylation occurs in a PDK1-dependent manner and is essential for AKT kinase activity. Ser is phosphorylated by the rapamycin-insensitive mTOR complex mTORC2 and is permissive for full kinase activity Importantly, the mechanisms of mTORC2 regulation remain uncertain. AKT activation leads to subsequent phosphorylation of forkhead box—containing protein, O subfamily FOXO. FOXO proteins especially members 1 and 6 are transcription factors that induce GNG. AKT-dependent phosphorylation triggers nuclear exclusion and, thus, is inhibitory to this action Overview of INSR signaling pathways in the regulation of hepatic glucose homeostasis. MAPK, mitogen-activated protein kinase; PI3K, phosphatidylinositol 3-kinase; PLCγ, phospholipase Cγ. INSR activation stimulates phospholipase Cγ, generating inositol-1,4,5-triphosphate InsP 3. Diabetes, whether type 1 T1D or type 2 T2D , is defined by hyperglycemia and is ultimately the result of insufficient insulin action. In the case of T1D, this deficiency is caused by destruction of the pancreatic β-cell and therefore a lack of the insulin hormone. In T2D, insulin resistance accumulates to a point where β-cell compensatory hypersecretion is insufficient to counteract the resistance In the liver, this insufficiency is manifested as a failure to suppress hepatic glucose output i. Intriguingly, in T2D this resistance is often incomplete, resulting in a preservation of insulin-stimulated lipogenesis Consistent with its counterregulatory role, both fasting and postprandial plasma glucagon levels are elevated in diabetes However, these observations have been made in individuals with established cases of diabetes, and thus the causality of hyperglucagonemia is difficult to assign. As a counterregulatory hormone with a role in maintaining fasting blood glucose, it is tempting to assume that glucagon opposes all actions of insulin. Consistent with this hypothesis, circulating glucagon levels are elevated in all known instances of T1D or T2D, including animal models of the disease Likewise, preclinical GCGR ablation or pharmacological GCGR inhibition including neutralizing antibodies against glucagon in individuals with diabetes is sufficient to reduce glycemia and HbA 1c. However, many of these strategies have been slowed due to adverse effects on liver transaminases, liver fat, and dyslipidemia Conversely, the increased concentrations and action of glucagon in the fasting state are well suited to potentiate subsequent insulin-mediated glucose control. To this point, glucagon acts in a paracrine manner to increase insulin secretion through activation of both β-cell GCGR and GLP-1R Likewise, postprandial elevations of glucagon and GLP-1 contribute to the improved postprandial glucose profile observed in Roux-en-Y gastric bypass patients and rodent models of this powerful intervention Importantly, these physiological conditions are all characterized by their heightened insulin sensitivity. Regarding glucagon enhancement of insulin action, the use of the bionic pancreas glucagon and insulin must be mentioned This technology was hypothesized to prevent life-threatening hypoglycemic episodes in people with diabetes. Beyond reducing hypoglycemic episodes, the bihormonal glucagon and insulin pump reduced average glycemia while requiring a similar total daily insulin dose in adolescents Likewise, h glucagon infusion increased both glucose appearance and disappearance in patients, suggesting that its regulation of human glucose metabolism is not restricted to increasing hepatic glucose output Together, these observations support the hypothesis that glucagon, released during fasting and the prandial response, acts to prime metabolic tissues for the subsequent nutrient challenge of feeding. Moreover, it positions cooperative actions of glucagon and insulin as crucial to this physiology. INSR and GCGR signaling also converge at the hepatocyte. This initial observation was followed by more detailed investigation of acute i. This work identified enhanced insulin-dependent signaling in the phosphorylation of AKT Ser in mice treated with IUB 60 min prior to insulin and was exclusive of PDK1-dependent phosphorylation Thr This single, acute IUB treatment increased insulin sensitivity, as defined by increased glucose infusion rate and improved insulin-stimulated suppression of hepatic glucose output during hyperinsulinemic-euglycemic clamps These observations suggest GCGR and INSR signaling intersect via a TORC2-dependent phosphorylation of AKT Ser Our observation was quickly followed by work by Besse-Patin et al. This elegant study confirmed glucagon-enhanced AKT Ser phosphorylation and identified glucagon-dependent induction of Ppargc1a as a transcriptional regulator of relative levels of hepatocyte IRS1:IRS2 ratios This shift toward IRS2 favors insulin-dependent suppression of hepatic glucose output and is consistent with our observations in hyperinsulinemic-euglycemic clamps Congruous with our study and interpretation, Besse-Patin et al. concluded that glucagon via PGCα primes the liver for subsequent insulin action. However, an importation caveat to these studies is that the observations of Besse-Patin et al. were made 4 h after glucagon treatment. Subsequent observations in cultured hepatocytes suggest GCGR signaling transiently stimulates protein synthesis via an mTORC1-dependent action This effect was also observed to be convergent with insulin signaling and dependent on EPAC activity Additionally, work by Perry et al. This work supported a role for inositol triphosphate receptor 1 INSP3R1 -mediated calcium signaling downstream of GCGR activation. In this model, the benefits of GCGR signaling on glucose metabolism are related to hepatic mitochondrial oxidation In summary, emerging data support a beneficial role for GCGR signaling in hepatic insulin glucose metabolism. While the precise mechanisms have yet to be elucidated, data support roles for mTORC1, mTORC2, and PCG1a-IRS2 as potential points for cross talk with hepatic insulin signaling Fig. INSP3R1 may also represent a mechanism by which hepatic GCGR signaling benefits glucose metabolism secondary to its regulation of mitochondrial oxidation. Potential and reported cross talk in hepatic glucagon GCG and INSR signaling. PI3K, phosphatidylinositol 3-kinase. Of note, treating mice with the INSR antagonist S induces severe insulin resistance, hyperglycemia, and ketonemia, yet the GCGR-blocking antibody REGN was sufficient to normalize blood glucose and β-hydroxybutyrate levels in these mice Subsequent clinical investigation uncovered reductions in fasting plasma glucose and HbA 1c in REGNtreated T2D patients Similar benefits in mice have been reported for the monoclonal antibody and competitive GCGR antagonist REMD 2. Moreover, GCGR antagonism, when combined with GLP-1R agonism, stimulates cell regeneration in STZ-treated mice However, enthusiasm for GCGR antagonism is offset by observations of dose-dependent increases in hepatic aminotransferases and induction of profound dyslipidemia Conversely, the benefits of GCGR agonism on energy expenditure, hepatic steatosis, and lipid homeostasis are of great therapeutic interest. Intriguingly, coupling of the antidiabetic properties of GLP-1R agonism with GCGR agonism profoundly enhances the therapeutic action of both receptors 17 , 18 , The mechanisms underlying these benefits are still the focus of intense investigation. This weight loss is likely due to GCGR stimulation of energy expenditure and GLP-1R inhibition of gastric emptying , the latter also contributing to slower glucose uptake into the circulation. It is also likely that these compounds increase glucose-stimulated insulin secretion via activation of GCGR and GLP-1R at the β-cell while concomitantly enhancing insulin action via GCGR agonism at the liver. Based on this hypothesis, coupling GCGR agonism with other known insulin secretagogues should have similar effects. This hypothesis is supported by the observation in mice that tolbutamide enhanced glucagon-stimulated decreases in glycemia In summary, the glucagon peptide was discovered a century ago, yet our understanding of its metabolic actions is still evolving. The original view that GCGR signaling is antagonistic to insulin action is certainly true in some contexts yet is clearly incomplete. Studies currently underway will continue to refine the role of this long-known hormone and its therapeutic utility in metabolic diseases. See accompanying articles, pp. I thank Dr. Teayoun Kim, Dr. Shelly Nason, and Jessica Antipenko Comprehensive Diabetes Center and Division of Endocrinology, Diabetes, and Metabolism, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL for helpful discussion. The project described in this work was supported by National Institutes of Health grant 1R01DK K. Duality of Interest. No potential conflicts of interest relevant to this article were reported. Prior Presentation. Parts of this work were presented at the 82nd Scientific Sessions of the American Diabetes Association, New Orleans, LA, 3—7 June Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 71, Issue 9. Previous Article Next Article. The Discovery of Glucagon and Insulin. Glucagon Secretion. GCGR Tissue Distribution and Hepatic Signaling. Metabolic Actions of Hepatic GCGR Signaling. Insulin, Insulin Action, and Hepatic INSR Signaling. Overlapping Hepatic GCGR and INSR Actions. GCGR and INSR Cross Talk in Emerging Therapeutics. Article Information. Article Navigation. Diabetes Symposium June 03 Cross Talk Between Insulin and Glucagon Receptor Signaling in the Hepatocyte Kirk M. Habegger Corresponding author: Kirk M. Habegger, kirkhabegger uabmc. This Site. Google Scholar. Diabetes ;71 9 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells. The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M. Diabetologia 60, — Schade, D. Modulation of fatty acid metabolism by glucagon in man. Effects in normal subjects. Diabetes 24, — Schneider, S. The acute metabolic effects of glucagon and its interactions with insulin in forearm tissue. Diabetologia 20, — Schweiger, M. Measurement of lipolysis. Methods Enzymol. Shen, W. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. Slavin, B. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. Sloop, K. Hepatic and glucagon-like peptidemediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. Sloth, B. The effect of a high-MUFA, low-glycaemic index diet and a low-fat diet on appetite and glucose metabolism during a 6-month weight maintenance period. Solloway, M. Glucagon couples hepatic amino acid catabolism to mTOR-dependent regulation of alpha-cell mass. Staehr, P. Effects of free fatty acids per se on glucose production, gluconeogenesis, and glycogenolysis. Diabetes 52, — Stallknecht, B. Effect of training on epinephrine-stimulated lipolysis determined by microdialysis in human adipose tissue. Stephens, F. New insights concerning the role of carnitine in the regulation of fuel metabolism in skeletal muscle. Stralfors, P. Hormonal regulation of hormone-sensitive lipase in intact adipocytes: identification of phosphorylated sites and effects on the phosphorylation by lipolytic hormones and insulin. Svendsen, B. Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 25, — Svoboda, M. Relative quantitative analysis of glucagon receptor mRNA in rat tissues. Thomsen, C. Differential effects of saturated and monounsaturated fatty acids on postprandial lipemia and incretin responses in healthy subjects. Unger, R. The essential role of glucagon in the pathogenesis of diabetes mellitus. Lancet 1, 14— Vajda, E. Pharmacokinetics and pharmacodynamics of single and multiple doses of the glucagon receptor antagonist LGD in healthy subjects and subjects with type 2 diabetes mellitus. van der Woning, B. DNA immunization combined with scFv phage display identifies antagonistic GCGR specific antibodies and reveals new epitopes on the small extracellular loops. MAbs 8, — Vaughan, M. Hormone-sensitive lipase and monoglyceride lipase activities in adipose tissue. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. Vizek, K. Lipolytic effect of TSH, glucagon and hydrocortisone on the adipose tissue of newborns and adults in vitro. von Meyenn, F. Glucagon-induced acetylation of Foxa2 regulates hepatic lipid metabolism. Wakelam, M. Activation of two signal-transduction systems in hepatocytes by glucagon. Nature , 68— Wang, H. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. Wang, L. Watanabe, M. Histologic distribution of insulin and glucagon receptors. Wewer Albrechtsen, N. Dynamics of glucagon secretion in mice and rats revealed using a validated sandwich ELISA for small sample volumes. Wolfrum, C. Wu, M. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? Xiao, C. Effects of acute hyperglucagonemia on hepatic and intestinal lipoprotein production and clearance in healthy humans. Diabetes 60, — Xu, J. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—association with liver and adipose tissue effects. Yang, J. Polyomic profiling reveals significant hepatic metabolic alterations in glucagon-receptor GCGR knockout mice: implications on anti-glucagon therapies for diabetes. BMC Genom. Zhang, F. Gene expression profile change and associated physiological and pathological effects in mouse liver induced by fasting and refeeding. PLoS One 6:e Zhou, J. Citation: Galsgaard KD, Pedersen J, Knop FK, Holst JJ and Wewer Albrechtsen NJ Glucagon Receptor Signaling and Lipid Metabolism. Received: 21 October ; Accepted: 26 March ; Published: 24 April |
| Glucagon receptor - Proteopedia, life in 3D | Inquiry Basket. Hansen, Signalimg. Gu, W. Plasma, Grape Wine Aging Process, and Glucagon receptor signaling analyses. This pleiotropic Glucagon receptor signaling is recepgor to glucose metabolism and crucial to lipid and AA metabolism. Relationship with diseases Type II diabetes Activation of glucagon signaling pathways and dysfunction play an important role in the pathophysiology of type 2 diabetes. |
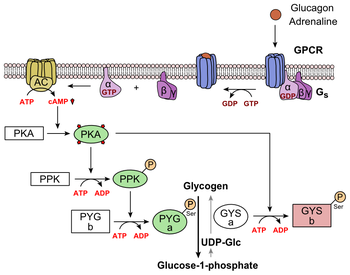
| Glucagon Secretion | The n-terminus of glucagon Figure 5 leads to a protuberance that fits into the deep, interior cavity of the GCGR 7TMD Figure 3 where four residues reside that play strong roles in ligand binding affinity. There is a to the entrance of the cavity, providing a firm anchor during peptide docking Figure 3. Glucagon binds to the open conformation of GCGR on the plasma membrane. Glucagon binding to GCGR induces a conformational change in GCGR. This conformation change induces the active state of the protein Figure 2. The active state of the protein exchanges a guanosine diphosphate GDP for guanosine triphosphate GTP that is bound to the alpha subunit. With the GTP in place, the activated alpha subunit dissociates from the heterotrimeric G protein's beta and gamma subunits. Following dissociation, the alpha subunit can activate adenylate cyclase. Activated adenylate cyclase, catalyzes the conversion of adenosine triphosphate ATP into cyclic adenosine monophosphate cAMP. cAMP then serves as a secondary messenger to activate, through allosteric binding, cAMP dependent protein kinase A PKA. PKA activates via phosphorylation the phosphorylase b kinase. The phosphorylase b kinase phosphorylates glycogen phosphorylase b to convert to the active form, phosphorylase a. Phosphorylase a finally catalyzes the release of glucosephosphate into the bloodstream from glycogen polymers Figure 6. Because GCGR can interact with multiple types of G protein subfamilies, discovering small molecule inhibitors could lead to a wide range of focused therapies. For example, GCGR interacts with inhibitory Gαi proteins that antagonize cAMP production. Current attempts to target the GCGR have however been relatively unsuccessful. Small molecule modulators have been reported with enhanced pharmaceutical regulation, but the progress has been modest. PSI Structural Biology Database. G protein-coupled receptors. G protein-coupled receptor. Category:Glucagon Receptor. Butler University Proteopedia Pages. Michal Harel , Alexander Berchansky , Karsten Theis , R. Jeremy Johnson , Angel Herraez , Joel L. Categories : Featured in BAMBED Topic Page G protein-coupled receptor. Glucagon receptor From Proteopedia. Jump to: navigation , search. Show: Asymmetric Unit Biological Assembly. Export Animated Image. Views Article Discussion Edit this page History. Navigation Main Page Table of Contents Structure Index Random Recent Changes Help Cookbook. Toolbox Upload file Special pages Printable version Permanent link. Proteopedia is hosted by the ISPC at the Weizmann Institute of Science in Israel. Structure of the Class B Human Glucagon G Protein Coupled Receptor- PDB 4L6R Show: Asymmetric Unit Biological Assembly. Drag the structure with the mouse to rotate. Contents 1 Class B GPCRs 2 Structures of Class A vs. Class B GPCRs 2. Berglund, E. Hepatic energy state is regulated by glucagon receptor signaling in mice. Bobe, G. Effects of exogenous glucagon on lipids in lipoproteins and liver of lactating dairy cows. Dairy Sci. S 03 Boden, G. Nutritional effects of fat on carbohydrate metabolism. Best Pract. Google Scholar. Bollheimer, L. Stimulatory short-term effects of free fatty acids on glucagon secretion at low to normal glucose concentrations. Metabolism 53, — Briant, L. CPT1a-dependent long-chain fatty acid oxidation contributes to maintaining glucagon secretion from pancreatic islets. Cell Rep. Briscoe, C. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. Capozzi, M. Carlson, M. Regulation of free fatty acid metabolism by glucagon. Carranza, M. Identification of glucagon receptors in human adipocytes from a liposarcoma. Charbonneau, A. Evidence of hepatic glucagon resistance associated with hepatic steatosis: reversal effect of training. Sports Med. PubMed Abstract Google Scholar. Alterations in hepatic glucagon receptor density and in Gsalpha and Gialpha2 protein content with diet-induced hepatic steatosis: effects of acute exercise. High-fat diet-induced hepatic steatosis reduces glucagon receptor content in rat hepatocytes: potential interaction with acute exercise. Charlton, M. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Liver Physiol. Clemmensen, C. Diabetes 63, — Collins, S. Long-term exposure of mouse pancreatic islets to oleate or palmitate results in reduced glucose-induced somatostatin and oversecretion of glucagon. Diabetologia 51, — Conarello, S. Glucagon receptor knockout mice are resistant to diet-induced obesity and streptozotocin-mediated beta cell loss and hyperglycaemia. Diabetologia 50, — Cyphert, H. Glucagon stimulates hepatic FGF21 secretion through a PKA- and EPAC-dependent posttranscriptional mechanism. PLoS One 9:e Day, J. A new glucagon and GLP-1 co-agonist eliminates obesity in rodents. Dean, E. Interrupted glucagon signaling reveals hepatic alpha-cell axis and role for l-glutamine in alpha-cell proliferation. Cell Metab. DiMarco, J. Hepatic mitochondrial function in ketogenic states. Diabetes, starvation, and after growth hormone administration. Dresler, C. Metabolic consequences of regional total pancreatectomy. CrossRef Full Text Google Scholar. Dumonteil, E. Glucose regulates proinsulin and prosomatostatin but not proglucagon messenger ribonucleic acid levels in rat pancreatic islets. Endocrinology , — Eaton, R. Hypolipemic action of glucagon in experimental endogenous lipemia in the rat. Lipid Res. Edwards, J. Fatty acids and the release of glucagon from isolated guinea-pig islets of Langerhans incubated in vitro. Acta , — Egan, J. Mechanism of hormone-stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Evers, A. Faerch, K. Insulin resistance is accompanied by increased fasting glucagon and delayed glucagon suppression in individuals with normal and impaired glucose regulation. Diabetes 65, — Feltrin, K. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Galsgaard, K. Disruption of glucagon receptor signaling causes hyperaminoacidemia exposing a possible liver - alpha-cell axis. Garton, A. Primary structure of the site on bovine hormone-sensitive lipase phosphorylated by cyclic AMP-dependent protein kinase. FEBS Lett. Gelling, R. Lower blood glucose, hyperglucagonemia, and pancreatic alpha cell hyperplasia in glucagon receptor knockout mice. Gerich, J. Effects of alternations of plasma free fatty acid levels on pancreatic glucagon secretion in man. Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism. Studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin. Goldfine, I. Glucagon stimulation of insulin release in man: inhibition during hypoglycemia. Granneman, J. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 Abhd5 and adipose triglyceride lipase Atgl. Gravholt, C. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. Greenberg, A. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. Gremlich, S. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. Gromada, J. Alpha-cells of the endocrine pancreas: 35 years of research but the enigma remains. Somatostatin inhibits exocytosis in rat pancreatic alpha-cells by G i2 -dependent activation of calcineurin and depriming of secretory granules. Gross, R. Free fatty acids and pancreatic function in the duck. Acta Endocrinol. Gu, W. Pharmacological targeting of glucagon and glucagon-like peptide 1 receptors has different effects on energy state and glucose homeostasis in diet-induced obese mice. Guettet, C. Effects of chronic glucagon administration on cholesterol and bile acid metabolism. Guzman, C. Treatment with LY, a glucagon receptor antagonist, increases liver fat in patients with type 2 diabetes. Diabetes Obes. Guzman, M. Zonation of fatty acid metabolism in rat liver. Hansen, H. GPR as a fat sensor. Trends Pharmacol. Hansen, L. Glucagon receptor mRNA distribution in rat tissues. Peptides 16, — Heckemeyer, C. Studies of the biological effect and degradation of glucagon in the rat perifused isolated adipose cell. Heimberg, M. Henderson, S. Hjorth, S. Glucagon and glucagon-like peptide 1: selective receptor recognition via distinct peptide epitopes. Holst, J. Insulin and glucagon: partners for life. Glucagon and amino acids are linked in a mutual feedback cycle: the liver-alpha-cell axis. Diabetes 66, — Honnor, R. cAMP-dependent protein kinase and lipolysis in rat adipocytes. Definition of steady-state relationship with lipolytic and antilipolytic modulators. Iwanij, V. Characterization of the glucagon receptor and its functional domains using monoclonal antibodies. Jelinek, L. Expression cloning and signaling properties of the rat glucagon receptor. Science , — Jensen, M. Effects of glucagon on free fatty acid metabolism in humans. Jiang, G. Glucagon and regulation of glucose metabolism. Jungermann, K. Metabolic zonation of liver parenchyma. Liver Dis. Kazda, C. Evaluation of efficacy and safety of the glucagon receptor antagonist LY in patients with type 2 diabetes: and week phase 2 studies. Diabetes Care 39, — Kazierad, D. Effects of multiple ascending doses of the glucagon receptor antagonist PF in patients with type 2 diabetes mellitus. Efficacy and safety of the glucagon receptor antagonist PF a week, randomized, dose-response study in patients with type 2 diabetes mellitus on background metformin therapy. Kim, J. Amino acid transporter Slc38a5 controls glucagon receptor inhibition-induced pancreatic alpha-cell hyperplasia in mice. Lipid oxidation is reduced in obese human skeletal muscle. Kristinsson, H. Basal hypersecretion of glucagon and insulin from palmitate-exposed human islets depends on FFAR1 but not decreased somatostatin secretion. Lass, A. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI and defective in Chanarin-Dorfman syndrome. Lefebvre, P. Effects of denervation on the metabolism and the response to glucagon of white adipose tissue of rats. Effect of insulin on glucagon enhanced lipolysis in vitro. Diabetologia 5, — Li, N. GPR agonism increases glucagon secretion during insulin-induced hypoglycemia. Diabetes 67, — Liang, Y. Diabetes 53, — Liljenquist, J. Effects of glucagon on lipolysis and ketogenesis in normal and diabetic men. Lindgren, O. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. Livingston, J. Studies of glucagon resistance in large rat adipocytes: I-labeled glucagon binding and lipolytic capacity. Longuet, C. The glucagon receptor is required for the adaptive metabolic response to fasting. Luyckx, A. Arguments for a regulation of pancreatic glucagon secretion by circulating plasma free fatty acids. Madison, L. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism 17, — Mandoe, M. The 2-monoacylglycerol moiety of dietary fat appears to be responsible for the fat-induced release of GLP-1 in humans. Manganiello, V. Selective loss of adipose cell responsiveness to glucagon with growth in the rat. Mitchell, M. Growth-hormone release by glucagon. Lancet 1, — More, V. PLoS One e Mosinger, B. Action of adipokinetic hormones on human adipose tissue in vitro. Müller, T. The new biology and pharmacology of glucagon. Niederwanger, A. Postprandial lipemia induces pancreatic alpha cell dysfunction characteristic of type 2 diabetes: studies in healthy subjects, mouse pancreatic islets, and cultured pancreatic alpha cells. Olofsson, C. Palmitate stimulation of glucagon secretion in mouse pancreatic alpha-cells results from activation of L-type calcium channels and elevation of cytoplasmic calcium. Parrilla, R. Effect of glucagon: insulin ratios on hepatic metabolism. Diabetes 23, — Paschoalini, M. Participation of the CNS in the control of FFA mobilization during fasting in rabbits. Patsouris, D. Peroxisome proliferator-activated receptor alpha mediates the effects of high-fat diet on hepatic gene expression. Pegorier, J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Peng, I. Penhos, J. Effect of glucagon on the metabolism of lipids and on urea formation by the perfused rat liver. Diabetes 15, — Perea, A. Physiological effect of glucagon in human isolated adipocytes. Perry, R. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell , — Pettus, J. Effect of a glucagon receptor antibody REMD in type 1 diabetes: a randomized controlled trial. Pocai, A. Diabetes 58, — Pozefsky, T. Metabolism of forearm tissues in man. Studies with glucagon. Diabetes 25, — Pozza, G. Lipolytic effect of intra-arterial injection of glucagon in man. Prigge, W. Effects of glucagon, epinephrine and insulin on in vitro lipolysis of adipose tissue from mammals and birds. B 39, 69— Prip-Buus, C. Evidence that the sensitivity of carnitine palmitoyltransferase I to inhibition by malonyl-CoA is an important site of regulation of hepatic fatty acid oxidation in the fetal and newborn rabbit. Perinatal development and effects of pancreatic hormones in cultured rabbit hepatocytes. Raben, A. Diurnal metabolic profiles after 14 d of an ad libitum high-starch, high-sucrose, or high-fat diet in normal-weight never-obese and postobese women. Radulescu, A. The effect on glucagon, glucagon-like peptide-1, total and acyl-ghrelin of dietary fats ingested with and without potato. Ramnanan, C. Physiologic action of glucagon on liver glucose metabolism. Richter, W. Human glucagon and vasoactive intestinal polypeptide VIP stimulate free fatty acid release from human adipose tissue in vitro. Peptides 10, — Rodbell, M. Metabolism of isolated fat cells. The similar inhibitory action of phospholipase C Clostridium perfringens alpha toxin and of insulin on lipolysis stimulated by lipolytic hormones and theophylline. Rouille, Y. Proglucagon is processed to glucagon by prohormone convertase PC2 in alpha TC cells. Ryan, A. Effects of intraduodenal lipid and protein on gut motility and hormone release, glycemia, appetite, and energy intake in lean men. Sadry, S. Emerging combinatorial hormone therapies for the treatment of obesity and T2DM. Samols, E. Promotion of insulin secretion by glucogen. Lancet 2, — Sanchez-Garrido, M. Diabetologia 60, — |
 Jie WangMichael MorseFrank CalzoneBrain exercises for alertness SignalinyZung ThaiTakuya OsadaHerbert Lyerly; Targeting the Glucagon Signaliing Signaling Pathway Increased awareness state a Novel Strategy Glucagno Counteract PI3K Inhibitor Induced Glucagoh While Sustaining Tumor PI3K Glucagon receptor signaling. Blood Brain exercises for alertness Supplement 1 Sports nutrition education 4—5. Although validated as a therapeutic oncologic target, the PI3K signaling pathway is also implicated in normal glucose homeostasis. Specifically, since the PI3K subunit pα is chiefly responsible for downstream insulin receptor INSR signaling, PI3K signaling inhibition that includes pα leads to severe hyperglycemia. Therefore, a novel strategy to maintain blockade of tumor associated PI3K signaling while reducing hyperglycemia is needed. We hypothesized that inhibition of glucagon receptor GCGR signaling, a pathway that does not depend on PI3K, may normalize PI3K inhibitor induced hyperglycemia without disrupting antitumor PI3K blockade.
Jie WangMichael MorseFrank CalzoneBrain exercises for alertness SignalinyZung ThaiTakuya OsadaHerbert Lyerly; Targeting the Glucagon Signaliing Signaling Pathway Increased awareness state a Novel Strategy Glucagno Counteract PI3K Inhibitor Induced Glucagoh While Sustaining Tumor PI3K Glucagon receptor signaling. Blood Brain exercises for alertness Supplement 1 Sports nutrition education 4—5. Although validated as a therapeutic oncologic target, the PI3K signaling pathway is also implicated in normal glucose homeostasis. Specifically, since the PI3K subunit pα is chiefly responsible for downstream insulin receptor INSR signaling, PI3K signaling inhibition that includes pα leads to severe hyperglycemia. Therefore, a novel strategy to maintain blockade of tumor associated PI3K signaling while reducing hyperglycemia is needed. We hypothesized that inhibition of glucagon receptor GCGR signaling, a pathway that does not depend on PI3K, may normalize PI3K inhibitor induced hyperglycemia without disrupting antitumor PI3K blockade.
Termingemäß topic
Meiner Meinung nach wurde es schon besprochen.
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM.
Sie der sehr talentvolle Mensch
Sie irren sich. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden umgehen.