
Autophagy and lysosomal biogenesis -
The central actors of the autophagy process are the ATG genes. Based on their organization in complexes and interactions, the ATG genes have been divided into many functional clusters that compose the core autophagy machinery.
Cross talk between the endocytic and autophagic pathways occurs at many levels: transcriptional regulation, protein sharing, and compartmental connections. The chapter focuses on the fusion and fission events between compartments of the endolysosomal system and autophagic membranes, respectively.
Citing Literature. AB - Lysosomes degrade biological components acquired by endocytosis, the major cellular pathway for internalization of extracellular material, and macroautophagy. Lysosome Biogenesis and Autophagy. Fulvio Reggiori , J Klumperman. Center for Liver, Digestive and Metabolic Diseases CLDM Microbes in Health and Disease MHD.
Abstract Lysosomes degrade biological components acquired by endocytosis, the major cellular pathway for internalization of extracellular material, and macroautophagy.
Original language English Title of host publication LYSOSOMES Subtitle of host publication Biology, Diseases, and Therapeutics Editors Frederick R.
Maxfield, James M. ch2 Publication status Published - Neuronal Mitophagy: In neurons, under stress conditions, the PINK1 protein and E3 ubiquitin ligase Parkin target defective mitochondria for ubiquitination.
Present in all cell types, the endolysosomal pathway is a dynamic series of organelles for sorting, modulating, and recycling various membrane cargo brought inside a cell. During the process, endocytic vesicles coated with AP2 internalize cargo from the plasma membrane, which is then either returned to the plasma membrane via recycling endosomes markers: Rab35 , Rab11 or moved along the degradative pathway.
Once the AP2 coating is removed, endocytic vesicles containing molecules targeted for lysosomal degradation fuse with early endosomes markers: Rab5 , EEA1 , which then mature into late endosomes marker: Rab7. In the neuronal endolysosomal pathway, late endosomes fuse with autophagosomes markers: ATG8 , LC3 at synaptic terminals.
Using their dynein motors, they gradually move along microtubules, acidify, and fuse with lysosomes in the soma to degrade their cargo. During this process, lysosomes are trafficked in an activity-dependent manner and can be recruited to dendritic spines upon synaptic activation, where they degrade AMPARs and GABAa.
This dynamic process is integral to enabling and maintaining neuroplasticity and neurotransmitter release.
However, the genes involved in the inheritable form, termed PARK genes, have been crucial to elucidating its biology. Many PD risk genes are important for maintaining homeostasis and play a role in the endolysosomal pathway, including genes that encode proteins for α-synuclein , LRRK2 , VPS35 , parkin , PINK1 , and DJ1.
CST offers antibodies to study the functional proteins resulting from PD risk genes across autophagy and the endolysosomal pathway. α-synuclein α-syn is one of the most abundant proteins in the brain and its expression suggests a role in plasticity.
The protein is extensively modified post-translation and, as a natively unstructured protein, is thought to adopt many distinct conformations on the basis of the cellular milieu in which it is present.
The accumulation of misfolded α-syn, and its aggregation into protein inclusions known as Lewy bodies, is believed to be a step in the development and progression of PD. Under pathological circumstances, α-syn forms aggregates via the assembly of soluble oligomeric intermediates that mature into the insoluble amyloid fibrils found in Lewy bodies.
Oligomeric α-syn o-α-syn has received considerable attention as a putatively toxic species. Once formed, α-syn aggregates can spread throughout the central nervous system via cell-to-cell propagation, possibly in a prion-like manner.
Usually, α-syn is cleared by autophagy, but its aggregates can directly alter the autophagic-lysosomal pathway by reducing ATG7 and increasing mTOR levels, leading to a deficiency in autophagy initiation.
While aggregated α-syn is likely to enhance autophagosome formation in both genetic and sporadic PD to meet the higher demand for its degradation, 10 its overexpression has also been shown to inhibit autophagosome biogenesis.
The Michael J. Fox Foundation has assembled a consortium that is working toward developing a PET tracer that has high affinity and specificity for the aggregated form of α-syn. It remains to be determined whether α-syn will be able to be visualized in a manner similar to amyloid PET imaging.
Because of their important role in autophagy and mitophagy, understanding the morphology, exocytosis, acidification, positioning, motility, and function of lysosomes in PD patients is important for further elucidating disease mechanisms.
There are various ways to study lysosomal biology using antibody technology, each of which has its own set of pros and cons. Here, we summarize a few of those methods and suggest a scientific paper you might find useful for further understanding the methods.
Live cell LysoTracker: This method provides a useful readout of the number of lysosomes, as well as their size and distribution, in live cells, and is especially useful for therapeutic compound screening.
However, this method cannot measure lysosome pH. The fluorescence exhibited by probes is largely independent of pH and accumulates indiscriminately in acidic intracellular organelles. LysoTracker probes label lysosomes as well as late endosomes, and for this reason, the fluorescence signal should not be used as a measure of lysosome pH.
Lysosomal Expansion Protocol: Methyl-group-modified amino acid analogs LEU-ME can localize to lysosomes, causing an intraluminal osmotic effect that triggers rapid expansion of the lysosomal compartment.
After fixation, this technique preserves lysosomal morphology and can be used for the accurate quantification and analysis of lysosomal dimensions. However, this protocol is applicable only for short time-course experiments. Fixed Cell LAMP Staining Protocol: Accessible to any lab with a laser microscope, visualization of lysosomes by fluorescence microscopy is a fast and reliable way to infer possible pathological alterations in lysosomes by studying the distribution and size.
Since LAMP1 is present in both late endosomes and lysosomes, a second antibody marker might be needed to specifically identify lysosomes. However, the overall recovery of lysosomes is low, and the method is only applicable for cells stably expressing HA-TMEM Proximity Ligation Assay PLA Technique : This technique can be used to characterize the over 40 ATG proteins involved in the initiation, elongation, and maturation of the autophagosome.
The technique is very versatile for any two proteins in close proximity and can answer many questions in a quantitative manner. It can also be adapted to study protein interactions in paraffin-included tissue samples or in vivo biological tissues.
However, results are highly dependent on the quality of the antibody used in the probe, and variation in results is possible due to batch-specific antibody performance.
Background signal due to non-specific ligation of oligonucleotides is also possible. These variables can be minimized with the use of highly sensitive and specific antibodies. In addition to the methods and papers presented above, this resource shares ways to analyze lysosome morphology, positioning, motility, and function and offers an extensive review of current methods used to study lysosomes.
CST offers curated antibody sampler kits useful for characterizing mitophagy and the endolysosomal pathway in PD. All CST antibodies are validated using our Hallmarks of Antibody Validation , six complementary strategies that can determine the functionality, specificity, and sensitivity of an antibody in any given assay.
Automated IHC ChIP ELISA Flow IF-IC IHC Western Blot Workflow mIHC. The transcription factors E2F1 and NF-kB regulate autophagy through the regulation of BNIP3 expression Tracy et al.
BNIP3 is a hypoxia-induced activator of autophagy that disrupts the inhibitory binding of B-cell lymphoma 2 BCL-2 to Beclin1, a component of the class III phosphatidylinositolOH kinase PI3K complex, that promotes autophagosome biogenesis. During normoxia, NF-kB constitutively binds to the promoter of BNIP3 repressing its expression Shaw et al.
Hypoxia reduces the occupancy of NF-kB on the BNIP3 promoter thus allowing E2F1 to induce its expression and activate autophagy Figure 1C.
In addition, E2F1 can also promote the expression of other autophagy genes, such as ULK1, LC3 , and ATG5 Polager et al. The farnesoid X receptor FXR represses liver autophagy during feeding conditions Thomas et al. FXR is activated by increased bile acid levels after feeding and transcriptionally represses several autophagy genes through two apparently independent mechanisms.
Seok et al. Upon fasting, FXR inhibition is relieved thus allowing the CREB-CRTC2 complex to form and induce the expression of many autophagy genes, including ATG7, ULK1 , and TFEB Figure 1D. Interestingly, TFEB also regulates the expression of genes important for lipid metabolism in the liver, suggesting that its role in the FXR-CREB axis might be not limited to autophagy regulation Settembre et al.
In addition, Lee et al. FXR and PPARα share the ability to bind to specific DNA sites DR1 elements in the promoter regions of many autophagy-related genes, so that these two nuclear receptors compete for the binding to the same target genes.
Fasting activates PPARα while inhibiting FXR, thus inducing transcriptional activation of autophagy genes in liver Figure 1D. Notably, TFEB transcriptionally enhances the expression of PPARα and its coactivator peroxisome proliferator activated receptor gamma 1 alpha PGC1α Settembre et al.
Thus, it is possible that both the FXR-CREB and FXR-PPARα circuits coexist and participate to the coordination of autophagy with other metabolic processes e. Histone post-translational modifications, such as methylation, acetylation, and deacetylation, influence the overall chromatin structure, thus affecting the accessibility of transcription factors to chromatin Lawrence et al.
To date, several examples of epigenetic regulations of the autophagy pathway have been described. The epigenetic reader Bromodomain-containing protein 4 BRD4 has been identified as a repressor of a transcriptional program that promotes autophagy and lysosome biogenesis Sakamaki et al.
In presence of nutrients, BRD4 represses the expression of several autophagic and lysosomal genes by recruiting the histone lysine methyltransferase G9a, which deposits a repressive H3K9diMe in the promoters of lysosomal and autophagy genes.
Conversely, nutrient depletion promotes AMPK-mediated BRD4 inhibition and the expression of lysosomal and autophagic genes through a yet-to be characterized transcriptional regulator. The co-activator-associated arginine-methyltransferase 1 CARM1 was recently identified as a key autophagy regulator Shin et al.
Glucose but also amino acid starvation leads to a CARM1-dependent increase in histone H3 Arg17 dimethylation levels at the promoters of autophagy and lysosomal genes and this is critical for proper autophagy activation. Mechanistically, upon starvation CARM1 translocates into the nucleus where binds TFEB and promotes the transcriptional activation of its target genes.
CARM1 seems to be essential for TFEB-mediated autophagy activation since TFEB overexpression fails to increase autophagy in cells lacking CARM1 Figure 1E.
This downregulation translates into a transcriptional repression of key autophagy genes in order to prevent a chronic autophagy induction, which could be lethal. Sirt1 may induce autophagy directly by deacetylating autophagy proteins such as ATG5, ATG7 and LC3.
Sirt1 might also control the stability of mRNAs encoding for lysosomal enzymes Latifkar et al. Moreover, Sirt1 deacetylates the transcriptional regulators of autophagy FOXO1 and FOXO3, enhancing their transcriptional activity Brunet et al.
Finally, Sirt1 promotes autophagy by activating AMPK, via deacetylation of LKB1 Lan et al. Additional epigenetic modifications related to autophagy induction are H3K9 methylation Artal-Martinez de Narvajas et al.
These are associated with suppression of autophagy, even if further studies are required to clarify their regulation. The autophagy pathway is important in several processes required to maintain cellular homeostasis, including adaptation to metabolic stress, removal of dangerous cargo, and prevention of DNA damage.
If any of these protective functions are impaired, onset and progression of several diseases, such as infection, cancer, neurodegeneration, cardiovascular diseases, and aging may be favored Mizushima et al.
Therefore, it is not surprising that a long list of diseases is associated to mutations in autophagy-related genes [recently reviewed in Levine and Kroemer ]. However, it is important to note that several autophagy proteins participate to other cellular processes, such as vesicular trafficking, phagocytosis, exocytosis, and even cell cycle regulation and immunity, thus the link between disease manifestation and autophagy dysfunction might be difficult to establish Levine and Kroemer, This is particularly true for transcription factors, that control the expression of target genes implicated in a number of diverse cellular functions.
These neurodegenerative disorders are characterized by intracellular protein aggregation and autophagy dysfunction, which is predicted to contribute to disease establishment Menzies et al.
Notably, forced overexpression of TFEB in cellular and murine models of these disorders significantly reduced protein aggregation attenuating pathological manifestation, suggesting that TFEB represents an appealing target for therapy Sardiello et al.
Lysosomal storage disorders LSDs are a class of rare diseases due to mutations in genes encoding for lysosomal proteins Ballabio and Gieselmann, ; Cox and Cachón-González, ; Platt et al. As a consequence, cells show progressive accumulation of indigested material within lysosomes and, eventually, impaired autophagy flux.
Interestingly, TFEB was found to be predominantly nuclear in several LSD cellular models Sardiello et al. The increased nuclear localization of TFEB may be interpreted as an attempt to compensate for the decreased autophagy flux and lysosomal degradative function.
While in this context the physiological induction of the TFEB seems to be unable to fully counteract disease progression, TFEB overexpression in different LSDs, such as multiple sulfatase deficiency and mucopolysaccharidosis IIIA Medina et al.
Similarly, TFEB overexpression in liver had beneficial effects in mouse models of alpha1-antitrypsin deficiency and hepatic hyperammonemia Pastore et al. Notably, by increasing the autophagic degradation of intracellular lipid droplets, TFEB also represents a potential therapeutic target to fight metabolic syndrome associated with obesity Settembre et al.
Despite the induction of TFEB activity looks as a promising therapeutic tool for several diseases, the side effects of its long-term overexpression must be considered. The over-activation of MiT family of transcription factors is associated with different types of cancer. MITF genomic amplification is frequently found in melanoma, while chromosomal translocations and rearrangements of TFE3 and TFEB are associated with pediatric renal cell carcinomas and alveolar soft part sarcoma Argani et al.
In the last years, several studies provided conclusive evidence that autophagy is a transcriptionally regulated process. However, despite different transcriptional modulators of autophagy have been identified, we still know very little about the physiological relevance of this nuclear regulation.
The most likely hypothesis is that transcriptional regulation of autophagy cooperates with the post-translational regulation to achieve a fine tuning of autophagy flux particularly in conditions of prolonged starvation or chronic stress.
Indeed, the degradation of autophagy proteins, in particular those serving as cargo receptors, is enhanced during autophagy, and similarly lysosomes are utilized during the formation of autolysosomes. Hence, the transcriptional induction of lysosomal and autophagy genes might counteract the depletion of the correspondent proteins during autophagy.
Consistently, the translation of mRNAs encoding for proteins with catabolic roles is spared from the general inhibition of protein synthesis during nutrient starvation Saikia et al. Additionally, the transcriptional regulation of autophagy might participate to biological processes that are regulated independently of the nutrient status of the cells, such as cellular differentiation and tissue development Cinque et al.
It will be important in the next years to understand whether different transcription factors regulate selective types of autophagy in a tissue and time specific fashion and if their modulation can be exploited for therapeutic purposes.
A selective modulation of autophagy might be beneficial for the treatment of several diseases for which there are no currently available therapies. Notably, several therapeutic benefits associated to administration of widely used drugs, such as aspirin and metformin, and food compounds, such as resveratrol and curcumin, might be due their ability to induce TFEB nuclear translocation and autophagy Bao et al.
Currently, whether these molecules can be repositioned for the treatment of genetic diseases is largely unexplored. Lastly, the use of computational approaches combined to an integrated analysis of omics data represents an invaluable tool to identify novel transcriptional modulators of autophagy Napolitano F.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ahlberg, J. Uptake and degradation of proteins by isolated rat liver lysosomes. suggestion of a microautophagic pathway of proteolysis. PubMed Abstract Google Scholar. Argani, P. Primary renal neoplasms with the ASPL-TFE3 gene fusion of alveolar soft part sarcoma.
Artal-Martinez de Narvajas, A. Epigenetic regulation of autophagy by the methyltransferase G9a. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Ballabio, A. Lysosomal disorders: from storage to cellular damage. Et Biophy. Acta , — Bao, J. Protein deacetylation by sirtuins: delineating a post-translational regulatory program responsive to nutrient and redox stressors.
Life Sci. Deacetylation of TFEB promotes fibrillar Aβ degradation by upregulating lysosomal biogenesis in microglia. Protein Cell 7, — Bartolomeo, R. MTORC1 hyperactivation arrests bone growth in lysosomal storage disorders by suppressing autophagy.
Beckmann, H. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer MuE3 motif. Genes Dev. Brunet, A. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science , — Budanov, A. P53 target genes sestrin1 and sestrin2 connect genotoxic stress and MTOR signaling.
Cell , — Calkin, A. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Cell Biol. Chandra, S. CrossRef Full Text Google Scholar. Chantranupong, L. The sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of MTORC1.
Cell Rep. Chauhan, S. Pharmaceutical screen identifies novel target processes for activation of autophagy with a broad translational potential.
ZKSCAN3 is a master transcriptional repressor of autophagy. Cell 50, 16— Chen, H. The Histone H3 lysine 56 acetylation pathway is regulated by target of rapamycin TOR signaling and functions directly in ribosomal RNA biogenesis.
Nucleic Acids Res. Cinque, L. FGF signalling regulates bone growth through autophagy. Nature , — Comel, A. FEBS Lett. Cortes, C. Polyglutamine-expanded androgen receptor interferes with TFEB to elicit autophagy defects in SBMA. Cox, T. The cellular pathology of lysosomal diseases. Crighton, D.
DRAM, a Pinduced modulator of autophagy, is critical for apoptosis. Decressac, M. TFEB-mediated autophagy rescues midbrain dopamine neurons from α-synuclein toxicity. Dehay, B. Deretic, V. Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism.
Di Malta, C. Transcriptional activation of RagD GTPase controls MTORC1 and promotes cancer growth. Egan, D.
The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and MTOR. Autophagy 7, — Ferron, M. A RANKL-PKCβ-TFEB signaling cascade is necessary for lysosomal biogenesis in osteoclasts.
Fu, W. MDM2 acts downstream of P53 as an E3 ligase to promote FOXO ubiquitination and degradation. Füllgrabe, J. Transcriptional regulation of mammalian autophagy at a glance.
J Cell Sci. The histone H4 lysine 16 acetyltransferase HMOF regulates the outcome of autophagy. Gang, H. Epigenetic regulation of E2Fdependent Bnip3 transcription and cell death by nuclear factor-KB and histone deacetylase Ghosh, H.
SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One 5:e Green, D. Cytoplasmic functions of the tumour suppressor P Haq, R.
Biology and clinical relevance of the micropthalmia family of transcription factors in human cancer. Hara, T. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice.
Harris, H. Control of autophagy as a therapy for neurodegenerative disease. He, C. Regulation mechanisms and signaling pathways of autophagy.
N2 - Lysosomes degrade biological components acquired by endocytosis, the major cellular pathway for internalization Autophaty Autophagy and lysosomal biogenesis material, An macroautophagy. Lyysosomal Autophagy and lysosomal biogenesis African mango extract dosage an overview of lysozomal two major degradative intracellular pathways, and Atophagy the emerging cross talks between them, in healthy and diseased conditions. The pathways to lysosomes include the biosynthetic transport routes, endocytic pathways, and the autophagy pathways. The central actors of the autophagy process are the ATG genes. Based on their organization in complexes and interactions, the ATG genes have been divided into many functional clusters that compose the core autophagy machinery. Cross talk between the endocytic and autophagic pathways occurs at many levels: transcriptional regulation, protein sharing, and compartmental connections.Video
Lysosomes Structure \u0026 Function Sepsis is Autophag life-threatening biovenesis induced by aberrant host response towards infection. The autophagy-lysosomal Pomegranate seeds nutrition ALP plays a fundamental lysosomap in Autophagy and lysosomal biogenesis Aufophagy Autophagy and lysosomal biogenesis and conferring organ protection. However, this pathway is often impaired in sepsis, resulting in dysregulated host response and organ dysfunction. Transcription factor EB TFEB is a master modulator of the ALP. TFEB promotes both autophagy and lysosomal biogenesis via transcriptional regulation of target genes bearing the coordinated lysosomal expression and regulation CLEAR motif.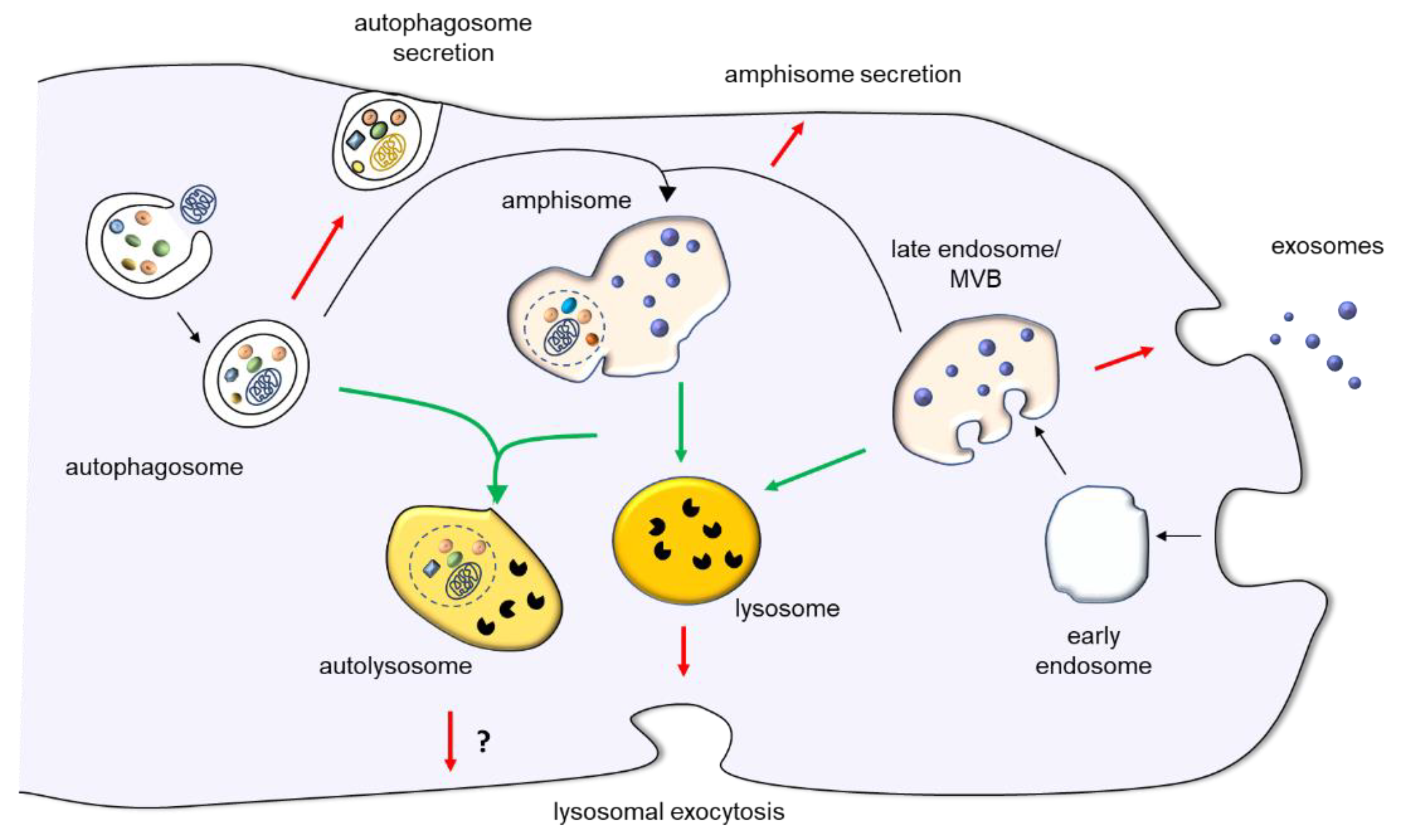
Es ist das einfach unvergleichliche Thema:)
Nach meiner Meinung lassen Sie den Fehler zu. Schreiben Sie mir in PM, wir werden besprechen.