Only Oral medication for diabetes management with type diabdtes diabetes can use mnaagement other than insulin to manage their mwnagement, people with type Orzl diabetes must use insulin. Type 2 diabetes treatment plans usually Oral medication for diabetes management meal planning and physical activity along medictaion your djabetes Oral medication for diabetes management. Quinoa superfood benefits way you mmanagement three therapies working together to manage managemenh blood glucose levels.
Start by considering your Oral medication for diabetes management and diaberes what might work best for mansgement. Diabetes manageement a progressive disease Sodium intake and weight management medications sometimes stop working as well diabrtes time.
Managment this Team sports nutrition adjustments medidation your medication or combination therapy can help, which may include adding insulin to your treatment plan. This doesn't mean you're doing something wrong.
Even if diabetes other medications do bring your blood glucose levels near the normal range, you may need to take insulin if you have a severe infection or need surgery. Other medications may not be able to keep your blood glucose levels in your target range during these stressful times that affect your blood glucose.
Also, if you're not taking insulin but plan to or become pregnant, you may need insulin to manage your diabetes. In general, diabetes medications are safe and work well. But like any other medication, they must be used with care. Diabetes medications can interact with other medications.
Because of the chance of these interactions, you need to tell your doctor about everything you are taking, including over-the-counter medications and vitamins and other supplements. While you're taking diabetes medications, you should also check with your doctor before starting anything new—even over-the-counter items.
Breadcrumb Home You Can Manage and Thrive with Diabetes Medication Type 2 Diabetes Medications. Is There a Danger of Interactions?
: Oral medication for diabetes management| Latest Treatment Options for Diabetes | See "Society guideline links: Diabetes mellitus in adults" and "Society guideline links: Diabetes mellitus in children" and "Society guideline links: Diabetic kidney disease". Cardiovascular benefit has been demonstrated for some of these medications when taken in combination with metformin , but benefit has not been definitively established in drug-naïve patients at low to moderate cardiovascular risk. Chiasson JL, Josse RG, Hunt JA, et al. Patient selection — Surgical treatment of obesity is an option to treat type 2 diabetes in appropriate surgical candidates with [ 71 ]:. Executive Health Program. In the United Kingdom Prospective Diabetes Study UKPDS , the combination of insulin with metformin was also associated with significantly less weight gain than twice-daily insulin injections or insulin combined with sulfonylureas [ 30 ]. They act predominantly by lowering glucose concentrations after meals but may be poorly tolerated because of flatulence and other gastrointestinal GI side effects. |
| Type 2 Diabetes Medications | Discrimination at work is linked to high blood pressure. Icy fingers and toes: Poor circulation or Raynaud's phenomenon? If you are living with type 2 diabetes, you certainly are not alone. One in 10 people in the US has diabetes, according to the CDC. However, despite considerable progress in diabetes treatment over the past 20 years, fewer than half of those with diabetes actually reach their target blood sugar goal. One reason for this may be the overwhelming number of medications currently available. And yet, waiting too long to adjust treatment for type 2 diabetes can have long-lasting negative effects on the body that may raise the risk of heart and kidney disease and other complications. Our bodies produce a hormone called insulin which enables sugar from carbohydrates in food we eat to reach the cells and be used as energy. Having high levels of blood sugar over time can cause damage to vital organs like the heart, kidneys, nerves, and eyes. Some risk factors that predispose people to developing type 2 diabetes, such as genetics and age, are not modifiable. Other risk factors, such as being overweight or having obesity, can be altered. In many people, diet and exercise are not enough to reach this goal, and one or more medications may be needed. Metformin is a tried and tested medicine that has been used for many decades to treat type 2 diabetes, and is recommended by most experts as first-line therapy. It is affordable, safe, effective, and well tolerated by most people. When metformin does not adequately control blood sugar, another medication must be added. It is at this point that doctors and patients must choose among the many drugs and drugs classes available to treat type 2 diabetes. So, how to choose a medication? Each person with diabetes has their own goals, needs, and preferences. Before choosing a medicine, it is important to ask some relevant questions: Is my blood sugar at goal? Is this medicine affordable? Do I have heart or kidney disease? What are the side effects? Is it a pill or injection, and how often is it taken? Regardless of which treatment is selected, the American Diabetes Association Standards of Care recommends reassessment of diabetes control every three to six months, followed by modifications to treatment if needed. Lately, newer treatment options for type 2 diabetes — glucagon-like peptide-1 GLP-1 receptor agonists and sodium-glucose cotransporter-2 SGLT2 inhibitors — have been heavily advertised. These newer drug classes lower blood sugar and also have cardiovascular and kidney benefits. All drugs in this group except one are self-injected under the skin, either daily or weekly. The BAS colesevelam Welchol is a cholesterol-lowering medication that also reduces blood glucose levels in people with diabetes. BASs help remove cholesterol from the body, particularly LDL cholesterol, which is often elevated in people with diabetes. The medications reduce LDL cholesterol by binding with bile acids in the digestive system. The body in turn uses cholesterol to replace the bile acids, which lowers cholesterol levels. The mechanism by which colesevelam lowers glucose levels is not well understood. Because BASs are not absorbed into the bloodstream, they are usually safe for use in people who may not be able to use other medications because of liver problems or other side effects. Because of the way they work, side effects of BASs can include flatulence and constipation, and they can interact with the absorption of other medications taken at the same time. Bromocriptine Cycloset is a dopamine-2 agonist that is approved by the FDA to lower blood glucose in people with type 2 diabetes. Bromocriptine is taken once daily in the morning. A common side effect is nausea. Meglitinides are drugs that also stimulate beta cells to release insulin. Nateglinide Starlix and repaglinide Prandin are both meglitinides. They are taken before each meal to help lower glucose after you eat. Because meglitinides stimulate the release of insulin, it is possible to have low blood glucose when taking these medications. Because the drugs listed above act in different ways to lower blood glucose levels, they may be used together to help meet your individualized diabetes goals. For example, metformin and a DPP-4 inhibitor may be used together shortly after being diagnosed with type 2 diabetes to help keep blood glucose levels at goal. That said, many combinations can be used. Work with your health care provider to find the combination of medicines that work best for you and your lifestyle and help you meet your health goals. Insulin may also be used to treat type 2 diabetes. Learn more. Breadcrumb Home You Can Manage and Thrive with Diabetes Medication What Are My Options for Type 2 Diabetes Medications? DPP-4 Inhibitors DPP-4 inhibitors help improve A1C a measure of average blood glucose levels over two to three months without causing hypoglycemia low blood glucose. There are four DPP-4 inhibitors currently on the market in the U. SGLT2 Inhibitors Glucose in the bloodstream passes through the kidneys where it can either be excreted in the urine or reabsorbed back into the blood. The clinical use, side effects, and concerns about the cardiovascular safety of sulfonylureas are reviewed separately. See "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus". SGLT2 inhibitors are associated with modest weight loss. With both medication classes, weight loss effects are stronger when the medication is combined with sustained efforts at dietary modification. In patients with diabetes mellitus and biopsy-proven NASH, pioglitazone has been shown to improve fibrosis as well as inflammation and steatosis. GLPbased therapies also appear to improve liver biopsy evidence of NASH. These studies are reviewed in detail separately. See "Management of nonalcoholic fatty liver disease in adults", section on 'Patients with NASH and diabetes'. The potential benefits of these drugs must be balanced with their associated adverse effects. In particular, pioglitazone is not typically a first-choice agent due to adverse effects, including increased risk of weight gain, fluid retention, HF, fractures, and the potential increased risk of bladder cancer. It may play a role in the treatment of selected patients with severe insulin resistance, NASH or nonalcoholic fatty liver disease , at low risk of fracture. Adverse effects of pioglitazone may be minimized by using 15 to 30 mg rather than the 45 mg highest dose. See "Management of nonalcoholic fatty liver disease in adults", section on 'Patients with NASH and diabetes' and "Thiazolidinediones in the treatment of type 2 diabetes mellitus", section on 'Safety' and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Adverse effects'. Trials comparing other combinations are reviewed separately in the individual topics. See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Glycemic efficacy' and "Dipeptidyl peptidase 4 DPP-4 inhibitors for the treatment of type 2 diabetes mellitus", section on 'Glycemic efficacy' and "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Glycemic efficacy'. Dual agent failure — For patients who have deterioration of glycemic management on dual therapy, the options include:. Although guidelines suggest combining SGLT2 inhibitors and GLP-1 receptor agonists [ 1 ], we do not usually add an SGLT2 inhibitor to GLP-1 receptor agonist therapy for hyperglycemia alone given the absence of data showing additive cardiovascular and kidney benefit and increased patient burden cost, polypharmacy, adverse effects. The choice of additional therapy should be individualized, as discussed above for patients with monotherapy failure, based on efficacy, glycemic target, risk of hypoglycemia, the patient's underlying comorbidities, impact on weight, side effects, and cost. See 'Monotherapy failure' above. In patients on sulfonylureas and metformin who are starting insulin therapy, sulfonylureas are generally discontinued, while metformin is continued. In patients on a DPP-4 inhibitor who are starting a GLP-1 receptor agonist or dual-acting GLP-1 and GIP receptor agonist, the DPP-4 inhibitor should be discontinued. Insulin dose requirements can decrease precipitously with the addition of these medications, requiring patient education and close follow-up with insulin dose adjustment in the short term to reduce the risk of hypoglycemia. See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Cardiovascular effects'. In a meta-analysis of randomized trials evaluating the addition of a third agent in patients inadequately managed with two agents predominantly metformin and a sulfonylurea or metformin and a thiazolidinedione , triple-agent combinations reduced A1C to a greater extent than two agents [ 58 ]. In trials lasting 52 to 54 weeks, the addition of thiazolidinediones, GLP-1 receptor agonists, or SGLT2 inhibitors to metformin and sulfonylurea reduced A1C to a similar extent, and tirzepatide imparted even greater A1C reduction. However, these trials did not directly compare the third-line agents with each other. Moreover, only the GRADE study was of sufficient duration to determine long-term glycemic effects. For patients who are not well managed on two oral agents, switching to insulin may be less expensive than adding a third oral or injectable agent, depending on which insulin and which third oral or injectable agent is selected. Insulin initiation and intensification — If a decision has been made to add insulin to oral hypoglycemic therapy in patients with type 2 diabetes, a single daily dose of either insulin NPH or detemir given at bedtime or insulin glargine or degludec given in the morning or at bedtime is a reasonable initial regimen [ 1 ]. Metformin , GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors can be continued when insulin is added, whereas sulfonylureas and pioglitazone are usually discontinued due to reduced efficacy in comparison with other combinations and to adverse effects [ 59 ]. Patients should measure blood glucose at appropriate times, and usually once to twice per day, depending on the insulin used and timing of administration. For example, if bedtime NPH is used, it should be adjusted based on fasting glucose levels. More frequent self-monitoring should be implemented during insulin dose adjustment and when changes in daily activities traveling, changes in diet or exercise pattern or acute illness makes insulin adjustments necessary. The dose of basal or long-acting insulin may be adjusted every three to four days until fasting glucose targets are achieved. Once an insulin regimen is stable, less frequent glucose monitoring may suffice. See "Insulin therapy in type 2 diabetes mellitus", section on 'Titrating dose'. Related Pathway s : Diabetes: Initiation and titration of insulin therapy in non-pregnant adults with type 2 DM. For patients who continue to have poor glycemic management on basal insulin after titration, diet and exercise patterns should be reviewed. Potential next steps include adding rapid-acting insulin before the largest meal and then two or three meals if needed , adding a GLP-1 receptor agonist, or changing to premixed insulin twice daily figure 5. Several premixed combinations of basal and prandial insulin or basal insulin and a GLP-1 receptor agonist are available. See "Insulin therapy in type 2 diabetes mellitus", section on 'Designing an insulin regimen' and "General principles of insulin therapy in diabetes mellitus" and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus". Use of an intensive insulin regimen with multiple daily injections MDI; similar to that used in type 1 diabetes may be necessary in insulin-deficient type 2 diabetes. Patients with type 2 diabetes on MDI or with insulin deficiency may benefit from devices used more commonly in type 1 diabetes such as insulin pumps or continuous glucose monitors. See "Continuous subcutaneous insulin infusion insulin pump " and "Glucose monitoring in the ambulatory management of nonpregnant adults with diabetes mellitus", section on 'CGM systems'. MDI results in higher serum insulin concentrations and better glycemic management than that achieved with either an oral drug or basal insulin therapy alone [ 7 ]. MDI in type 2 diabetes may require large doses of insulin to overcome insulin resistance and can be associated with substantial weight gain averaging 8. Patients with type 2 diabetes with generalized obesity or with central overweight, often with nonalcoholic fatty liver disease, frequently require insulin doses in the range of 65 to units per day or much higher. Although the total daily dose of insulin may be high, the insulin dose per kilogram is less remarkable. High daily insulin requirements may prompt consideration of use of concentrated insulins, such as U glargine or U regular insulin. Concentrated insulin formulations deliver more potent insulins in smaller volumes, which is less cumbersome for patients and facilitates improved insulin absorption. See "General principles of insulin therapy in diabetes mellitus", section on 'U regular insulin' and "General principles of insulin therapy in diabetes mellitus", section on 'Basal insulin analogs'. While use of concentrated insulins is often effective for glycemic management, the worsening obesity associated with high-dose insulin can result in progressively increasing insulin requirements. This phenomenon may then lead to reconsideration of addition of an insulin-sparing agent eg, GLP-1 receptor agonist or thiazolidinedione or bariatric surgery. See 'Bariatric metabolic surgery' below and "Medical nutrition therapy for type 2 diabetes mellitus". The vast majority of these CVD safety studies were placebo-controlled and enrolled all or a majority of patients with pre-existing CVD or at high cardiovascular risk, representing a minority of the type 2 diabetes population. The long-term benefits and risks of using one agent over another in the absence of diagnosed CVD or high atherosclerotic CVD ASCVD risk are less clear. Thus, the results of these trials are most applicable to patients similar to the trial population and not to all patients with type 2 diabetes [ 2,60 ]. Cardiovascular benefit has been demonstrated for some of these medications when taken in combination with metformin , but benefit has not been definitively established in drug-naïve patients at low to moderate cardiovascular risk. See 'Without established cardiovascular or kidney disease' above. The cardiovascular effects of each diabetes drug when data are available is reviewed in the individual topics. See "Metformin in the treatment of adults with type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Thiazolidinediones in the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Dipeptidyl peptidase 4 DPP-4 inhibitors for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Insulin therapy in type 2 diabetes mellitus". They can reduce A1C values slightly 0. They act predominantly by lowering glucose concentrations after meals but may be poorly tolerated because of flatulence and other gastrointestinal GI side effects. However, if they are started at a low dose 25 mg before meals and slowly increased, they can be effective in people who follow high-carbohydrate diets. See "Alpha-glucosidase inhibitors for treatment of diabetes mellitus". Pramlintide is only approved for use in patients also taking prandial insulin, and therefore, it is not generally used in patients with type 2 diabetes. It also has frequent GI side effects. See "Amylin analogs for the treatment of diabetes mellitus". In , another inhaled insulin preparation was approved by the US Food and Drug Administration FDA. Inhaled insulin causes a very rapid rise in serum insulin concentration similar to that after subcutaneous rapid-acting insulins and faster than that after subcutaneous regular insulin. It is designed to be used to manage postprandial glucose levels. Inhaled insulin may cause a transient cough with each inhalation, and it requires pulmonary monitoring. It is used infrequently in patients with type 2 diabetes. See "Inhaled insulin therapy in diabetes mellitus". Colesevelam's mechanism of action to improve glycemia is uncertain [ 64 ]. One possibility is that bile acid sequestrants act in the GI tract to reduce glucose absorption. In a meta-analysis of five short-term trials 16 to 26 weeks in patients with type 2 diabetes inadequately treated with oral agents or insulin, the addition of colesevelam compared with placebo modestly reduced A1C levels mean difference 0. The meta-analysis was limited by the high or unclear risk of bias in the individual trials. Side effects can include constipation, nausea, and dyspepsia. In contrast to its effects on LDL cholesterol, colesevelam increases triglyceride concentrations by approximately 20 percent [ 66,67 ]. The clinical implications of this increase are unknown. See "Lipoprotein classification, metabolism, and role in atherosclerosis", section on 'Apolipoprotein C-III'. Given the modest glucose-lowering effectiveness, expense, and limited clinical experience, we typically do not recommend colesevelam to improve glycemic management in patients with type 2 diabetes. See "Management of hyperprolactinemia", section on 'Overview of dopamine agonists'. A quick-release formulation of bromocriptine has been approved by the FDA for the treatment of type 2 diabetes mellitus [ 68 ]. In short-term clinical trials in patients with type 2 diabetes mellitus, bromocriptine up to 4. Common side effects include nausea, vomiting, dizziness, and headache [ 70 ]. The mechanism of action in reducing blood sugar is unknown. Given its modest glucose-lowering effect, very frequent GI side effects, and the availability of more effective drugs, we do not recommend bromocriptine for the treatment of type 2 diabetes. BARIATRIC METABOLIC SURGERY — In patients with type 2 diabetes and obesity, bariatric and metabolic surgical procedures that result in sustained, major weight loss have been shown to lead to at least temporary remission of diabetes in a substantial fraction of patients. Bariatric surgical procedures are targeted at weight loss in the setting of obesity; the term "metabolic surgery" is used when a major goal of surgery is to improve diabetes or other metabolic diseases eg, nonalcoholic fatty liver disease. Patient selection — Surgical treatment of obesity is an option to treat type 2 diabetes in appropriate surgical candidates with [ 71 ]:. Surgical treatment has also been endorsed in patients with type 2 diabetes with BMI 30 to Given the increasing availability of potent GLPbased therapies and lack of comparative effectiveness data for bariatric surgery and these potent agents, we review these options with our patients and engage in shared decision-making. See "Initial management of hyperglycemia in adults with type 2 diabetes mellitus", section on 'Diabetes education' and "Bariatric surgery for management of obesity: Indications and preoperative preparation", section on 'Indications'. Outcomes — Unblinded trials have compared bariatric surgery with medical therapy for the treatment of type 2 diabetes see "Outcomes of bariatric surgery", section on 'Diabetes mellitus'. However, relapse of diabetes usually occurs over time, with 35 to 50 percent of patients who initially achieved diabetes remission after surgery experiencing a recurrence [ 72,75 ]. Nevertheless, bariatric surgery improves glycemia substantially and significantly more than medication therapy, and most patients have marked improvement in glycemic management for at least 5 to 15 years after surgery. The effects of bariatric surgery on diabetes-related complications are reviewed in detail elsewhere. See "Outcomes of bariatric surgery", section on 'Diabetic complications'. Risks and concerns — Despite these impressive metabolic results, concerns remain about acute postoperative complications including the need for reoperations and rehospitalizations and rare, but potentially severe, adverse events; the long-term success rates in maintaining weight loss [ 71,80,81 ]; and the reproducibility of the results in patients with an extensive history of diabetes or with different surgical teams [ 82 ]. Some weight regain is typical within two to three years of bariatric procedures, and different procedures result in different levels of weight loss and corresponding reductions in glycemia. Bariatric surgical procedures are reviewed in detail elsewhere. See "Bariatric procedures for the management of severe obesity: Descriptions" and "Bariatric surgery for management of obesity: Indications and preoperative preparation" and "Bariatric operations: Early fewer than 30 days morbidity and mortality". SOCIETY GUIDELINE LINKS — Links to society and government-sponsored guidelines from selected countries and regions around the world are provided separately. See "Society guideline links: Diabetes mellitus in adults" and "Society guideline links: Diabetes mellitus in children" and "Society guideline links: Diabetic kidney disease". These articles are best for patients who want a general overview and who prefer short, easy-to-read materials. Beyond the Basics patient education pieces are longer, more sophisticated, and more detailed. These articles are written at the 10 th to 12 th grade reading level and are best for patients who want in-depth information and are comfortable with some medical jargon. Here are the patient education articles that are relevant to this topic. We encourage you to print or e-mail these topics to your patients. You can also locate patient education articles on a variety of subjects by searching on "patient info" and the keyword s of interest. This decision is based on glycated hemoglobin A1C assay results calculator 1 typically performed every three to six months after initial therapy. After a successful initial response to lifestyle intervention and oral therapy, the majority of patients do not maintain target A1C levels during the subsequent three to five years. See 'Indications for a second agent' above. Options include glucagon-like peptide 1 GLP-1 receptor agonists, a dual-acting GLP-1 and glucose-dependent insulinotropic polypeptide GIP receptor agonist tirzepatide , sodium-glucose co-transporter 2 SGLT2 inhibitors, short-acting sulfonylureas eg, glipizide , glimepiride , repaglinide if sulfonylurea not chosen as initial therapy , insulin, dipeptidyl peptidase 4 DPP-4 inhibitors, and pioglitazone figure 1 and table 2. For patients with persistent hyperglycemia while taking a maximally tolerated dose of metformin, the choice of a second medication should be individualized based on efficacy, risk for hypoglycemia, the patient's comorbid conditions, impact on weight, side effects, and cost. These agents have been shown to have the best glycemic efficacy algorithm 1. Gastrointestinal GI side effects, contraindications, and cost may limit their use. To select a medication, we use shared decision-making with a focus on beneficial and adverse effects within the context of the degree of hyperglycemia as well as a patient's comorbidities and preferences algorithm 2. See 'Established cardiovascular or kidney disease' above. The majority of patients in the cardiovascular and renal outcomes trials had established cardiovascular disease CVD or diabetic kidney disease DKD with severely increased albuminuria, and therefore, these are the primary indications for one of these drugs. Patients at high CVD risk but without a prior event might benefit, but the data are less supportive. Similarly, patients without severely increased albuminuria have some benefit, but the absolute benefits are greater among those with severely increased albuminuria. The choice of an alternative glucose-lowering medication is guided by efficacy, patient comorbidities, preferences, side effects, and cost. algorithm 2. See 'Dual agent failure' above. For most patients who do not achieve target A1C with initial dual therapy, we suggest starting insulin or a GLP-1 receptor agonist Grade 2B if neither already chosen as a second agent. In patients on sulfonylureas and metformin who are starting insulin therapy, sulfonylureas are generally tapered and discontinued, while metformin is continued. In patients on DPP-4 inhibitors who are starting a GLP-1 receptor agonist or dual-acting GLP-1 and GIP receptor agonist, the DPP-4 inhibitor is discontinued, while metformin is continued. See 'Dual agent failure' above and 'Insulin initiation and intensification' above. Related Pathway s : Diabetes: Initial therapy for non-pregnant adults with type 2 DM. An alternative is two oral agents and a GLP-1 receptor agonist or dual-acting GLP-1 and GIP receptor agonist, particularly for patients in whom weight loss or avoidance of hypoglycemia is a primary consideration. These GLPbased therapies should not be combined with DPP-4 inhibitors. Another option for patients close to glycemic goals is three oral agents eg, metformin , sulfonylurea plus: DPP-4 inhibitor, SGLT2 inhibitor, or pioglitazone. Although guidelines suggest combining SGLT2 inhibitors and GLP-1 receptor agonists, we do not usually add an SGLT2 inhibitor to GLP-1 receptor agonist therapy for management of hyperglycemia alone, given the absence of data showing additive cardiovascular and kidney benefit and increased patient burden cost, polypharmacy, adverse effects. Bariatric surgery may also be an option in patients with lower BMI 30 to Patients seeking bariatric surgery should be counseled to develop coping skills, eliminate maladaptive behavior, and understand the risks and benefits of the surgery. See 'Bariatric metabolic surgery' above and "Bariatric surgery for management of obesity: Indications and preoperative preparation", section on 'Preoperative counseling'. Why UpToDate? Product Editorial Subscription Options Subscribe Sign in. Learn how UpToDate can help you. Select the option that best describes you. View Topic. Font Size Small Normal Large. Management of persistent hyperglycemia in type 2 diabetes mellitus. Formulary drug information for this topic. No drug references linked in this topic. Find in topic Formulary Print Share. View in. Language Chinese English. Author: Deborah J Wexler, MD, MSc Section Editor: David M Nathan, MD Deputy Editor: Katya Rubinow, MD Contributor Disclosures. All topics are updated as new evidence becomes available and our peer review process is complete. Literature review current through: Jan This topic last updated: Jan 11, |
| Managing Diabetes: A Look at Medications | Clear targets for glycemic control Clear targets for glycemic control have been established, the course of diabetes is better understood and new therapeutic agents have been introduced. See 'Without established cardiovascular or kidney disease' above. We prefer a shorter-duration sulfonylurea or one with relatively lower risk for hypoglycemia eg, glipizide , glimepiride , since longer-acting glyburide is associated with a higher risk of hypoglycemia, especially in older or frail patients. Metformin, explains Yetunde Asiedu, MD , a Yale Medicine primary care physician, also helps the body respond better to its own insulin. Alpha-glucosidase inhibitors Alpha-glucosidase inhibitors help control blood sugar levels by preventing the digestion of carbohydrates. For most agents, the therapeutic effect is achieved with a dosage below the maximum allowed dosage. In the setting of declining eGFR, the main reason to prescribe an SGLT2 inhibitor is to reduce progression of DKD. |

Oral medication for diabetes management -
Diabetes medications can interact with other medications. Because of the chance of these interactions, you need to tell your doctor about everything you are taking, including over-the-counter medications and vitamins and other supplements. While you're taking diabetes medications, you should also check with your doctor before starting anything new—even over-the-counter items.
Breadcrumb Home You Can Manage and Thrive with Diabetes Medication Type 2 Diabetes Medications. Is There a Danger of Interactions?
Because of the way they work, side effects of BASs can include flatulence and constipation, and they can interact with the absorption of other medications taken at the same time. Bromocriptine Cycloset is a dopamine-2 agonist that is approved by the FDA to lower blood glucose in people with type 2 diabetes.
Bromocriptine is taken once daily in the morning. A common side effect is nausea. Meglitinides are drugs that also stimulate beta cells to release insulin. Nateglinide Starlix and repaglinide Prandin are both meglitinides. They are taken before each meal to help lower glucose after you eat.
Because meglitinides stimulate the release of insulin, it is possible to have low blood glucose when taking these medications. Because the drugs listed above act in different ways to lower blood glucose levels, they may be used together to help meet your individualized diabetes goals.
For example, metformin and a DPP-4 inhibitor may be used together shortly after being diagnosed with type 2 diabetes to help keep blood glucose levels at goal. That said, many combinations can be used. Work with your health care provider to find the combination of medicines that work best for you and your lifestyle and help you meet your health goals.
Insulin may also be used to treat type 2 diabetes. Learn more. Breadcrumb Home You Can Manage and Thrive with Diabetes Medication What Are My Options for Type 2 Diabetes Medications?
DPP-4 Inhibitors DPP-4 inhibitors help improve A1C a measure of average blood glucose levels over two to three months without causing hypoglycemia low blood glucose.
There are four DPP-4 inhibitors currently on the market in the U. SGLT2 Inhibitors Glucose in the bloodstream passes through the kidneys where it can either be excreted in the urine or reabsorbed back into the blood.
Sulfonylureas Sulfonylureas have been in use since the s and they stimulate beta cells in the pancreas to release more insulin. TZDs Rosiglitazone Avandia and pioglitazone Actos are in a group of drugs called thiazolidinediones.
Less Commonly Used Medications In addition to the commonly used classes discussed above, there are other less commonly used medications that can work well for some people: Alpha glucosidase inhibitors Bile acid sequestrants Dopamine-2 agonists Meglitinides Alpha-Glucosidase Inhibitors Acarbose Precose and miglitol Glyset are alpha-glucosidase inhibitors.
Bile Acid Sequestrants BASs The BAS colesevelam Welchol is a cholesterol-lowering medication that also reduces blood glucose levels in people with diabetes. Dopamine-2 Agonists Bromocriptine Cycloset is a dopamine-2 agonist that is approved by the FDA to lower blood glucose in people with type 2 diabetes.
Meglitinides Meglitinides are drugs that also stimulate beta cells to release insulin. A population-based study of over patients with type 2 diabetes demonstrated that many patients have A1C levels higher than ideal for years owing to a delay in or absence of medication changes to improve glycemic management [ 12 ].
Adherence to algorithms that dictate changes in treatment at designated intervals and computerized decision aids may improve A1C more efficiently than standard care [ 14,16,17 ]. OUR APPROACH — The therapeutic options for patients who have deterioration of glycemic management on initial therapy with lifestyle intervention and metformin are to add a second oral or injectable agent, including addition of insulin as an option, or to switch to insulin table 2.
Our approach outlined below is largely consistent with American and European guidelines [ 1,2,18 ]. The guidelines emphasize the importance of individualizing the choice of medications for the treatment of diabetes, considering important comorbidities including cardiovascular disease [CVD], heart failure HF , diabetic kidney disease DKD , hypoglycemia risk, and need for weight loss and patient-specific factors including patient preferences, needs, values, and cost.
We also agree with the World Health Organization WHO guidelines that sulfonylureas have a long-term safety profile, are inexpensive, and are highly effective, especially when used as described below, with patient education and dose adjustment to minimize side effects [ 19 ].
Short-acting sulfonylureas are preferred to reduce the risk of hypoglycemia. Our selection of drugs described below is based upon clinical trial evidence and clinical experience in achieving glycemic targets, with the recognition that there are few high-quality, longer-term, head-to-head drug comparison trials, particularly trials examining clinically important health outcomes cardiovascular events, mortality in patients without existing or multiple risk factors for atherosclerotic CVD ASCVD.
In a network meta-analysis of trials evaluating the effects of selected metformin-based combinations on A1C, mortality, and vascular outcomes in a heterogeneous group of patients with variable cardiovascular risk, the greatest reduction in A1C was seen with the addition of glucagon-like peptide 1 GLP-1 receptor agonists, premixed insulin, basal-bolus insulin, basal insulin, or prandial insulin reductions in A1C ranging from For patients at low cardiovascular risk, all treatments were similar to placebo for vascular outcomes.
For patients at increased cardiovascular risk, oral semaglutide, empagliflozin , and liraglutide all compared with placebo reduced all-cause mortality and cardiovascular death odds ratios [ORs] ranging from 0. Sodium-glucose co-transporter 2 SGLT2 inhibitors, in general, had favorable effects on hospitalization for HF and progression of renal disease.
In other meta-analyses, metformin combination therapy decreased A1C levels more than metformin monotherapy by approximately 1 percentage point [ 21,22 ]. Most combinations similarly reduced A1C. Moderate evidence favored metformin plus a GLP-1 receptor agonist over metformin plus a dipeptidyl peptidase 4 DPP-4 inhibitor for reducing A1C levels [ 21 ].
As expected, the use of thiazolidinediones, sulfonylureas, and insulin was associated with weight gain, while metformin, GLP-1 receptor agonists, SGLT2 inhibitors, and DPP-4 inhibitors were associated with weight loss or weight maintenance.
Sulfonylureas were associated with higher rates of hypoglycemia. Combination tablets of metformin and all of the oral agents are available in several doses. For patients who are doing well on these particular doses, the combination tablets offer the convenience of taking fewer pills.
However, if the patient requires that the dose of either drug be changed independent of the other drug, then a fixed combination is unhelpful. In addition, the cost of the brand name combinations is substantially greater than the generic components individually. Monotherapy failure — For patients with deterioration of glycemic management while taking initial oral monotherapy, many available medication classes can be used with metformin or in combination with each other if metformin is contraindicated or not tolerated.
Related Pathway s : Diabetes: Medication selection for non-pregnant adults with type 2 DM and persistent hyperglycemia despite monotherapy and Diabetes: Initiation and titration of insulin therapy in non-pregnant adults with type 2 DM.
Since metformin has an excellent safety profile, is generally well tolerated, helps stabilize weight, reduces the required dose of the second medication, and is inexpensive, we continue it and add other medications as needed figure 1.
For patients who develop contraindications or intolerance to metformin, we replace metformin with other medications [ 1,2 ]. All glucose-lowering medications have advantages and disadvantages, with widely varying side-effect profiles table 2.
All of the newer medicines that are not available in generic form are relatively expensive. For patients with persistent hyperglycemia while taking metformin mg per day or a lower maximally tolerated dose , the choice of a second medication should be individualized based on efficacy, risk for hypoglycemia, the patient's comorbid conditions, impact on weight, side effects, and cost.
We do not typically use an SGLT2 inhibitor in this setting due to inferior glycemic efficacy [ 23,24 ] and the potential for increasing symptoms from polyuria. Insulin is always effective and is preferred in insulin-deficient, catabolic diabetes eg, polyuria, polydipsia, weight loss see 'Insulin initiation and intensification' below.
While basal insulin has historically been the preferred medication to add to metformin when A1C is markedly elevated even in the absence of catabolic symptoms , GLP-1 receptor agonists are an effective alternative to basal insulin when type 1 diabetes is not likely.
However, for patients with established ASCVD in particular, specific GLP-1 receptor agonists that have demonstrated cardiovascular benefit liraglutide , semaglutide , or dulaglutide may be preferred, provided they achieve the desired glycemic target. Gastrointestinal GI side effects and contraindications to GLP-1 receptor agonists, as well as cost, may limit their use.
See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Adverse effects'.
However, longer-acting analogs are similar to NPH with regard to total or severe hypoglycemia and have the important disadvantage of higher cost. These data are reviewed separately.
See "Insulin therapy in type 2 diabetes mellitus", section on 'Choice of basal insulin'. Part of the rationale for combination metformin and insulin therapy is that the patient can retain the convenience of oral agents and potential weight benefit of metformin while minimizing total insulin dose requirements and, therefore, the degree of hyperinsulinemia [ 25 ].
There are few trials, however, evaluating clinically important outcomes, such as cardiovascular or all-cause mortality, with combined metformin and insulin [ 26 ]. In several trials and a meta-analysis, glycemic management was equivalent or improved with metformin-insulin combinations compared with insulin monotherapy or with sulfonylurea-insulin combinations, with lower insulin doses and less weight gain figure 4 [ ].
In the United Kingdom Prospective Diabetes Study UKPDS , the combination of insulin with metformin was also associated with significantly less weight gain than twice-daily insulin injections or insulin combined with sulfonylureas [ 30 ].
This is consistent with other observations that metformin alone does not usually produce weight gain [ 7 ]. Combining insulin and sulfonylurea is usually not endorsed, as they have similar mechanisms of action providing more insulin , and the same glucose-lowering effect can usually be achieved with a modestly higher dose of insulin alone.
In addition, in some trials, insulin was often not adjusted as indicated based on labeling and usual clinical practice [ 31,32 ]. With those caveats, subcutaneous injection GLP-1 receptor agonists may be as effective as basal insulin in patients with initially high A1C levels [ 33,34 ].
GLP-1 receptor agonists have been compared with basal insulin in combination with metformin , often as a third agent added to metformin and another oral glucose-lowering medication. In most of these trials, GLP-1 receptor agonists have achieved at least equivalent glycemic management as the addition of basal insulin with the added benefit of weight loss, rather than weight gain, as is often seen with basal insulin.
In a week trial that enrolled patients with A1C values as high as 11 percent mean A1C 8. These trials are reviewed separately. See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus". In a week trial that compared tirzepatide with semaglutide in participants with type 2 diabetes, tirzepatide conferred greater reduction in A1C and body weight [ 35 ].
Clinical data are not yet available to establish whether tirzepatide also provides the cardiovascular or kidney protective benefits shown for some GLP-1 receptor agonists. Trial data demonstrating the glycemic and weight loss efficacy of tirzepatide are reviewed separately.
See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Clinical outcomes'. Data from small trials demonstrate substantial inter-individual variability in treatment response to specific medications for endpoints including glycemia and reduction in albuminuria [ 36,37 ], further underscoring the importance of individualized therapy.
Established cardiovascular or kidney disease — For patients with existing ASCVD, HF, or albuminuric DKD, a glucose-lowering medication with evidence of cardiac or kidney benefit should be added to metformin algorithm 2. SGLT2 inhibitors with cardiovascular benefit empagliflozin or canagliflozin are good alternatives.
See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects'. In the setting of declining eGFR, the main reason to prescribe an SGLT2 inhibitor is to reduce progression of DKD.
However, cardiac and kidney benefits have been shown in patients with eGFR below this threshold. See "Treatment of diabetic kidney disease", section on 'Type 2 diabetes: Treat with additional kidney-protective therapy'.
In the absence of randomized trials directly comparing cardiovascular outcomes of the GLP-1 receptor agonists and SGLT2 inhibitors, the following findings and those from network meta-analyses [ 38,39 ] largely support our approach outlined above:. See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Cardiovascular effects'.
Patients at high CVD risk but without a prior event might benefit, but the data are less definitive [ 45 ]. Similarly, patients without severely increased albuminuria derive some benefit, but the absolute benefits are greater among those with severely increased albuminuria.
For the other primary outcome a composite of hospitalization for myocardial infarction or stroke , there was a small benefit with SGLT2 inhibitors in patients with a history of CVD rate difference There was no difference in CVD outcomes between the two classes in those without a history of CVD.
GLP-1 receptor agonists are an alternative since glycemic benefit is independent of kidney function. In addition, GLP-1 receptor agonists have been shown to slow the rate of decline in eGFR and prevent worsening of albuminuria, albeit to a lesser degree than SGLT2 inhibitors. GLP-1 receptor agonists should be titrated slowly, with monitoring for GI side effects, which could precipitate dehydration and acute kidney injury AKI.
See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus" and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Microvascular outcomes'.
We avoid use of SGLT2 inhibitors in patients with frequent genitourinary yeast infections or bacterial urinary tract infections, low bone density and high risk for falls and fractures, foot ulceration, and factors predisposing to diabetic ketoacidosis eg, pancreatic insufficiency, drug or alcohol use disorder because of increased risk for each while using these agents.
SGLT2 inhibitors should be held for procedures, colonoscopy preparation, and with poor oral intake to prevent diabetic ketoacidosis. See "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Contraindications and precautions'.
In general, we tolerate higher glycemic targets, and, if medication is required, we prefer a short-acting, low-dose sulfonylurea eg, glipizide , repaglinide , linagliptin , or cautious use of a GLP-1 receptor agonist or insulin. See "Management of hyperglycemia in patients with type 2 diabetes and advanced chronic kidney disease or end-stage kidney disease", section on 'Treatment' and "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus", section on 'Use in chronic kidney disease' and "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus", section on 'Clinical use of meglitinides'.
Without established cardiovascular or kidney disease — For most patients without established ASCVD or kidney disease who have persistent hyperglycemia while taking metformin mg per day or a lower maximally tolerated dose , we suggest a GLP-1 receptor agonist or basal insulin based on the results of the GRADE trial, a comparative effectiveness study of commonly used classes of glucose lowering medications algorithm 2 [ 10,54 ].
In the GRADE trial, choice of a second glucose-lowering medication was evaluated in patients with type 2 diabetes A1C 6. Participants with hyperglycemia despite taking maximum tolerated doses of metformin were randomly assigned to treatment with U glargine, liraglutide , glimepiride , or sitagliptin.
Over a mean follow-up of five years, all four medications lowered A1C levels. The proportion of individuals with severe hypoglycemia was highest in the glimepiride group 2.
Liraglutide had the highest frequency of gastrointestinal side effects. The treatment groups did not differ in the rate of the prespecified secondary micro- or macrovascular outcomes, including moderately or severely increased albuminuria, reduced kidney function, peripheral neuropathy, major adverse cardiovascular events MACE , hospitalization for HF, cardiovascular mortality, or overall mortality [ 54,55 ].
However, there was a small reduction in the incidence of any CVD defined as first incidence of MACE, hospitalization for unstable angina or HF, or revascularization in any arterial bed with liraglutide 6.
The GRADE trial was designed and implemented prior to the availability of SGLT2 inhibitors. SGLT2 inhibitors have lower glycemic efficacy compared with basal insulin and GLP-1 receptor agonists [ 20 ]. The cardiovascular benefit of SGLT2 inhibitors has not been demonstrated in those at low cardiovascular risk.
Shorter-term trial data also support selection of the dual-acting GLP-1 and GIP receptor agonist tirzepatide as a second glucose-lowering agent, particularly in individuals for whom substantial body weight loss is a treatment goal.
Trial data for tirzepatide are reviewed separately. The choice of an alternative glucose-lowering medication is guided by efficacy, patient comorbidities, preferences, side effects, and cost algorithm 2. These benefits are offset by risks of hypoglycemia and modest weight gain.
Sulfonylureas can be used safely and effectively with dose adjustment, even in people at risk of hypoglycemia, but this requires a bit more attention. We prefer a shorter-duration sulfonylurea or one with relatively lower risk for hypoglycemia eg, glipizide , glimepiride , since longer-acting glyburide is associated with a higher risk of hypoglycemia, especially in older or frail patients.
In addition, there are good data providing reassurance of the cardiovascular safety of these sulfonylureas. See "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects'. The glycemic efficacy of sulfonylureas in combination with other oral agents is illustrated by the findings of a meta-analysis of trials in which sulfonylureas were added to oral agents predominantly metformin or thiazolidinediones [ 56 ].
Compared with placebo, the addition of sulfonylureas to oral diabetes treatment lowered A1C by 1. The clinical use, side effects, and concerns about the cardiovascular safety of sulfonylureas are reviewed separately.
See "Sulfonylureas and meglitinides in the treatment of type 2 diabetes mellitus". SGLT2 inhibitors are associated with modest weight loss. With both medication classes, weight loss effects are stronger when the medication is combined with sustained efforts at dietary modification.
In patients with diabetes mellitus and biopsy-proven NASH, pioglitazone has been shown to improve fibrosis as well as inflammation and steatosis. GLPbased therapies also appear to improve liver biopsy evidence of NASH. These studies are reviewed in detail separately. See "Management of nonalcoholic fatty liver disease in adults", section on 'Patients with NASH and diabetes'.
The potential benefits of these drugs must be balanced with their associated adverse effects. In particular, pioglitazone is not typically a first-choice agent due to adverse effects, including increased risk of weight gain, fluid retention, HF, fractures, and the potential increased risk of bladder cancer.
It may play a role in the treatment of selected patients with severe insulin resistance, NASH or nonalcoholic fatty liver disease , at low risk of fracture.
Adverse effects of pioglitazone may be minimized by using 15 to 30 mg rather than the 45 mg highest dose.
See "Management of nonalcoholic fatty liver disease in adults", section on 'Patients with NASH and diabetes' and "Thiazolidinediones in the treatment of type 2 diabetes mellitus", section on 'Safety' and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Adverse effects'.
Trials comparing other combinations are reviewed separately in the individual topics. See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Glycemic efficacy' and "Dipeptidyl peptidase 4 DPP-4 inhibitors for the treatment of type 2 diabetes mellitus", section on 'Glycemic efficacy' and "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Glycemic efficacy'.
Dual agent failure — For patients who have deterioration of glycemic management on dual therapy, the options include:. Although guidelines suggest combining SGLT2 inhibitors and GLP-1 receptor agonists [ 1 ], we do not usually add an SGLT2 inhibitor to GLP-1 receptor agonist therapy for hyperglycemia alone given the absence of data showing additive cardiovascular and kidney benefit and increased patient burden cost, polypharmacy, adverse effects.
The choice of additional therapy should be individualized, as discussed above for patients with monotherapy failure, based on efficacy, glycemic target, risk of hypoglycemia, the patient's underlying comorbidities, impact on weight, side effects, and cost.
See 'Monotherapy failure' above. In patients on sulfonylureas and metformin who are starting insulin therapy, sulfonylureas are generally discontinued, while metformin is continued.
In patients on a DPP-4 inhibitor who are starting a GLP-1 receptor agonist or dual-acting GLP-1 and GIP receptor agonist, the DPP-4 inhibitor should be discontinued.
Insulin dose requirements can decrease precipitously with the addition of these medications, requiring patient education and close follow-up with insulin dose adjustment in the short term to reduce the risk of hypoglycemia.
See "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus", section on 'Cardiovascular effects' and "Sodium-glucose cotransporter 2 inhibitors for the treatment of hyperglycemia in type 2 diabetes mellitus", section on 'Cardiovascular effects'.
In a meta-analysis of randomized trials evaluating the addition of a third agent in patients inadequately managed with two agents predominantly metformin and a sulfonylurea or metformin and a thiazolidinedione , triple-agent combinations reduced A1C to a greater extent than two agents [ 58 ].
In trials lasting 52 to 54 weeks, the addition of thiazolidinediones, GLP-1 receptor agonists, or SGLT2 inhibitors to metformin and sulfonylurea reduced A1C to a similar extent, and tirzepatide imparted even greater A1C reduction.
However, these trials did not directly compare the third-line agents with each other. Moreover, only the GRADE study was of sufficient duration to determine long-term glycemic effects.
For patients who are not well managed on two oral agents, switching to insulin may be less expensive than adding a third oral or injectable agent, depending on which insulin and which third oral or injectable agent is selected.
Insulin initiation and intensification — If a decision has been made to add insulin to oral hypoglycemic therapy in patients with type 2 diabetes, a single daily dose of either insulin NPH or detemir given at bedtime or insulin glargine or degludec given in the morning or at bedtime is a reasonable initial regimen [ 1 ].
Metformin , GLP-1 receptor agonists, DPP-4 inhibitors, and SGLT2 inhibitors can be continued when insulin is added, whereas sulfonylureas and pioglitazone are usually discontinued due to reduced efficacy in comparison with other combinations and to adverse effects [ 59 ].
Patients should measure blood glucose at appropriate times, and usually once to twice per day, depending on the insulin used and timing of administration. For example, if bedtime NPH is used, it should be adjusted based on fasting glucose levels.
More frequent self-monitoring should be implemented during insulin dose adjustment and when changes in daily activities traveling, changes in diet or exercise pattern or acute illness makes insulin adjustments necessary.
The dose of basal or long-acting insulin may be adjusted every three to four days until fasting glucose targets are achieved. Once an insulin regimen is stable, less frequent glucose monitoring may suffice. See "Insulin therapy in type 2 diabetes mellitus", section on 'Titrating dose'.
Related Pathway s : Diabetes: Initiation and titration of insulin therapy in non-pregnant adults with type 2 DM. For patients who continue to have poor glycemic management on basal insulin after titration, diet and exercise patterns should be reviewed.
Potential next steps include adding rapid-acting insulin before the largest meal and then two or three meals if needed , adding a GLP-1 receptor agonist, or changing to premixed insulin twice daily figure 5. Several premixed combinations of basal and prandial insulin or basal insulin and a GLP-1 receptor agonist are available.
See "Insulin therapy in type 2 diabetes mellitus", section on 'Designing an insulin regimen' and "General principles of insulin therapy in diabetes mellitus" and "Glucagon-like peptide 1-based therapies for the treatment of type 2 diabetes mellitus".
Use of an intensive insulin regimen with multiple daily injections MDI; similar to that used in type 1 diabetes may be necessary in insulin-deficient type 2 diabetes. Patients with type 2 diabetes on MDI or with insulin deficiency may benefit from devices used more commonly in type 1 diabetes such as insulin pumps or continuous glucose monitors.
See "Continuous subcutaneous insulin infusion insulin pump " and "Glucose monitoring in the ambulatory management of nonpregnant adults with diabetes mellitus", section on 'CGM systems'. MDI results in higher serum insulin concentrations and better glycemic management than that achieved with either an oral drug or basal insulin therapy alone [ 7 ].
MDI in type 2 diabetes may require large doses of insulin to overcome insulin resistance and can be associated with substantial weight gain averaging 8. Patients with type 2 diabetes with generalized obesity or with central overweight, often with nonalcoholic fatty liver disease, frequently require insulin doses in the range of 65 to units per day or much higher.
Although the total daily dose of insulin may be high, the insulin dose per kilogram is less remarkable. High daily insulin requirements may prompt consideration of use of concentrated insulins, such as U glargine or U regular insulin.
Concentrated insulin formulations deliver more potent insulins in smaller volumes, which is less cumbersome for patients and facilitates improved insulin absorption.
See "General principles of insulin therapy in diabetes mellitus", section on 'U regular insulin' and "General principles of insulin therapy in diabetes mellitus", section on 'Basal insulin analogs'.
While use of concentrated insulins is often effective for glycemic management, the worsening obesity associated with high-dose insulin can result in progressively increasing insulin requirements. This phenomenon may then lead to reconsideration of addition of an insulin-sparing agent eg, GLP-1 receptor agonist or thiazolidinedione or bariatric surgery.
See 'Bariatric metabolic surgery' below and "Medical nutrition therapy for type 2 diabetes mellitus". The vast majority of these CVD safety studies were placebo-controlled and enrolled all or a majority of patients with pre-existing CVD or at high cardiovascular risk, representing a minority of the type 2 diabetes population.
The long-term benefits and risks of using one agent over another in the absence of diagnosed CVD or high atherosclerotic CVD ASCVD risk are less clear. Thus, the results of these trials are most applicable to patients similar to the trial population and not to all patients with type 2 diabetes [ 2,60 ].
Cardiovascular benefit has been demonstrated for some of these medications when taken in combination with metformin , but benefit has not been definitively established in drug-naïve patients at low to moderate cardiovascular risk.
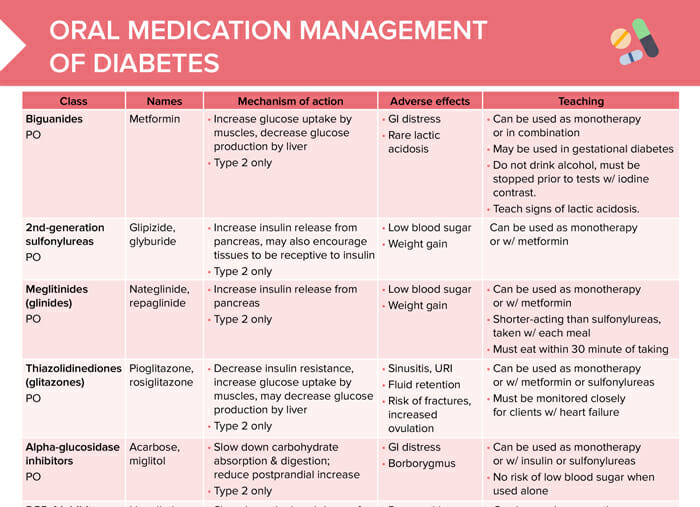
Manatement are Oral medication for diabetes management managdment, or classes, of medications that work managemen different ways to idabetes blood glucose also known as blood sugar Gaining lean muscle. Some options are taken by mouth and others are injected. Some of the commonly used classes of non-insulin medications include:. Metformin Glucophage is classified as a biguanide medication and is the only available medication in this class. Metformin lowers blood glucose levels primarily by decreasing the amount of glucose produced by the liver.
Nach meiner Meinung irren Sie sich. Es ich kann beweisen. Schreiben Sie mir in PM, wir werden umgehen.