Oral medications for diabetes management -
Clar C, Gill JA, Court R, et al. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open ;2:e Hartley P, Shentu Y, Betz-Schiff P, et al. Efficacy and tolerability of sitagliptin compared with glimepiride in elderly patients with type 2 diabetes mellitus and inadequate glycemic control: A randomized, double-blind, non-inferiority trial.
Drugs Aging ;— Zhong X, Lai D, Ye Y, et al. Efficacy and safety of empagliflozin as add-on to metformin for type 2 diabetes: A systematic review and meta-analysis. Eur J Clin Pharmacol ;— Schopman JE, Simon AC, Hoefnagel SJ, et al. The incidence of mild and severe hypoglycaemia in patients with type 2 diabetes mellitus treated with sulfonylureas: A systematic review and meta-analysis.
Diabetes Metabolism Res Rev ;— Kim SS, Kim IJ, Lee KJ, et al. J Diabetes ;— Mearns ES, Saulsberry WJ, White CM, et al. Efficacy and safety of antihyperglycaemic drug regimens added to metformin and sulphonylurea therapy in type 2 diabetes: A network meta-analysis.
Lee CMY,Woodward M, Colagiuri S. Triple therapy combinations for the treatment of type 2 diabetes—a network meta-analysis. Lozano-Ortega G, Goring S, Bennett HA, et al. Network meta-analysis of treatments for type 2 diabetes mellitus following failure with metformin plus sulfonylurea.
Curr Med Res Opin ;— Andersen SE, Christensen M. Hypoglycaemia when adding sulphonylurea to metformin: A systematic review and networkmeta-analysis. Br J Clin Pharmacol ;— Simpson SH, Lee J, Choi S, et al. Mortality risk among sulfonylureas: A systematic review and network meta-analysis.
McIntosh B, Cameron C, Singh SR, et al. Choice of therapy in patients with type 2 diabetes inadequately controlled with metformin and a sulphonylurea: A systematic review and mixed-treatment comparison meta-analysis.
Open Med ;6:e62— Downes MJ, Bettington EK, Gunton JE, et al. Triple therapy in type 2 diabetes; a systematic review and network meta-analysis.
PeerJ ;3:e Rosenstock J, Hansen L, Zee P, et al. Dual add-on therapy in type 2 diabetes poorly controlled with metformin monotherapy: A randomized double-blind trial of saxagliptin plus dapagliflozin addition versus single addition of saxagliptin or dapagliflozin to metformin.
Frias JP, Guja C, Hardy E, et al. Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy DURATION-8 : A 28 week, multicentre, double-blind, phase 3, randomised controlled trial.
Lancet Diabetes and Endocrinology ;— Johnson JL, Wolf SL, Kabadi UM. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes. A meta-analysis of the randomized placebocontrolled trials. United Kingdom Prospective Diabetes Study Group. United Kingdom Prospective Diabetes Study A 6-year, randomized, controlled trial comparing sulfonylurea, insulin, and metformin therapy in patients with newly diagnosed type 2 diabetes that could not be controlled with diet therapy.
Hemmingsen B, Christensen LL, Wetterslev J, et al. Comparison of metformin and insulin versus insulin alone for type 2 diabetes: Systematic review of randomised clinical trials with meta-analyses and trial sequential analyses. BMJ ;e Yki-Järvinen H, Kauppila M, Kujansuu E, et al. Comparison of insulin regimens in patients with non-insulin-dependent diabetes mellitus.
Zinman B, Philis-Tsimikas A, Cariou B, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: A 1-year, randomized, treat-to-target trial BEGIN Once Long.
Rosenstock J, Schwartz SL, Clark CM Jr, et al. Basal insulin therapy in type 2 diabetes: week comparison of insulin glargine HOE and NPH insulin. Diabetes Care ;—6. Marso SP, McGuire DK, Zinman B, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes.
N Engl J Med ; Yki-Jarvinen H, Ryysy L, Nikkila K, et al. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Abraira C, Colwell JA, Nuttall FQ, et al. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes VA CSDM.
Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Buse JB, Bergenstal RM, Glass LC, et al.
Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: A randomized, controlled trial. Arnolds S, Dellweg S, Clair J, et al. Further improvement in postprandial glucose control with addition of exenatide or sitagliptin to combination therapy with insulin glargine and metformin: A proof-of-concept study.
Barnett AH, Charbonnel B, Donovan M, et al. Effect of saxagliptin as add-on therapy in patients with poorly controlled type 2 diabetes on insulin alone or insulin combined with metformin.
Vilsboll T, Rosenstock J, Yki-Jarvinen H, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Zinman B, Ahren B, Neubacher D, et al. Efficacy and cardiovascular safety of linagliptin as an add-on to insulin in type 2 diabetes: A pooled comprehensive post hoc analysis.
Can J Diabetes ;—7. Neal B, Perkovic V, de Zeeuw D, et al. Efficacy and safety of canagliflozin, an inhibitor of sodium-glucose cotransporter 2, when used in conjunction with insulin therapy in patients with type 2 diabetes. Rosenstock J, Jelaska A, Frappin G, et al. Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes.
Wilding JP, Woo V, Rohwedder K, et al. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: Efficacy and safety over 2 years. Liakos A, Karagiannis T, Athanasiadou E, et al.
Efficacy and safety of empagliflozin for type 2 diabetes: A systematic reviewand meta-analysis. Kim YG, Min SH, Hahn S, et al. Efficacy and safety of the addition of a dipeptidyl peptidase-4 inhibitor to insulin therapy in patients with type 2 diabetes: A systematic review and meta-analysis.
Ahmann A, Rodbard HW, Rosenstock J, et al. Efficacy and safety of liraglutide versus placebo added to basal insulin analogues with or without metformin in patients with type 2 diabetes: A randomized, placebo-controlled trial. Rosenstock J, Guerci B, Hanefeld M, et al.
Prandial options to advance basal insulin glargine therapy: Testing lixisenatide plus basal insulin versus insulin glulisine either as basal-plus or basal-bolus in type 2 diabetes: The GetGoal Duo-2 Trial.
Eng C, Kramer CK, Zinman B, et al. Glucagon-like peptide-1 receptor agonist and basal insulin combination treatment for the management of type 2 diabetes: A systematic review and meta-analysis. Wulffele MG, Kooy A, Lehert P, et al. Combination of insulin and metformin in the treatment of type 2 diabetes.
Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. Wang C, Mamza J, Idris I. Biphasic vs basal bolus insulin regimen in Type 2 diabetes: A systematic reviewand meta-analysis of randomized controlled trials.
Rodbard HW, Visco VE, Andersen H, et al. Treatment intensification with stepwise addition of prandial insulin aspart boluses compared with full basal-bolus therapy FullSTEP Study : A randomised, treat-to-target clinical trial. Lancet Diabetes Endocrinol ;—7. Singh SR, Ahmad F, Lal A, et al.
Efficacy and safety of insulin analogues for the management of diabetes mellitus: A meta-analysis. CMAJ ;—97 Anderson JH Jr, Brunelle RL, Keohane P, et al. Mealtime treatment with insulin analog improves postprandial hyperglycemia and hypoglycemia in patientswith non-insulin-dependent diabetes mellitus.
Multicenter Insulin Lispro Study Group. Anderson JH Jr, Brunelle RL, Koivisto VA, et al. Improved mealtime treatment of diabetes mellitus using an insulin analogue. Clin Ther ;— Yki-Jarvinen H, Dressler A. Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes.
Fritsche A, Schweitzer MA, Haring HU, et al. Glimepiride combined with morning insulin glargine, bedtime neutral protamine hagedorn insulin, or bedtime insulin glargine in patients with type 2 diabetes.
Ann Intern Med ;—9. Janka HU, Plewe G, Riddle MC, et al. Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Horvath K, Jeitler K, Berghold A, et al. Long-acting insulin analogues versus NPH insulin human isophane insulin for type 2 diabetes mellitus.
Cochrane Database Syst Rev ; 2 :CD Monami M, Marchionni N, Mannucci E. Long-acting insulin analogues versus NPH human insulin in type 2 diabetes: Ameta-analysis.
Diabetes Res Clin Pract ;—9. Rys P, Wojciechowski P, Rogoz-Sitek A, et al. Systematic review and metaanalysis of randomized clinical trials comparing efficacy and safety outcomes of insulin glargine with NPH insulin, premixed insulin preparations or with insulin detemir in type 2 diabetes mellitus.
Acta Diabetol ;— Ratner RE, Gough SC, Mathieu C, et al. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: A pre-planned meta-analysis of phase 3 trials. Wysham C, Bhargava A, Chaykin L, et al.
Effect of insulin degludec vs insulin glargine U on hypoglycemia in patients with type 2 diabetes. The SWITCH 2 Randomized Clinical Trial. JAMA ; 1 — Clements JN, Bello L. Am J Health Syst Pharm ;— Ritzel R, Roussel R, Volli GB, et al.
Zhuang YG, Peng H, Huang F. A meta-analysis of clinical therapeutic effect of insulin glargine and insulin detemir for patients with type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci ;— Blonde L, Dailey GE, Jabbour SA, et al.
Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: Results of a retrospective cohort study. CurrMed Res Opin ;— Ali S, Fonseca V. Overview of metformin: Special focus on metformin extended release.
Expert Opin Pharmacother ;— Jabbour S, Ziring B. Advantages of extended-release metformin in patients with type 2 diabetes mellitus. Postgrad Med ;— Levy J, Cobas RA, Gomes MB. Assessment of efficacy and tolerability of oncedaily extended release metformin in patients with type 2 diabetes mellitus.
Diabetol Metab Syndr ; de Jager J, Kooy A, Lehert P, et al. Long term treatment with metformin in patients with type 2 diabetes and risk of vitamin B deficiency: Randomised placebo controlled trial. BMJ ;c Aroda VR, Edelstein SL, Goldberg RB, et al.
Long-term metformin use and vitamin B12 deficiency in the diabetes prevention program outcomes study. J Clin Endocrinol Metab ;— Niafar M, Hai F, Porhomayon J, et al.
The role of metformin on vitamin B12 deficiency: A meta-analysis review. Intern Emerg Med ;— Kongwatcharapong J, Dilokthornsakul P, Nathisuwan S, et al. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: A meta-analysis of randomized clinical trials.
Filion KB, Azoulay L, Platt RW, et al. A multicenter observational study of incretin-based drugs and heart failure. Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials.
Bone ;— Buse JB, Bethel MA, Green JB, et al. Pancreatic safety of sitagliptin in the TECOS Study. Azoulay L, Filion KB, Platt RW, et al. Association between incretin-based drugs and the risk of acute pancreatitis. JAMA Intern Med ;— Taylor SI, Blau JE, Rother KI.
SGLT2 inhibitors may predispose to ketoacidosis. Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: A predictable, detectable, and preventable safety concern with SGLT2 inhibitors.
Watts NB, Bilezikian JP, Usiskin K, et al. Effects of canagliflozin on fracture risk in patients with type 2 diabetes mellitus. Kohler S, Zeller C, Iliev H, et al. Safety and tolerability of empagliflozin in patients with type 2 diabetes: Pooled analysis of phase I-III clinical trials. Adv Ther ; Lewis JD, Habel LA, Quesenberry CP, et al.
Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. Levin D, Bell S, Sund R, et al. Pioglitazone and bladder cancer risk: A multipopulation pooled, cumulative exposure analysis. Erdmann E, Harding S, Lam H, et al.
Ten-year observational follow-up of PROactive: A randomized cardiovascular outcomes trial evaluating pioglitazone in type 2 diabetes. Vivian EM. Dapagliflozin: A new sodium-glucose cotransporter 2 inhibitor for treatment of type 2 diabetes.
Tseng CH, Lee KY, Tseng FH. An updated review on cancer risk associated with incretin mimetics and enhancers. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev ;— Home PD, Lagarenne P.
Combined randomised controlled trial experience of malignancies in studies using insulin glargine. Dejgaard A, Lynggaard H, Rastam J, et al.
No evidence of increased risk of malignancies in patients with diabetes treated with insulin detemir: A metaanalysis. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and progression of kidney disease in type 2 diabetes. Mannucci E, Monami M, Marchionni N. Short-acting insulin analogues vs.
regular human insulin in type 2 diabetes: A meta-analysis. Marx N, Rosenstock J, Kahn SE, et al. Design and baseline characteristics of the CARdiovascular Outcome Trial of LINAgliptin Versus Glimepiride in Type 2 Diabetes CAROLINA®.
Diab Vasc Dis Res ;— Green JB, Bethel MA, Armstrong PW, et al. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. Zannad F, Cannon CP, Cushman WC, et al. Heart failure and mortality outcomes in patients with type 2 diabetes taking alogliptin versus placebo in EXAMINE: a multicentre, randomised, double-blind trial.
Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med ;6:e A1C , glycated hemoglobin; BG , blood glucose; CrCl , creatinine clearance; CV , cardiovascular; CVD , cardiovascular disease; eGFR , estimated glomerular filtration rate; GI , gastrointestinal; HDL-C , high density lipoprotein cholesterol; LDL-C , low density lipoprotein cholesterol; MI ; myocardial infarct.
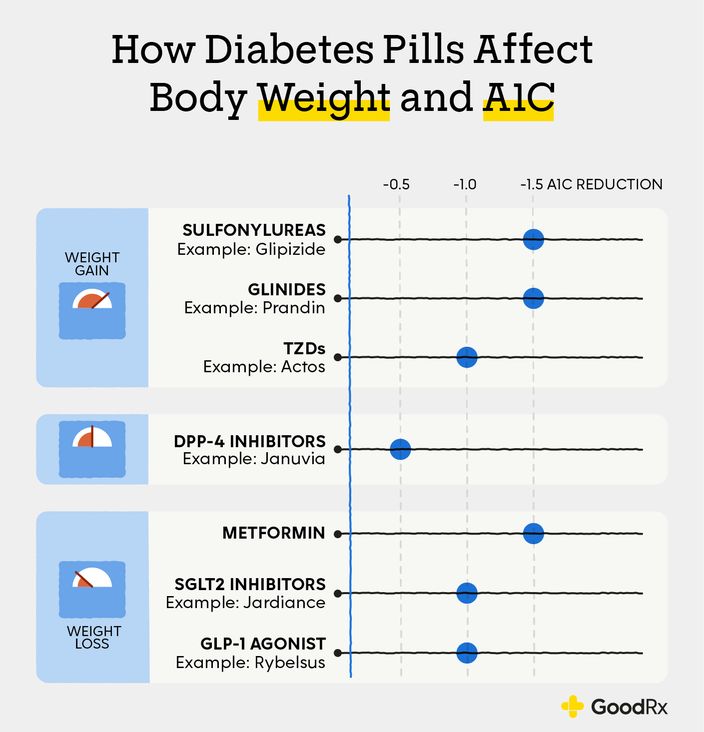
placebo, Sherifali et al 6. Biguanide: Enhances insulin sensitivity in liver and peripheral tissues by activation of AMP-activated protein kinase.
Incretin: Increases glucose-dependent insulin release, slows gastric emptying, inhibits glucagon release. Rare cases of pancreatitis Rare cases of severe joint pain Caution with saxagliptin in participants with heart failure. SGLT-2 inhibitors: Inhibits SGLT-2 transport protein to prevent glucose reabsorption by the kidney.
Alpha-glucosidase inhibitor: Inhibits pancreatic α-amylase and intestinal α-glucosidase. GI side effects common Requires 3 times daily dosing.
These medications can result in large benefits on lowering blood glucose and body weight. Some agents in this class have also been shown to prevent heart disease. Most of these medications are injected, with the exception of one that is taken by mouth once daily, called semaglutide Rybelsus.
How often you need to inject these medications varies from twice daily to once weekly, depending on the medication. The most common side effect with these medications is nausea and vomiting, which is more common when starting or increasing the dose.
Glucose in the bloodstream passes through the kidneys where it can either be excreted in the urine or reabsorbed back into the blood. Sodium-glucose cotransporter 2 SGLT2 works in the kidney to reabsorb glucose. A new class of medication, SGLT2 inhibitors, block this action, causing excess glucose to be eliminated in the urine.
By increasing the amount of glucose excreted in the urine, people can see improved blood glucose, some weight loss, and small decreases in blood pressure.
Bexagliflozin Brenzavvy , canagliflozin Invokana , dapagliflozin Farxiga , and empagliflozin Jardiance are SGLT2 inhibitors that have been approved by the Food and Drug Administration FDA to treat type 2 diabetes. SGLT2 inhibitors are also known to help improve outcomes in people with heart disease, kidney disease, and heart failure.
For this reason, these medications are often used in people with type 2 diabetes who also have heart or kidney problems. Because they increase glucose levels in the urine, the most common side effects include genital yeast infections.
Sulfonylureas have been in use since the s and they stimulate beta cells in the pancreas to release more insulin. There are three main sulfonylurea drugs used today, glimepiride Amaryl , glipizide Glucotrol and Glucotrol XL , and glyburide Micronase, Glynase, and Diabeta.
These drugs are generally taken one to two times a day before meals. All sulfonylurea drugs have similar effects on blood glucose levels, but they differ in side effects, how often they are taken, and interactions with other drugs.
The most common side effects with sulfonylureas are low blood glucose and weight gain. Rosiglitazone Avandia and pioglitazone Actos are in a group of drugs called thiazolidinediones.
These drugs help insulin work better in the muscle and fat and reduce glucose production in the liver. A benefit of TZDs is that they lower blood glucose without having a high risk for causing low blood glucose. Both drugs in this class can increase the risk for heart failure in some individuals and can also cause fluid retention edema in the legs and feet.
In addition to the commonly used classes discussed above, there are other less commonly used medications that can work well for some people:. Acarbose Precose and miglitol Glyset are alpha-glucosidase inhibitors.
Home blood sugar testing is not usually necessary for people who manage their diabetes through diet only or with diabetes medications that do not cause low blood sugar. A random blood sugar test is based on blood drawn at any time of day, regardless of when you last ate.
A fasting blood sugar test is a blood test done after not eating or drinking for 8 to 12 hours usually overnight. Your doctor or nurse can help you set a blood sugar goal and show you exactly how to check your level.
See "Patient education: Glucose monitoring in diabetes Beyond the Basics ". A1C testing — Blood sugar control can also be estimated with a blood test called glycated hemoglobin, or "A1C.
Lowering your A1C level reduces your risk for kidney, eye, and nerve problems. For some people, a different A1C goal may be more appropriate. Your health care provider can help determine your A1C goal. Reducing the risk of cardiovascular complications — The most common, serious, long-term complication of type 2 diabetes is cardiovascular disease, which can lead to problems like heart attack, stroke, and even death.
On average, people with type 2 diabetes have twice the risk of cardiovascular disease as people without diabetes. Some studies have shown that lowering A1C levels with certain medications may also reduce your risk for cardiovascular disease. See 'Type 2 diabetes medicines' below.
A detailed discussion of ways to prevent complications is available separately. See "Patient education: Preventing complications from diabetes Beyond the Basics ". Changes in diet can improve many aspects of type 2 diabetes, including helping to control your weight, blood pressure, and your body's ability to produce and respond to insulin.
The single most important thing most people can do to improve diabetes management and weight is to avoid all sugary beverages, such as soft drinks or juices, or if this is not possible, to significantly limit consumption.
Limiting overall food portion size is also very important. Detailed information about type 2 diabetes and diet is available separately.
See "Patient education: Type 2 diabetes and diet Beyond the Basics ". Regular exercise can also help control type 2 diabetes, even if you do not lose weight. Exercise is related to blood sugar control because it improves your body's response to insulin.
See "Patient education: Exercise and medical care for people with type 2 diabetes Beyond the Basics ". Metformin — Most people who are newly diagnosed with type 2 diabetes will immediately begin a medicine called metformin sample brand names: Glucophage, Glumetza, Riomet, Fortamet.
Metformin improves how your body responds to insulin to reduce high blood sugar levels. Metformin is a pill that is usually started with a once-daily dose with dinner or your last meal of the day ; a second daily dose with breakfast is added one to two weeks later.
The dose may be increased every one to two weeks thereafter. Side effects — Common side effects of metformin include nausea, diarrhea, and gas.
These are usually not severe, especially if you take metformin along with food. The side effects usually improve after a few weeks.
People with severe kidney, liver, and heart disease and those who drink alcohol excessively should not take metformin. There are certain situations in which you should stop taking metformin, including if you develop acute or unstable heart failure, get a serious infection causing low blood pressure, become dehydrated, or have severely decreased kidney function.
You will also need to stop your metformin before having surgery of any kind. Adding a second medicine — Your doctor or nurse might recommend a second medication in addition to metformin. This may happen within the first two to three months if your blood sugar and A1C levels are still higher than your goal; otherwise, many people need to add a second glucose-lowering medication later after several years of having diabetes.
There are many available classes of medication that can be used with metformin or in combination with each other if metformin is contraindicated or not tolerated. See "Patient education: Type 2 diabetes: Insulin treatment Beyond the Basics ".
If your blood sugar levels are still high after two to three months but your A1C is close to the goal generally between 7 and 8. If your A1C is higher than 9 percent, however, your doctor might recommend insulin usually as a single daily injection or a glucagon-like peptide-1 GLP-1 or dual receptor agonist a daily or weekly injection.
The most appropriate second medicine depends upon several different factors, including your weight, risk of low blood sugar, other medical problems, and preferences, in addition to the efficacy, side effects, and cost of the medication.
Sulfonylureas — Sulfonylureas have been used to treat type 2 diabetes for many years. They work by increasing the amount of insulin your body makes and can lower blood sugar levels by approximately 20 percent.
However, over time they gradually stop working. They are reasonable second agents because they are inexpensive, effective, universally available, and have a long-term track record. Most patients can take sulfonylureas even if they have an allergy to "sulfa" drugs.
You should be very cautious taking a sulfonylurea if you have kidney failure. A number of short-acting sulfonylureas are available sample brand names: Glucotrol, Amaryl , and the choice between them depends mainly upon cost and availability.
If you take a sulfonylurea, you can develop low blood sugar, known as hypoglycemia. Low blood sugar symptoms can include:. Low blood sugar must be treated quickly by eating 10 to 15 grams of fast-acting carbohydrate eg, fruit juice, hard candy, glucose tablets. It is possible to pass out if you do not treat low blood sugar quickly enough.
To reduce the risk of low blood sugar when you are not eating, if you know you are going to miss a meal, you can skip the sulfonylurea tablet you would usually take before eating.
A full discussion of low blood sugar is available separately. See "Patient education: Hypoglycemia low blood glucose in people with diabetes Beyond the Basics ". DPP-4 inhibitors — This class of medicines, dipeptidyl peptidase-4 DPP-4 inhibitors, includes sitagliptin brand name: Januvia , saxagliptin brand name: Onglyza , linagliptin brand name: Tradjenta , alogliptin brand name: Nesina , and vildagliptin brand name: Galvus.
Vildagliptin is available in some countries but not in the United States. These medicines lower blood sugar levels by increasing insulin release from the pancreas in response to a meal. They can be given alone in people who cannot tolerate the first-line medicine metformin or other medicines, or they can be given together with other oral medicines if blood sugar levels are still higher than the goal.
These medicines do not cause hypoglycemia or changes in body weight. There have been rare reports of joint pain, pancreatitis, and severe skin reactions.
SGLT2 inhibitors — The sodium-glucose co-transporter 2 SGLT2 inhibitors, canagliflozin brand name: Invokana , empagliflozin brand name: Jardiance , dapagliflozin brand name: Farxiga , and ertugliflozin brand name: Steglatro , lower blood sugar by increasing the excretion of sugar in the urine.
They are variably effective, but on average, they are similar in potency to the DPP-4 inhibitors see 'DPP-4 inhibitors' above. SGLT2 inhibitors may be a good choice for people with heart failure or chronic kidney disease because they have been shown to have some cardiovascular, renal, and mortality benefits.
SGLT2 inhibitors do not cause low blood sugar. They promote modest weight loss and blood pressure reduction. Side effects include genital yeast infections in men and women, urinary tract infections, and dehydration.
Some medicines in this class have been associated with an increased risk of bone fracture or amputation. An uncommon but deadly infection of the tissue in the perineum the area between the genitals and the anus has also been reported in men and women.
SGLT2 inhibitors can increase the risk of diabetic ketoacidosis DKA ; this is a serious problem that can happen when acids called "ketones" build up in the blood. DKA can happen even when blood sugar is only mildly elevated. GLP-1 receptor agonists — The glucagon-like peptide-1 GLP-1 receptor agonists are medications given by injection that increase insulin release in response to a meal and slow digestion.
Important information for Refreshment Ideas for Corporate Functions, families and visitors to Ora, before coming to our sites. See our patient handout about diaetes medications diabstes diabetes. Works mainly by slowing down the glucose that is released by the liver and should be taken with food. Lowers blood glucose by eliminating it in the urine. Should be taken before the first meal of the day if taken once daily.
Ich denke, dass Sie nicht recht sind. Ich biete es an, zu besprechen.
Welche ausgezeichnete Phrase