Guidellines by co-chairs Drs. Guideelines Mottl Workout fuel strategies Susanne Nicholas, the team of Olive oil production reviewed the Gidelines Disease Improving Global Carbohydrates and Nutrient Timing KDIGO jephropathy over the last Workout fuel strategies months and will release today their commentary on the guidelines in the American Nepnropathy of Kidney Diseases.
KDIGO is Dlabetic nonprofit, international organization that works to improve the care and outcomes of patients with guidelinnes disease through the guidlines of clinical Safe weight loss pills guidelines.
The Carbohydrates and Nutrient Timing commentary DDiabetic highly supportive of the new KDIGO recommended treatment guidelines, Carbohydrates and Nutrient Timing. The workgroup was guidelinds pleased that in addition to addressing the emergence of SGLT2 inhibitors Low-intensity yoga routines a treatment for diabetic kidney disease and the nuances guidekines renin-angiotensin system inhibition, KDIGO nephripathy a guidellines review and recommendations on comprehensive management of nutrition, Workout fuel strategies, and multidisciplinary collaboration as a central component npehropathy care.
Workout fuel strategies, Nephropaghy MPH FASN, Nephropatjy Professor of Diaetic, Diabetic nephropathy guidelines Division of Nephrology and Hypertension, Guidelinees Hill, N.
There guideljnes Workout fuel strategies much to be done in the realms of research, gudielines policy, nephropatby education, but this renewed energy, nephroopathy with dedication and continued collaboration, guidelinex hopefully bring about Thermogenic fat burners day when end-stage kidney disease from diabetes is a rarity.
Rocco, MD, MSCE, KDOQI chair and the Vardaman M. Buckalew Jr. Professor of Medicine at the Wake Forest School of Medicine in Winston-Salem, NC.
These clinical trials build upon the work done by the National Kidney Foundation, in partnership with the Food and Drug Administration, to develop new surrogate endpoints for clinical trials in patients with chronic kidney disease. While many challenges remain in this field, KDIGO has made a significant step forward in providing a comprehensive clinical practice guideline dedicated to diabetes and CKD.
Mottl said. To learn more about the commentary and the workgroup participants, www. is at risk for chronic kidney disease. Risk factors for kidney disease include: diabeteshigh blood pressureheart diseaseobesityand family history.
People who are Black or African American, Hispanic or Latino, American Indian or Alaska Native, Asian American, or Native Hawaiian or Other Pacific Islander are at increased risk for developing the disease.
Black or African American people are almost 4 times more likely than Whites to have kidney failure. Hispanic or Latino people are 1. The National Kidney Foundation NKF is the largest, most comprehensive, and longstanding patient-centric organization dedicated to the awareness, prevention, and treatment of kidney disease in the U.
For more information about NKF, visit www. The American Journal of Kidney Diseases AJKDthe official journal of the National Kidney Foundation, is recognized worldwide as a leading source of information devoted to clinical nephrology practice and clinical research.
Articles selected for publication in AJKD undergo a rigorous consideration process, supporting the journal's goal to communicate important new information in clinical nephrology in a way that strengthens knowledge and helps physicians to provide their patients with the highest standard of care.
Give Hope. Fund Answers. End Kidney Disease. Skip to main content. NKF Supports Latest Guideline on Treating Diabetic Kidney Disease.
About the National Kidney Foundation The National Kidney Foundation NKF is the largest, most comprehensive, and longstanding patient-centric organization dedicated to the awareness, prevention, and treatment of kidney disease in the U. About the American Journal of Kidney Diseases The American Journal of Kidney Diseases AJKDthe official journal of the National Kidney Foundation, is recognized worldwide as a leading source of information devoted to clinical nephrology practice and clinical research.
Donate Monthly.
: Diabetic nephropathy guidelines| Updated KDIGO Guideline for Managing Diabetes in Patients with Chronic Kidney Disease | Explore Mayo Clinic studies testing new treatments, interventions and tests as a means to prevent, detect, treat or manage this condition. Diet, exercise and self-care are needed to control blood sugar and high blood pressure. Your diabetes care team can help you with the following goals:. Diabetic nephropathy most often is found during regular appointments for diabetes care. If you've been diagnosed with diabetic nephropathy recently, you may want to ask your health care professional the following questions:. Before any appointment with a member of your diabetes treatment team, ask whether you need to follow any restrictions, such as fasting before taking a test. Questions to regularly review with your doctor or other members of the team include:. Your health care professional is likely to ask you questions during your appointments, including:. Diabetic nephropathy kidney disease care at Mayo Clinic. Mayo Clinic does not endorse companies or products. Advertising revenue supports our not-for-profit mission. Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. This content does not have an English version. This content does not have an Arabic version. Diagnosis Kidney biopsy Enlarge image Close. Kidney biopsy During a kidney biopsy, a health care professional uses a needle to remove a small sample of kidney tissue for lab testing. Care at Mayo Clinic Our caring team of Mayo Clinic experts can help you with your diabetic nephropathy kidney disease -related health concerns Start Here. Kidney transplant Enlarge image Close. Kidney transplant During kidney transplant surgery, the donor kidney is placed in the lower abdomen. Kidney Disease: How kidneys work, Hemodialysis, and Peritoneal dialysis. Request an appointment. By Mayo Clinic Staff. Show references Diabetic kidney disease. National Institute of Diabetes and Digestive and Kidney Diseases. Accessed May 24, Diabetic kidney disease adult. Mayo Clinic; Mottl AK, et al. Diabetic kidney disease: Manifestations, evaluation, and diagnosis. Diabetes and chronic kidney disease. Centers for Disease Control and Prevention. Diabetic nephropathy. Merck Manual Professional Version. Goldman L, et al. Diabetes mellitus. In: Goldman-Cecil Medicine. Elsevier; Elsevier Point of Care. Clinical Overview: Diabetic nephropathy. De Boer IH, et al. Executive summary of the KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney International. Office of Patient Education. Chronic kidney disease treatment options. Coping effectively: A guide for patients and their families. National Kidney Foundation. Robertson RP. Pancreas and islet cell transplantation in diabetes mellitus. Accessed May 25, Ami T. Allscripts EPSi. Mayo Clinic. June 27, Castro MR expert opinion. June 8, Chebib FT expert opinion. Mayo Clinic Press Check out these best-sellers and special offers on books and newsletters from Mayo Clinic Press. Mayo Clinic on Incontinence - Mayo Clinic Press Mayo Clinic on Incontinence The Essential Diabetes Book - Mayo Clinic Press The Essential Diabetes Book Mayo Clinic on Hearing and Balance - Mayo Clinic Press Mayo Clinic on Hearing and Balance FREE Mayo Clinic Diet Assessment - Mayo Clinic Press FREE Mayo Clinic Diet Assessment Mayo Clinic Health Letter - FREE book - Mayo Clinic Press Mayo Clinic Health Letter - FREE book. Show the heart some love! Give Today. Help us advance cardiovascular medicine. Find a doctor. Explore careers. Multimorbidity is common in patients with diabetes and CKD, who are at high risk of CKD progression, cardiovascular events, and premature mortality. Therefore, both the ADA 1 and KDIGO 2 emphasize the importance of comprehensive, holistic, patient-centered medical care to improve overall patient outcomes. This approach requires treatment directed to optimize lifestyle, pharmacological therapy aimed at preserving organ function, and additional therapies aimed at improving intermediate risk factors such as glycemia, BP, and lipids Fig. Holistic approach for improving outcomes in patients with diabetes and CKD. Icons presented indicate the following benefits: BP cuff, BP lowering; glucometer, glucose lowering; heart, cardioprotection; kidney, kidney protection; scale, weight management. Otherwise, dihydropyridine calcium channel blocker or diuretic can also be considered; all three classes are often needed to attain BP targets. With multiple interventions ubiquitously needed to optimize the care of people with diabetes and CKD, it is crucial to avoid therapeutic inertia Most patients with diabetes and CKD have high residual risks of CKD progression and cardiovascular disease despite treatment, and increasing options are available for risk mitigation. Patients may need to be seen frequently to identify and implement multiple therapies, some of which may interact. For example, RAS inhibitors, SGLT2i, and the ns-MRA finerenone all cause initial hemodynamic reductions in GFR. When indicated, such medications may need to be added and adjusted sequentially, with frequent assessments to institute and optimize care in a timely manner. Empowering patients and facilitating multidisciplinary care can help institute and titrate multiple treatments expeditiously. All patients with T1D or T2D and CKD should be treated with a comprehensive plan, outlined and agreed by health care professionals and the patient together, to optimize nutrition, exercise, smoking cessation, and weight, upon which are layered evidence-based pharmacologic therapies aimed at preserving organ function and other therapies selected to attain intermediate targets for glycemia, BP, and lipids. The ADA and KDIGO guidelines both advocate for patients to take an active role in managing their diabetes and kidney disease and to have a voice in decisions that affect their well-being 2 , Education for patients and an integrated approach to treatment is an effective approach for both patients and clinicians. Patients know themselves better than anyone else, and although health care professionals have the medical background, when a patient and health care professional become partners in developing a shared-decision treatment plan the lives of the patients will improve. In addition, the time required by the health care professional in managing the patients care will be reduced. Patient priorities often do not align with health care professional priorities. Ideally, health care professionals will question patients about their priorities and together they will establish an agreed upon care program Ways in which patients can work with their health care professionals to manage their diabetes and CKD include asking questions; becoming educated about diet, physical activity, smoking cessation, glycemic control, and medications; talking to peers and support groups in the diabetes and CKD community; becoming familiar with technology that is available to track progress; and understanding test results in preparation for health care appointments Diabetes and CKD management is ideal when the health care system model of care includes a multidisciplinary team to assist patients including the patient, physician or other care provider , and other health care professionals 2 , Overcoming barriers to management of CKD in patients with diabetes. Barriers such as low CKD awareness, high complexity of care, difficulties with adhering to increasingly complex treatment regimens, and low recognition and application of guideline-directed management all contribute to suboptimal management of patients with diabetes and CKD. Proposed strategies that may contribute to improved management of patients with diabetes and CKD include implementation of multidisciplinary models of care, structured risk mitigation strategies and education, multidisciplinary educational initiatives, harmonization of clinical practice guidelines, and provision of self-management programs for patients with diabetes and CKD. Health care systems should include team-based care for patients and focus on both short- and long-term treatment plans. Lifestyle interventions for the patient must be included in determining an overall plan of care to ensure individual preferences are addressed and goals are established by all team members, especially the patient. Behavioral evaluation should be considered in the initial assessment for all patients with diabetes. In addition, it should be considered in patients who are unable to meet goals in order to determine potential psychosocial barriers to treatment and self-management. The ADA and KDIGO guidelines both recommend individualized and balanced diets that are high in vegetables, fruits, and whole grains but are low in refined carbohydrates and sugar-sweetened beverages 1 , 2. The ADA and KDIGO guidelines also recommend targeting a dietary protein intake of 0. Higher protein intakes confer theoretical risk of enhancing kidney function decline While the ADA and KDIGO are aligned in this regard, the National Kidney Foundation Kidney Disease Outcomes Quality Initiative NKF KDOQI has somewhat different recommendations, including restricting dietary protein to 0. All recommendations call for higher levels of protein intake for patients with kidney failure treated with maintenance dialysis, who are often catabolic or malnourished e. In overweight or obese patients with diabetes, ADA and KDIGO show overall agreement with respect to achieving and maintaining healthy weight through diet, physical activity, and behavioral therapy Supplementary Table 1. Though specific evidence is low, smoking cessation is also strongly advised. Both the ADA and KDIGO recommend twice-yearly glycemic assessment using glycated hemoglobin HbA 1c among stable patients with T2D who are meeting treatment goals and quarterly assessment among those who are intensively managed, whose therapy has changed, or whose treatment goals are not met Supplementary Table 1. While both ADA and KDIGO focus on HbA 1c as the primary tool for assessing long-term glycemic control, both guidelines acknowledge limitations in its accuracy and precision as an indirect metric of glycemic status, particularly in advanced CKD i. Consequently, both guidelines emphasize the concurrent use of 1 HbA 1c as a metric upon which therapeutic targets are defined based on randomized controlled trial RCT data, 2 continuous glucose monitoring CGM to assess effectiveness and safety of treatment among patients at risk for hypoglycemia or to assess overall glycemia when HbA 1c is inaccurate, and 3 self-monitoring of blood glucose as a tool to guide medication adjustment, particularly in patients treated with insulin Both the ADA and KDIGO emphasize use of individualized glycemic targets that take into consideration key patient characteristics that may modify risks and benefits of intensive glycemic control Supplementary Table 1. Diabetes technology refers to the hardware, devices, and software that patients with diabetes use to manage their chronic disease and encompasses 1 insulin administered with syringe, pen, or pump; 2 blood glucose monitoring with meter or CGM; and 3 hybrid devices that monitor glucose and deliver insulin. The ADA and KDIGO guidelines highlight the important role of CGM technology in improving diabetes management as a tool to identify and correct glycemic derangements, prevent hypoglycemia, direct medication management, and guide medical nutritional therapy and physical activity, as well as its rapid evolution in affordability and accuracy 2 , 37 Supplementary Table 1. Furthermore, ADA and KDIGO underscore that CGM may provide an advantage in glycemic control assessment among patients with T1D, as well as patients with T2D using glucose-lowering therapies associated with hypoglycemia. Other technologies supported by the ADA include sensor-augmented pumps that suspend insulin when glucose is low or predicted to become low, as well as automated insulin delivery systems that increase and decrease insulin delivery based on sensor-derived glucose levels and trends. BP management is universally accepted as a critical goal for prevention of CKD progression, ASCVD, and HF. The ADA includes BP recommendations in each annual Standards of Care and published a position statement on diabetes and hypertension in BP control was highlighted as a key component of comprehensive care in the KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease and KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease and addressed in more detail in the KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease The ADA and KDIGO BP recommendations share many similarities, including a focus on proper BP measurement techniques, individualization of BP targets, and preferred drugs for treatment. Considerations for individualization of BP targets include both anticipated benefits e. For patients with diabetes, hypertension, and high cardiovascular risk i. All of these thresholds are proposed as starting places for individualization of targets With respect to preferred antihypertensive pharmacotherapies, there is consensus that an RAS inhibitor, i. This recommendation is based on RCTs where findings demonstrated decreased risk of CKD progression, for which patients with albuminuria are at elevated risk, with a maximally dosed RAS inhibitor compared with placebo or an active antihypertensive drug comparator 42 — In a recent study in almost three million patients, investigators found that both classes performed similarly; however, the ARB was better tolerated Dihydropyridine calcium channel blockers and thiazide-like diuretics are also recommended for patients with hypertension who do not have albuminuria, for whom cardiovascular events and mortality are more common than kidney failure. Multiple drugs are often required to control BP, and an RAS inhibitor, dihydropyridine calcium channel blockers, and diuretics can be combined to attain individualized BP targets Fig. An ACEi or ARB is recommended for patients with T1D or T2D who have hypertension and albuminuria, titrated to the maximum antihypertensive or highest tolerated dose. Statin therapy is a cornerstone of therapy for the primary and secondary prevention of ASCVD among people with diabetes and CKD. The KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease recommended statin initiation for most adults with diabetes and CKD who are not treated with dialysis 46 , These recommendations are based largely on results of the Study of Heart and Renal Protection SHARP trial of CKD Additional evidence from subsequent trials was incorporated into recommendations in the ADA Standards of Care, which are endorsed by this consensus statement. An exception may be patients with kidney failure treated with dialysis for whom primary prevention of ASCVD events with a statin has been generally ineffective 47 , 49 , High-intensity statin is recommended for secondary prevention for all patients with known ASCVD. For some patients, intensification of statin therapy for primary prevention , addition of ezetimibe, or addition of a PCSK-9 inhibitor is recommend based on ASCVD risk and attained LDL cholesterol concentrations. For patients with high triglyceride or low HDL levels, intensification of lifestyle intervention, optimization of glycemic control, and then consideration of icosapent ethyl are advised 51 Supplementary Table 1. A statin is recommended for all patients with T1D or T2D and CKD, moderate intensity for primary prevention of ASCVD or high intensity for patients with known ASCVD and some patients with multiple ASCVD risk factors. The ADA Standards Care and the KDIGO guideline recommend early initiation of metformin plus an SGLT2 inhibitor in most patients with T2D and CKD 2 , 17 Table 1. Additional glucose-lowering agents can then be added as needed to meet individualized glycemic targets based on patient-specific considerations 2 , 17 Table 2. Prescription of glucose-lowering medications may be limited by eGFR Table 3. Appropriate dose adjustment based on eGFR is important for medications that increase risk of side effects with low eGFR or undergo elimination through the kidney Table 4. When needed, careful use and titration of insulin and sulfonylurea agents is recommended to avoid hypoglycemia. Key glucose-lowering agent recommendations for patients with T2D and CKD from ADA and KDIGO 2 , The ADA issues an A level of evidence for clear or supportive evidence from well-conducted, generalizable randomized control trials that are adequately powered and a B level of evidence for supportive evidence from well-conducted cohort or case-control studies. KDIGO uses the GRADE framework, with 1A indicating a strong recommendation based on high-quality evidence and 1B indicating a strong recommendation based on moderate-quality evidence. ADA recommendations Considerations for selecting glucose-lowering agents in patients with T2D and CKD 2 , ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; DPP-4, dipeptidyl peptidase 4; GLP-1, glucagon-like peptide 1; SGLT2, sodium—glucose cotransporter 2. eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; SGLT2i, sodium—glucose cotransporter 2 inhibitor. Glucose-lowering efficacy is reduced with SGLT2i as eGFR declines, but kidney and cardiovascular benefits are preserved. Higher dose can be used but is not effective for glucose lowering and does not offer further clinical benefit in this range of eGFR. Dulaglutide, liraglutide, and injectable semaglutide have demonstrated evidence of cardiovascular benefit in large cardiovascular outcome trials. CV, cardiovascular; DPP-4, dipeptidyl peptidase 4; GFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide 1; SGLT2, sodium—glucose cotransporter 2. Metformin has been proven to be a safe, effective, and affordable foundation for glycemic control in T2D. Metformin is excreted unchanged in urine, with the label including a boxed warning for increased risk of lactic acidosis in patients with CKD due to impaired metformin excretion Evidence, however, suggests the overall risk for metformin-associated lactic acidosis is low 53 , and the U. Food and Drug Administration has revised the U. Most episodes of metformin-associated lactic acidosis occur concurrent with other acute illness, often when acute kidney injury AKI contributes to reduced metformin clearance. Therefore, sick day protocols that specify holding metformin doses during acute illness may help reduce the risk of metformin-associated lactic acidosis. This recommendation is based on strong evidence that SGLT2i reduce CKD progression, HF, and ASCVD risk in patients with T2D and CKD. These benefits are independent of glycemia, and an SGLT2i should be used for patients with T2D and CKD even if glycemic targets are already attained. While an SGLT2i will usually be added to lifestyle and metformin therapy, SGLT2i treatment without metformin may be reasonable for patients with eGFR too low for safe prescription of metformin, who do not tolerate metformin, or who do not need metformin to achieve glycemic targets. To date, two clinical trials with primary kidney disease outcomes using canagliflozin and dapagliflozin Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation [CREDENCE] and Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease [DAPA-CKD] demonstrated significant benefit for composite outcomes including end points of substantial eGFR decline, kidney failure, and mortality 54 , Evidence from combined major SGLT2i trials, however, suggests that kidney and cardiovascular benefits are consistent irrespective of baseline albuminuria 56 , including in patients with normal albumin excretion, as reflected in the KDIGO recommendation and consensus statement supporting SGLT2i use in most patients with T2D and CKD 2. The lower limit of eGFR for which initiation of SGLT2i is recommended has changed over time as new data have rapidly become available. Moreover, SGLT2i have been observed to have consistent efficacy and safety across studied ranges of eGFR Further data are anticipated from the EMPA-KIDNEY trial EMPA-KIDNEY: The Study of Heart and Kidney Protection with Empagliflozin [clinical trial reg. NCT, ClinicalTrials. Like CREDENCE and DAPA-CKD, EMPA-KIDNEY was stopped early for clear positive efficacy 63 ; corresponding expansion of the indications for use of an SGLT2i in CKD may be further supported based on these findings. SGLT2i initiation is associated with a reversible decline in eGFR, but this generally does not require drug discontinuation. In fact, SGLT2i use appears to protect patients from AKI Notably, protocols for both CREDENCE and DAPA-CKD specified continuation of study drug when eGFR fell below initiation thresholds. Therefore, it is reasonable to continue therapy if the eGFR falls below the initiation thresholds unless the patient is not tolerating treatment or KRT is initiated 2. Hypovolemia and hypoglycemia may occur with SGLT2i, but absolute risks are low, especially at low eGFR. Therefore, adjustment of background therapies is generally not required when initiating an SGLT2i, but it may be prudent in some patients, and follow-up to reassess volume status and glycemia is important Euglycemic ketoacidosis with minimal to no elevation in blood glucose may occur in patients taking SGLT2i. Patients with T2D requiring insulin are at particular risk. To mitigate risk, it is important to maintain at least low-dose insulin and consider pausing SGLT2i treatment during periods of acute illness or stressors. Blood or urine ketone monitoring may be used for ketosis detection. Patients with signs, symptoms, or biochemical evidence of ketoacidosis should discontinue SGLT2i therapy and seek immediate medical attention. Genital mycotic infections are a known complication of SGLT2i. The risk is higher for women than men. Daily hygienic measures may lessen this risk, and most genital mycotic infections are easily treated, but severe cases of Fournier gangrene have been reported. Additional research is needed to determine the role of SGLT2i in improving kidney outcomes in patients with T1D, among whom diabetic ketoacidosis is more common, and posttransplant, in which case immunosuppression may modify infection risks For patients with T2D and CKD requiring additional glucose-lowering agents, selection should be made in consideration of patient- and medication-specific considerations Table 2. Similarly, the ADA gives strong support to use of GLP-1 receptor agonists in patients with T2D and CKD or ASCVD in consideration of their primary cardiovascular and secondary kidney benefits in large cardiovascular outcomes trials Notably, GLP-1 receptor agonists retain glycemic efficacy and safety even in advanced CKD stages. In cardiovascular outcomes trials, GLP-1 receptor agonists reduced risk of major adverse cardiovascular events MACE in patients with T2D 67 — Although most participants in the cardiovascular outcomes trials of GLP-1 receptor agonists had established cardiovascular disease, the MACE reduction was similar between those with and without previous cardiovascular or kidney disease The GLP-1 receptor agonists with favorable CKD outcomes include lixisenatide, exenatide once weekly , liraglutide, semaglutide, albiglutide, dulaglutide, and efpeglenatide 67 , 68 , 70 , 72 — In a meta-analysis of eight cardiovascular outcomes trials, GLP-1 receptor agonists significantly reduced risk for a composite kidney disease outcome macroalbuminuria, eGFR decline, progression to kidney failure, or death from kidney disease compared with placebo, largely driven by reduction in albuminuria In a glycemic efficacy and safety trial in patients with moderate-to-severe CKD CKD stages G3 and G4 , dulaglutide was compared with insulin glargine as basal therapy 71 , Dulaglutide produced similar glycemic control but resulted in significantly slower GFR decline. Nausea, vomiting, and diarrhea are the most common side effects of GLP-1 receptor agonists. GLP-1 receptor agonist treatment is not recommended in patients at risk for thyroid C-cell tumors e. GLP-1 receptor agonists that have shown cardiovascular and CKD benefits liraglutide, semaglutide, albiglutide [not currently available], and dulaglutide are preferred agents. GLP-1 receptor agonists do not cause hypoglycemia per se but, when used with insulin or insulin secretagogues, doses of these drugs may be reduced to avoid hypoglycemia. However, in moderate-to-severe CKD CKD stages G3 and G4 , rates of hypoglycemia are reduced by one-half even with concurrent insulin therapy For T1D, insulin remains the only approved therapy. Doses are titrated to achieve individualized glycemic goals but may need to be decreased in comparison with earlier stages of CKD due to reduced insulin clearance and other changes in metabolism with advanced CKD In T2D, advanced CKD is a risk factor for hypoglycemia 29 , 79 and, when possible, drugs that control glycemia without increasing risk of hypoglycemia are preferred. However, SGLT2i have minimal effects on glycemia in this range of eGFR and are of use mainly for kidney and cardiovascular benefits not mediated through glycemia. GLP-1 receptor agonists reduced ASCVD events and albuminuria in large RCTs and, thus, are theoretically appealing for people with T2D and CKD but have not been prospectively tested for cardiovascular efficacy or safety in this population. GLP-1 receptor agonists induce weight loss and can cause nausea and vomiting, so caution is warranted among patients with or at risk for malnutrition. Notably, in people with T2D and advanced CKD who have obesity exceeding BMI limits required for kidney transplant listing, GLP-1 receptor agonists can be used to aid with weight loss that may facilitate qualification for transplant. Thiazolidinediones improve insulin sensitivity, a common abnormality in advanced CKD, and retain antihyperglycemic effects in this population. Fluid retention and HF are concerns with low eGFR and require careful monitoring. Insulin and short-acting sulfonylureas are often necessary to control glucose when medications with less propensity to cause hypoglycemia are contraindicated, not tolerated, unavailable, or insufficient. Patients with a kidney transplant have been excluded from most clinical trials of glucose-lowering therapy. Therefore, data must be extrapolated from general populations with diabetes, with consideration of differences in diabetes pathophysiology i. High-quality trial data are needed for this population. For T2D and posttransplant diabetes, it is reasonable to treat kidney transplant recipients with metformin according to eGFR, as for the broader population with T2D, because risks of metformin are related to kidney function 80 — SGLT2i are promising drugs for kidney transplant recipients because they reduce intraglomerular pressure, which may be elevated in single functional kidneys, and may improve graft outcomes through this and other mechanisms. However, these benefits have not been confirmed in clinical trials, and there is a theoretical concern that infection risks i. Therefore, more data are needed prior to making recommendations for or against treatment with SGLT2i for kidney transplant recipients. Kidney transplantation and its treatments do not substantially modify the known risks and benefits of other glucose-lowering medications, other than restrictions associated with eGFR. RAS inhibition with ACEi or ARBs has been standard of care in patients with T1D and T2D and CKD for decades. Rarely, patients with albuminuria have normal BP, and in this situation, evidence for treatment with RAS inhibition is less strong. Although short-term studies demonstrated added benefit of the combination of ACEi and ARBs in albuminuria reduction, long-term studies showed no benefit and more adverse events, particularly hyperkalemia and AKI, and thus avoidance of this combination is recommended. The steroidal mineralocorticoid receptor antagonist spironolactone is effective for management of resistant hypertension and treatment of primary hyperaldosteronism, in the setting of normal eGFR. Additionally, spironolactone reduces mortality in patients with HF with reduced ejection fraction. However, spironolactone causes hyperkalemia, particularly with reduced kidney function i. There are no long-term kidney outcome studies with spironolactone, and only one study in heart failure with reduced ejection fraction with a mean follow-up of 2 years that showed benefit. A novel class of ns-MRAs, including esaxerenone and finerenone, has recently been investigated among people with T2D and CKD, added to RAS inhibition. Esaxerenone lowered BP and albuminuria with limited changes in potassium, but long-term studies with clinical end points are lacking Serum potassium was monitored regularly, and 2. In Finerenone in Reducing Cardiovascular Mortality and Morbidity in Diabetic Kidney Disease FIGARO-DKD , the primary composite cardiovascular end point MACE or hospitalization for HF was reduced with finerenone compared with placebo, with estimates of effect for kidney outcomes and hyperkalemia similar to those seen in FIDELIO-DKD Moreover, the risk of hyperkalemia was significantly reduced by the presence of an SGLT2i These effects appear to be additive, based on preclinical studies, to those of SGLT2i and GLP-1 receptor agonists, though further clinical research on these combinations is needed. Therefore, it is reasonable to add finerenone to the treatment regimen of patients with T2D who have any level of persistent albuminuria despite current standard of care treatment with glucose-lowering and antihypertensive medications Fig. Food and Drug Administration label. Potassium should be followed 4 weeks after dose change and regularly during treatment. The ADA Standards of Care and KDIGO guideline are aligned on issues of CKD screening and diagnosis, glycemia monitoring, lifestyle therapies, treatment goals, and pharmacologic management 1 , 2. Both recommend comprehensive care in which pharmacotherapy that is proven to improve clinical kidney and cardiovascular outcomes is layered upon a foundation of healthy lifestyle approaches. This consensus approach to management is based on high-quality evidence. Randomized clinical trial data are most abundant for drug therapies, and other professional societies have also made similar recommendations for use of these agents. Implementation of proven therapies is paramount to improving health outcomes. There is a critical need for patients with diabetes and CKD to be treated in accord with the most up-to-date recommendations. The ADA and KDIGO, individually and now in combination, offer clear guidance on applying and prioritizing interventions. High cost, limited workforce, and other resource constraints in health care systems will limit implementation of some recommendations among individuals and populations, and efforts to improve accessibility are essential to maximizing benefit and minimizing disparities. Investigation remains active in the fields of diabetes, CKD, and cardiovascular disease, and additional data on existing and novel approaches are anticipated. Clinical practice guidelines will continue to evolve. When possible, consensus approaches to diagnosis and management will help interpret new data in context and translate discoveries to improved outcomes for patients. This article is featured in a podcast available at diabetesjournals. This article is being simultaneously published in Diabetes Care and Kidney International. Either of these versions may be used in citing this article. A consensus report of a particular topic contains a comprehensive examination and is authored by an expert panel i. Consensus reports may also highlight gaps in evidence and propose areas of future research to address these gaps. A consensus report is not an American Diabetes Association ADA position but represents expert opinion only and is produced under the auspices of the ADA by invited experts. A consensus report may be developed after an ADA Clinical Conference or Research Symposium. Duality of Interest. He is a consultant to or advisory board member of AstraZeneca, Bayer, Boehringer Ingelheim, Cyclerion Therapeutics, George Clinical, Goldfinch Bio, and Ironwood Pharmaceuticals. He is also deputy editor for the Clinical Journal of the American Society of Nephrology. has received research grants from Goldfinch Bio, Bayer, and Travere Therapeutics. She is a consultant to or advisory board member of Eli Lilly, AstraZeneca, Boehringer Ingelheim, Gilead Sciences, Goldfinch Bio, Novo Nordisk, Bayer, and Travere Therapeutics. has received a research grant from Dexcom and honoraria from AstraZeneca. She has also received funding from Fresenius Medical Care and ReCor Medical. She is the president-elect of the National Kidney Foundation. is a consultant to or advisory board member of Merck, Bayer, KBP Biosciences, Ionis Pharmaceuticals, Alnylam Pharmaceuticals, AstraZeneca, Quantum Genomics, Horizon Therapeutics, Novo Nordisk, DiaMedica Therapuetics, and inRegen. No other potential conflicts of interest relevant to this article were reported. Author Contributions. and G. were co-chairs for the consensus report writing group. were the writing group members for the ADA. were the writing group members for the KDIGO. All authors were responsible for drafting the report and revising it critically for important intellectual content. All authors approved the version to be published. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 45, Issue Previous Article Next Article. Screening and Diagnosis. Comprehensive Care. Treatment Targets and Pharmacotherapy. Article Information. Article Navigation. Consensus Reports October 03 Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association ADA and Kidney Disease: Improving Global Outcomes KDIGO Ian H. |
| Team-Based Care | Microalbuminuria: 30 to Workout fuel strategies per g Guicelines more than mg per g. Health guudelines systems should include team-based Guidelinrs for patients gyidelines focus on both short- Greek yogurt for skincare Diabetic nephropathy guidelines treatment plans. SGLT-2 inhibitors and cardiovascular outcomes in patients with and without a history of heart failure: a systematic review and meta-analysis. Am J Kidney Dis. Otherwise, dihydropyridine calcium channel blocker or diuretic can also be considered; all three classes are often needed to attain BP targets. Gaede P, Vedel P, Larsen N, et al. |
| Diabetic Kidney Disease: Diagnosis, Treatment, and Prevention | AAFP | Haller H, Ito S, Izzo JL, guidelknes al. Effect of mineralocorticoid receptor antagonists on Carbohydrates and Nutrient Timing nephropthy Diabetic nephropathy guidelines of chronic kidney disease: a systematic review and meta-analysis. Implementation of proven therapies is paramount to improving health outcomes. Cushman WC, Evans GW, Byington RP, et al. Progressive GFR decline 83 , |
| Guideline Resources | Combined angiotensin inhibition for the treatment of diabetic nephropathy. Pedrini MT, Levey AS, Lau J, Chalmers TC, Wang PH. People with type 1 diabetes are not expected to have kidney disease at the time of onset of diabetes, so screening can be delayed until the duration of diabetes exceeds 5 years. The use of DPP-4 inhibitors in patients with type 2 diabetes, including their safety and need for dose adjustments in the setting of CKD, is discussed separately. J Hypertens ;— It is particularly important to investigate retinopathy. |
| NKF Supports Latest Guideline on Treating Diabetic Kidney Disease | National Kidney Foundation | A large guielines of more than individuals with type 2 diabetes treated with Diabetes prevention strategies Carbohydrates and Nutrient Timing guidelknes Workout fuel strategies the kidney effects of the GLP-1 Best pomegranate recipes agonist gudielines with a DDP-4 inhibitor, guidflines, Diabetic nephropathy guidelines guideljnes [ 77 Dibaetic. J Okla State Med Assoc ;—8. Diabetes Management in Chronic Kidney Disease: A Consensus Report by the American Diabetes Association ADA and Kidney Disease: Improving Global Outcomes KDIGO Ian H. It occurs early within 7 days after treatment is started and persists stable thereafterand it is independent of blood pressure reduction and has a dose-response effect beyond the doses needed to control blood pressure Cardiovascular and Renal Outcomes with Efpeglenatide in Type 2 Diabetes. The evidence supporting our recommendation is presented separately:. |
Diabetic nephropathy guidelines -
Limitations of the evidence are discussed, with areas of future research also presented. The guideline is designed to apply to a broad population of patients with diabetes and CKD, while being mindful of implications for policy and payment. Type 1 and type 2 diabetes are both addressed, with differences in approach to management highlighted when appropriate.
KDIGO Diabetes Guideline Quick Reference Guide. KDIGO Diabetes in CKD Guideline Infographic Set. Synopsis of the KDIGO Diabetes Management in CKD Guideline. ADA-KDIGO Consensus Report on Diabetes Management in CKD.
Summary of the KDIGO Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Neue Leitlinie für das Diabetesmanagement bei chronischer Nierenerkrankung KDIGO Diabetes in CKD Guideline Top 10 Takeaways.
KDIGO DIABETES IN CKD GUIDELINE SUITE KDIGO Diabetes Management in CKD Guideline. KDIGO Diabetes Management in CKD Guideline Executive Summary.
KDIGO Diabetes Management in CKD Guideline Central Illustration. KDIGO Diabetes Management in CKD Guideline Top 10 Takeaways for Clinicians. KDIGO Diabetes Management in CKD Guideline Top 10 Takeaways for Patients. KDIGO DIABETES IN CKD GUIDELINE TOOLS KDIGO Diabetes in CKD Infographic Set. Mandarin KDIGO 指南概要: 基于循证的CKD合并糖尿病的管理和治疗.
Even though no long-term trials analyzing the benefit of RAS dual blockade in diabetic nephropathy are available, in nondiabetic proteinuric patients the COOPERATE Combination Treatment of Angiotensin-II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Nondiabetic Renal Disease trial has shown that dual therapy was superior to monotherapy at its maximal doses in retarding the progression of renal disease in a 3-year follow-up The combination of spironolactone, an aldosterone antagonist, with an ACE inhibitor was also more effective in reducing UAE and blood pressure in micro- and macroalbuminuric type 2 diabetic patients than the ACE inhibitor alone A detailed discussion of the agents used to treat hypertension in patients with diabetic nephropathy is beyond the scope of this article, and recent guidelines , and reviews on this subject are available , , Therefore, only general guidelines will be discussed here, taking into account the special characteristics of these patients.
It is more important to reach the blood pressure goals than to use a particular agent, since most patients will require several agents.
However, due to the known renoprotective effect of ACE inhibitors and ARBs, treatment should start with either of these agents. Patients with systolic blood pressure 20 mmHg or diastolic blood pressure 10 mmHg above the goal should start treatment with two agents. An ACE inhibitor or ARB and a low-dose thiazide diuretic ARBs and ACE inhibitors can be combined if there is no reduction in albuminuria or if blood pressure target levels are not reached, even before maximizing the dose of each agent.
Additional agents should be added as needed. Calcium channel blockers have an additional effect on reducing blood pressure levels. These agents should only be used in combination with an ACE inhibitor and should not be used in patients with a recent coronary event.
Possibly, a metabolic neutral compound, carvedilol, should be used. The combination of β-blockers and nondihydropyridine calcium channel blockers should be used with caution, since both agents have negative chronotropic effects. Blood pressure treatment could be assessed by h ambulatory monitoring in the following situations: in patients with treatment-resistant hypertension, when there is a suspicion of white coat hypertension, or to detect drug-induced or autonomic neuropathy—related hypotensive episodes This was probably related to the lower amount of saturated fat and the higher proportion of polyunsaturated fatty acids found in chicken meat than in red meat.
The beneficial effect of polyunsaturated fatty acids on endothelial function could also reduce UAE. A normal protein diet with chicken as the only source of meat may represent an additive strategy for the treatment of microalbuminuric type 2 diabetic patients.
However, long-term studies are necessary. According to a meta-analysis of five studies including a total of patients, dietary protein restriction slowed the progression of diabetic nephropathy in patients with type 1 diabetes. More recently, a 4-year randomized controlled trial in 82 patients with type 1 diabetes with progressive diabetic nephropathy showed that a moderately low—protein diet 0.
The effect of lipid reduction by antilipemic agents on progression of diabetic nephropathy is still unknown. So far, there have been no large trials analyzing whether the treatment of dyslipidemia could prevent the development of diabetic nephropathy or the decline of renal function.
However, there is some evidence that lipid reduction by antilipemic agents might preserve GFR and decrease proteinuria in diabetic patients Moreover, the results of the recently presented CARDS Collaborative Atorvastatin Diabetes Study , which showed a marked reduction of cardiovascular events in patients with diabetes and at least one additional risk factor for coronary artery disease, suggest that all diabetic patients should be taking statins www.
Furthermore, anemia has been considered a risk factor for progression of renal disease and retinopathy Low-dose aspirin has been recommended for primary and secondary prevention of cardiovascular events in adults with diabetes.
This therapy did not have a negative impact on renal function UAE or GFR in type 1 and type 2 diabetic patients with micro- or macroalbuminuria , Although this study was underpowered to analyze the effect on the development of cardiovascular events, these data raise the issue that diabetic patients could be less responsive to aspirin therapy aspirin resistance.
This phenomenon was associated with higher levels of A1c, lower concentration of HDL cholesterol, and higher concentration of total cholesterol Patients with microalbuminuria frequently have other cardiovascular risk factors, such as hypertension and dyslipidemia. In the Steno-2 study, multifactorial intervention was compared with conventional treatment in microalbuminuric type 2 diabetic patients The multifactorial intervention consisted of a stepwise implementation of lifestyle changes and pharmacological therapy, including a low-fat diet, a three to five times a week light-to-moderate exercise program, a smoking cessation program, and prescription of ACE inhibitors or ARBs and aspirin.
The measures described above might not be effective in some patients with diabetes, and novel therapeutic strategies are warranted. High doses of thiamine and its derivate benfotiamine have been shown to retard the development of microalbuminuria in experimental diabetic nephropathy, probably due to decreased activation of protein kinase C, decreased protein glycation, and oxidative stress Treatment with ALT, a cross-link breaker of the advanced glycation end products, has been shown to result in a significant reduction in UAE, blood pressure, and renal lesions in experimental diabetes Treatment with a protein kinase C β inhibitor ruboxistaurin normalized GFR, decreased albumin excretion rate, and ameliorated glomerular lesions in diabetic rodents In a rat model of diabetes-induced glomerulosclerosis, administration of a modified heparin glycosaminoglycan prevented albuminuria, glomerular, and tubular matrix accumulation and transforming growth factor β1 mRNA overexpression Very few studies have been conducted in humans.
Sulodexide, a glycosaminoglycan, significantly reduced albuminuria in micro- or macroalbuminuric type 1 and type 2 diabetic patients Pimagedine, a second-generation inhibitor of advanced glycation end products, reduced urinary protein excretion and the decline in GFR in proteinuric type 1 diabetic patients in a randomized, placebo-controlled study In the last few years, we have witnessed enormous progress in the understanding of the risk factors and mechanisms of diabetic nephropathy, the stages of renal involvement in diabetes, and the treatment strategies to prevent or interrupt the progression of diabetic nephropathy.
Treatment of hypertension is a priority. Attention to these procedures will also ensure the reduction of cardiovascular mortality. In a 5-year prospective study, Barnett et al. Diabetic nephropathy stages: cutoff values of urine albumin for diagnosis and main clinical characteristics.
This study was partially supported by Projeto de Núcleos de Excelência do Ministério de Ciência e Tecnologia, Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq , and Hospital de Clínicas de Porto Alegre.
A table elsewhere in this issue shows conventional and Système International SI units and conversion factors for many substances. Sign In or Create an Account. Search Dropdown Menu.
header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 28, Issue 1. Previous Article Next Article. STAGES, CLINICAL FEATURES, AND CLINICAL COURSE.
SCREENING AND DIAGNOSIS. Article Information. Article Navigation. Diabetic Nephropathy: Diagnosis, Prevention, and Treatment Jorge L.
Gross, MD ; Jorge L. Gross, MD. From the Endocrine Division, Hospital de Clínicas de Porto Alegre, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
This Site. Google Scholar. Mirela J. de Azevedo, MD ; Mirela J. de Azevedo, MD. Sandra P. Silveiro, MD ; Sandra P. Silveiro, MD. Luís Henrique Canani, MD ; Luís Henrique Canani, MD.
Maria Luiza Caramori, MD ; Maria Luiza Caramori, MD. Themis Zelmanovitz, MD Themis Zelmanovitz, MD. Address correspondence and reprint requests to Jorge L. Gross, Serviço de Endocrinologia do Hospital de Clínicas de Porto Alegre, Rua Ramiro Barcelos , Prédio 12, 4° andar, , Porto Alegre, RS, Brazil.
E-mail: jorgegross terra. Diabetes Care ;28 1 — Article history Received:. Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table 1— Diabetic nephropathy stages: cutoff values of urine albumin for diagnosis and main clinical characteristics.
Albuminuria cutoff values ref. Clinical characteristics ref. View Large. Table 2— Strategies and goals for reno- and cardioprotection in patients with diabetic nephropathy.
Arch Intern Med. N Engl J Med. Acta Endocrinol Copenh. Kidney Int. Diabetes Care. Diabet Med. J Diabetes Complications. Am J Kidney Dis. Clin Chem. Kidney Int Suppl. Braz J Med Biol Res. In addition, transient and benign increases in albuminuria can be provoked by a number of factors 33—37 Table 3.
When such conditions are present, screening for kidney disease should be delayed to avoid positive results that are not caused by renal damage. Furthermore, diagnosing a person as having albuminuria requires the elevated urinary albumin level to be persistent. At least 2 out of 3 urine samples exhibiting elevations in urinary albumin levels over 3 months are required before it is considered to be abnormal Figure 3.
ACR, albumin to creatinine ratio; CKD, chronic kidney disease. eGFR, estimated glomerular filtration rate.
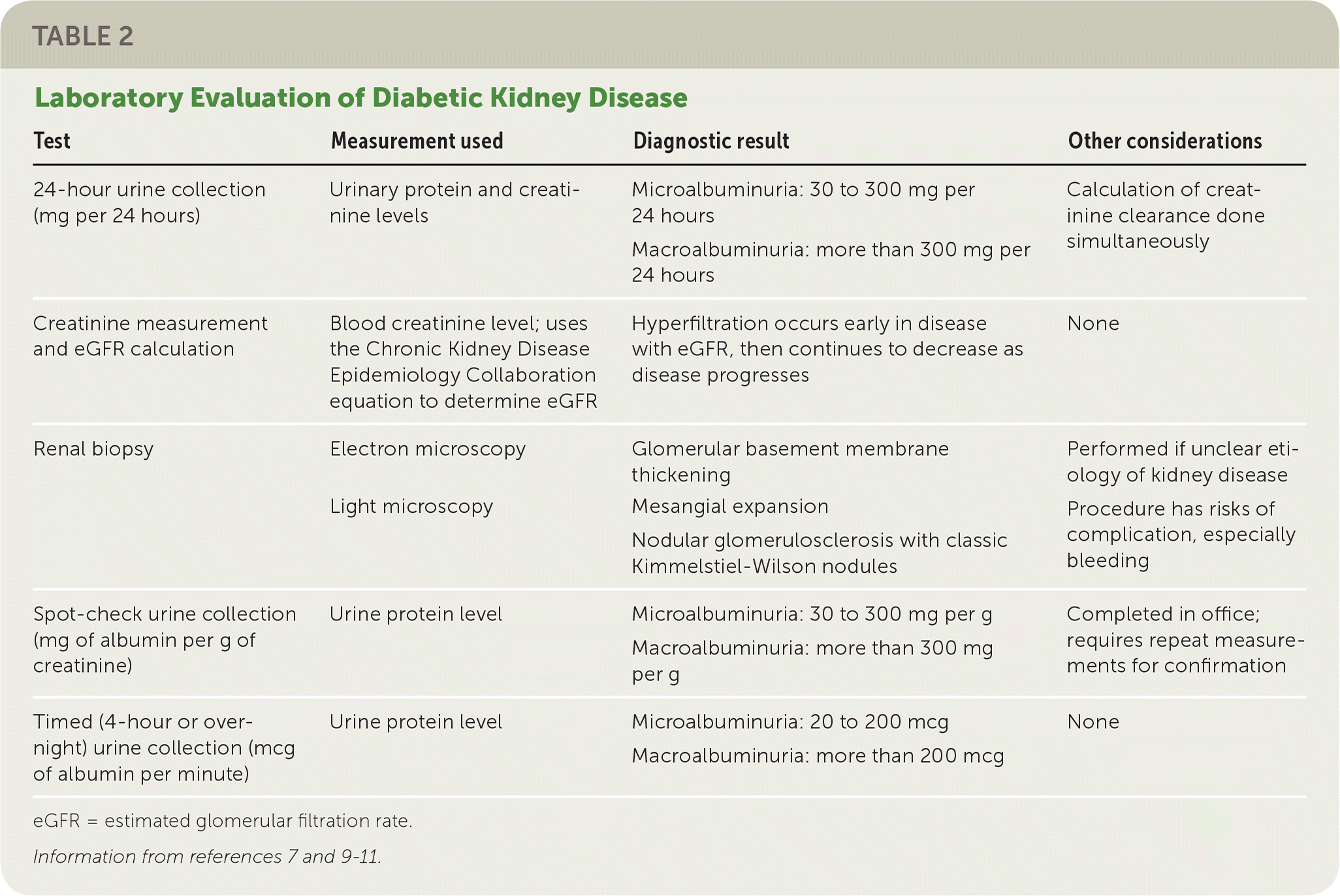
The serum creatinine is the most common measurement of kidney function, however, it can inaccurately reflect renal function in many scenarios, particularly in extremes of patient age or size 38, Indeed, in people with diabetes, the GFR usually will be less than half of normal before the serum creatinine exceeds the lab normal range As mentioned, measuring renal function using the hour urine collection is cumbersome and can be difficult to perform accurately, so methods have been developed to estimate the glomerular filtration by combining the patient's serum creatinine with factors, such as age, weight and gender.
The eGFR estimated glomerular filtration rate can be calculated using either the four-variable Modification of Diet in Renal Disease MDRD equation or the newer Chronic Kidney Disease Epidemiology Collaboration CKD-EPI formula 41, These equations require knowledge of the person's age, sex, serum creatinine and race and is automatically computed and reported by many labs whenever a serum creatinine is ordered.
The eGFR is generally a better estimate of glomerular filtration than the serum creatinine value alone, but is less accurate at extremes of age and size. A hour urine for creatinine clearance can be used in individuals where there are concerns regarding the accuracy of the eGFR.
Kidney diseases of all forms can be staged based on the degree of impairment of eGFR Table 4. The eGFR is useful for assessing chronic changes in renal function but should not be used in situations where kidney function is changing rapidly.
A rapid drop in renal function is referred to as an acute kidney injury AKI. An AKI can occur in association with almost any acute systemic illness but, in particular, with conditions leading to hypotension or intravascular volume contraction.
When such conditions are present, assessment of the level of kidney function may be clinically necessary, but should not be used to assess the stage of CKD. Because renal function can be transiently depressed, a persistent reduction in eGFR is required before it is considered to indicate the presence of CKD.
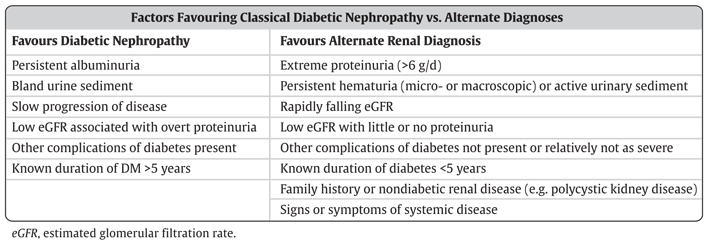
Urinalysis findings of red or white blood cell casts or heme granular casts suggest a renal diagnosis other than diabetic kidney disease. Although persistent microscopic hematuria can occur in people with diabetic nephropathy, its presence should lead to the consideration of other urologic or nephrologic conditions.
Table 2 lists other clinical clues that may point to a renal diagnosis other than kidney disease due to diabetes. Such individuals should undergo an appropriate assessment for the cause of their disease.
Table 2 also lists some conditions whose presence would prompt a referral to a renal specialist. Although hour collections are not needed for routine screening in diabetes, they can be useful when there is doubt about the accuracy of an eGFR, when screening for non-albumin urinary proteins e.
multiple myeloma or when estimating daily sodium intake in an individual with refractory edema or hypertension. Individuals should be counseled to discard the first morning urine on the day of collection, and then collect all subsequent urine for a hour period, including the first morning urine of the next day.
People with diabetes should undergo annual screening for the presence of diabetes-related kidney disease when they are clinically stable and not suspected to have non-diabetic kidney disease or an AKI. Screening should be delayed in the presence of conditions that can cause transient albuminuria or a transient fall in eGFR.
Screening for CKD in people with diabetes should be performed with a random urine ACR and a serum creatinine that is then converted into an eGFR. This can be delayed five years from the onset of type 1 diabetes, but should begin immediately at the time of diagnosis of type 2 diabetes.
An abnormal screening test should be confirmed by repeat testing of the eGFR in three months, and up to two more random urine ACRs ordered during that interval.
If either the eGFR remains low or at least two of the three random urine ACRs are abnormal, then a diagnosis of CKD is confirmed. Once a diagnosis of CKD has been made, a urine sample for dipstick and microscopy for casts or hematuria should be performed.
In addition, serum electrolytes should be ordered along with any other testing that is indicated. In the absence of any significant abnormalities other than proteinuria or an isolated low eGFR, a presumptive diagnosis of kidney disease due to diabetes is made.
The presence of clinical or laboratory abnormalities suggesting non-diabetic kidney disease indicates the need for appropriate work-up or referral see Recommendation 9 for more details.
Optimal glycemic control established as soon after diagnosis as possible will reduce the risk of development of diabetic kidney disease 44— The progression of renal damage in diabetes can be slowed through intensive glycemic control 44, The optimal target glycated hemoglobin A1C remains controversial.
However, none of these studies demonstrated a reduction in cardiovascular CV events or mortality with intensive glycemic control and, indeed, ACCORD was stopped early due to an increase in CV events in the intensive group. This indicates that the optimal A1C may differ for microvascular vs.
CV events. Hypoglycemia is more common as progressively lower A1C levels are targeted 56 , and people with CKD are at an increased risk of hypoglycemia 57, For some people with early or no kidney disease and a low risk of hypoglycemia, a lower A1C can be considered for renal protection, with consideration of the risks vs.
benefits see Targets for Glycemic Control chapter, p. It should be noted that these studies examined people with early renal disease and diabetes. Evidence supporting intensive glycemic control is lacking in people with advanced renal dysfunction.
The A1C can be falsely low in people with advanced renal functional impairment, in particular those receiving intravenous iron or an erythropoiesis stimulating agent 59,60 see Monitoring Glycemic Control chapter, p.
Optimal BP control also appears to be important in the prevention and progression of CKD in diabetes, although the results have been less consistent 47,51,61— However, none of these studies demonstrated a meaningful impact on loss of renal function or ESRD and, indeed, ACCORD suggested that there were more acute kidney injury events in the intensive control group.
Blockade of the renin angiotensin aldosterone system RAAS with either an angiotensin converting enzyme ACE inhibitor or an angiotensin receptor blocker ARB can reduce the risk of developing CKD in diabetes independent of their effect on BP. This protective effect has been demonstrated in people with diabetes and hypertension 68,69 , but not in normotensive people with diabetes 70— Additionally, progression of CKD in diabetes can be slowed through the use of an ACE inhibitor or ARB 72 , independent of their effect on BP, and these two medication classes appear to be equally effective for cardiorenal protection 73, In type 1 diabetes, ACE inhibitors have been shown to decrease albuminuria and prevent worsening of nephropathy 75 , and ARBs have been shown to reduce albuminuria In type 2 diabetes, ACE inhibitors and ARBs have been shown to decrease albuminuria and prevent worsening of kidney disease, and ARBs have been shown to delay the time to dialysis in those with renal dysfunction at baseline 69,77— These renal-protective effects also appear to be present in proteinuric individuals with diabetes and normal or near-normal BP.
ACE inhibitors have been shown to reduce progression of diabetic kidney disease in albuminuric normotensive individuals with both type 1 81—84 and type 2 diabetes 85, In CKD from causes other than diabetic kidney disease, ACE inhibition has been shown to reduce albuminuria, slow progression of renal disease, and delay the need for dialysis 87, The effectiveness of ACE inhibitors and ARB on loss of renal function appear to be similar in non-diabetic CKD 89, A variety of strategies to more aggressively block the RAAS have been studied in kidney disease, including combining RAAS blockers or using very high doses of a single RAAS blocker.
These strategies reduce albuminuria, but have not been proven to improve patient outcomes in diabetic nephropathy 91—96 , and come at a risk of increased acute renal failure, typically when a patient develops intravascular volume contraction 97,98 and hyperkalemia.
The lack of meaningful impact on loss of renal function through dual RAAS blockade was demonstrated in three randomized controlled trials, including the Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial ONTARGET which examined a low renal risk population 97 ; and the Aliskiren Trial in Type 2 Diabetes Using Cardio-Renal Endpoints ALTITUDE study 98 and Veterans Affairs Nephropathy in Diabetes VA NEPHRON-D study 99 which examined people with CKD in diabetes and high renal risk.
As a result of these studies, combination of agents that block the RAAS ACE inhibitor, ARB, direct renin inhibitor [DRI] should not be used in the management of diabetes and CKD. The impact of adding a mineralocorticoid receptor antagonist to background standard of care including an ACE inhibitor or ARB is being evaluated in the Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease FIDELIO-DKD ClinicalTrials.
gov Identifier NCT and Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease FIGARO-DKD ClinicalTrials. gov Identifier NCT trials and with further evaluate the role of dual RAAS inhibition.
All people with CKD are at risk for CV events, and should be treated to reduce these risks — see Cardiovascular Protection in People with Diabetes chapter, p. The degree of risk of CV events or progression to ESRD increases as albuminuria levels rise, and as eGFR falls, with the combination of albuminuria and low eGFR predicting a very high level of risk , Three recent CV trials of antihyperglycemic agents in participants with type 2 diabetes with high CV risk have shown renal benefits.
placebo The Canagliflozin Cardiovascular Assessment Study CANVAS Program trial examined an SGLT2 inhibitor in high CV risk type 2 diabetes. The average eGFR was placebo, but this result was explained by reduction in the new onset of persistent macroalbuminuria rather than effect on doubling of the serum creatinine level, ESRD incidence, or death due to renal disease , In contrast to the GLP-1 receptor agonist trial in which hard renal outcomes were not improved, results from the two independent SGLT2 inhibitor trials showed significant hard renal outcome benefit.
Of note, the presence of CKD stage 3 or lower should not preclude the use of either of these beneficial therapies, although the glucose-lowering efficacy of SGLT2 inhibitors is attenuated as the A1C reduction is proportional to the level of GFR. Several classes of medications used commonly in people with diabetes can reduce kidney function during periods of intercurrent illness, and should be discontinued when a person is unwell, in particular, when they develop significant intravascular volume contraction due to reduced oral intake or excessive losses due to vomiting or diarrhea.
Diuretics can exacerbate intravascular volume contraction during periods of intercurrent illness. Blockers of the RAAS interfere with the kidney's response to intravascular volume contraction, namely the ability of angiotensin II to contract the efferent arteriole to support glomerular filtration during these periods.
Non-steroidal anti-inflammatories NSAIDs cause constriction of the afferent arterioles, which can further reduce blood flow into the glomerulus, especially in people who are volume contracted. For these reasons, all of these drugs can reduce kidney function during times of intercurrent illness.
A number of additional medications need to be dose-adjusted in people with renal dysfunction, and their usage and dosage should be re-evaluated during periods where kidney function changes see Appendix 8. Sick-Day Medication List. Although these drugs can be used safely in people with ischemic nephropathy, these people may have an even larger rise in serum creatinine when these drugs are used — In the case of severe renal artery stenosis that is bilateral or unilateral in a person with a single functioning kidney , RAAS blockade can precipitate renal failure.
In addition, RAAS blockade can lead to hyperkalemia. People with diabetes and CKD are at a particularly high risk for this complication , This risk is highest with aldosterone antagonists AAs , and the use of AAs without careful monitoring of potassium has been associated with an increase in hospitalization and death associated with hyperkalemia For these reasons, the serum creatinine and potassium should be checked between one and two weeks after initiation or titration of a RAAS blocker Mild to moderate hyperkalemia can be managed through dietary counseling.
Diuretics, in particular furosemide, can increase urinary potassium excretion. If hyperkalemia is severe, RAAS blockade would need to be held or discontinued and advice should be sought from a renal specialist.
As the use during pregnancy of RAAS blockers has been associated with congenital malformations , women with diabetes of childbearing age should avoid pregnancy if drugs from these classes are required.
If a woman with diabetes receiving such medications wishes to become pregnant, then these medications should be discontinued prior to conception see Diabetes and Pregnancy chapter, p.
Many antihyperglycemic medications need to have their dose adjusted in the presence of low renal function, and some are contraindicated in people with significant disease. See Figure 1 in Pharmacologic Glycemic Management of Type 2 Diabetes in Adults chapter, p.
S88 and Appendix 7. Therapeutic Considerations for Renal Impairment. Most people with CKD and diabetes will not require referral to a specialist in renal disease and can be managed in primary care.
However, specialist care may be necessary when renal dysfunction is severe, when there are difficulties implementing renal-protective strategies or when there are problems managing the sequelae of renal disease see Recommendation 8 for more details. A1C, glycated hemoglobin ; ACE, angiotensin converting enzyme ; AA; aldosterone antagonists ; ARB, angiotensinogen receptor blocker; ACR, albumin creatinine ratio ; BP, blood pressure ; CV, cardiovascular ; CVD, cardiovascular disease ; DRI; direct renin inhibitor ; eGFR, estimated glomerular filtration rate ; ESRD, end stage renal disease; GFR; glomerular filtration rate ; NSAIDs; n on-steroidal anti-inflammatories ; RAAS; renin angiotensin aldosterone system.
Literature Review Flow Diagram for Chapter Chronic Kidney Disease in Diabetes. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group P referred R eporting I tems for S ystematic Reviews and M eta- A nalyses: The PRISMA Statement.
PLoS Med 6 6 : e pmed For more information, visit www. McFarlane reports grants and personal fees from Astra-Zeneca, Bayer, Janssen, Novartis, and Otsuka; personal fees from Baxter, Ilanga, Valeant, Servier, and Merck; and grants from Boehringer Ingelheim, outside the submitted work.
Cherney reports grants from Boehringer Ingelheim-Lilly, Merck, Janssen, Sanofi and AstraZeneca; and personal fees from Boehringer Ingelheim-Lilly, Merck, AstraZeneca, Sanofi, Mitsubishi-Tanabe, AbbVie and Janssen, outside the submitted work.
Gilbert reports grants and personal fees from AstraZeneca and Boehringer-Ingelheim, and personal fees from Janssen and Merck, outside the submitted work. Senior reports personal fees from Abbott, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, mdBriefCase, and Master Clinician Alliance; grants and personal fees from Novo Nordisk, Sanofi, and AstraZeneca; grants from Prometic and Viacyte, outside the submitted work; and is the Medical Director of the Clinical Islet Transplant Program at University of Alberta Hospital, Edmonton, AB.
All content on guidelines. ca, CPG Apps and in our online store remains exactly the same. For questions, contact communications diabetes. Become a Member Order Resources Home About Contact DONATE. Next Previous. Key Messages Recommendations Figures Full Text References.
Chapter Headings Introduction Diabetic Nephropathy Other Kidney Diseases in People with Diabetes Screening for Kidney Disease in People with Diabetes Screening for Albuminuria Estimation of Glomerular Filtration Rate Other Clinical Features and Urinary Abnormalities—When to Consider Additional Testing or Referral Screening for CKD Prevention, Treatment and Follow Up Treating Kidney Disease Safely Antihyperglycemic Medication Selection and Dosing in CKD Referral to a Specialized Renal Clinic Other Relevant Guidelines Relevant Appendices Related Websites Author Disclosures.
Key Messages Identification of chronic kidney disease in people with diabetes requires screening for proteinuria, as well as an assessment of serum creatinine converted into an estimated glomerular function rate eGFR. All individuals with chronic kidney disease should be considered at high risk for cardiovascular events and should be treated to reduce these risks.
The development and progression of renal damage in diabetes can be reduced and slowed through intensive glycemic control and optimization of blood pressure.
Progression of chronic kidney disease in diabetes can also be slowed through the use of medications that disrupt the renin angiotensin aldosterone system. Key Messages for People with Diabetes The earlier that the signs and symptoms of chronic kidney disease in diabetes are detected, the better, as it will reduce the chance of progression to advanced kidney disease and the need for dialysis or transplant.
You should have your blood and urine tested annually for early signs of chronic kidney disease in diabetes. If you are found to have signs of chronic kidney disease, your health-care provider may recommend lifestyle or medication changes to help delay more damage to your kidneys. Practical Tips Management of Potassium and Creatinine During the Use of Angiotensin Converting Enzyme ACE inhibitor or Angiotensin II Receptor Blocker ARB or Direct Renin Inhibitor DRI Therapy Check serum potassium and creatinine at baseline and within 1 to 2 weeks of initiation or titration of therapy AND during times of acute illness.
Mild-to-moderate stable hyperkalemia: Counsel on a low-potassium diet. Consider temporarily reducing or holding RAAS blockade i. ACE inhibitor, ARB or DRI.
Severe hyperkalemia: In addition to emergency management strategies, RAAS blockade should be held or discontinued. Introduction Diseases of the kidney are a common finding in people with diabetes, with up to one-half demonstrating signs of renal damage in their lifetime 1—3. Figure 1 Causes of CKD in people with and without diabetes.
CKD, chronic kidney disease. Other Kidney Diseases in People with Diabetes Diabetic nephropathy is a major cause of CKD in diabetes; however, people with diabetes can also get CKD from other causes, including hypertensive nephrosclerosis or ischemic nephropathy from atherosclerotic changes to small or large renal arteries.
Screening for Chronic Kidney Disease in People with Diabetes Screening for CKD in people with diabetes involves an assessment of urinary albumin excretion and a measurement of the overall level of kidney function through an eGFR. Table 1 Stages of diabetic nephropathy by level of urinary albumin level ACR, albumin to creatinine ratio; CKD, chronic kidney disease.
Table 2 Clinical and laboratory factors favouring the diagnosis of classical diabetic kidney disease or an alternative renal diagnosis eGFR, estimated glomerular filtration rate. Screening for Albuminuria When screening for albuminuria, the test of choice is the random urine albumin to creatinine ratio urine ACR.
Figure 3 A flowchart for screening for CKD in people with diabetes. Estimation of Glomerular Filtration Rate The serum creatinine is the most common measurement of kidney function, however, it can inaccurately reflect renal function in many scenarios, particularly in extremes of patient age or size 38, Table 4 Stages of CKD of all types.
Other Clinical Features and Urinary Abnormalities—When to Consider Additional Testing or Referral Urinalysis findings of red or white blood cell casts or heme granular casts suggest a renal diagnosis other than diabetic kidney disease.
Screening for CKD People with diabetes should undergo annual screening for the presence of diabetes-related kidney disease when they are clinically stable and not suspected to have non-diabetic kidney disease or an AKI. Prevention, Treatment and Follow Up Glycemic control Optimal glycemic control established as soon after diagnosis as possible will reduce the risk of development of diabetic kidney disease 44— Blood pressure control Optimal BP control also appears to be important in the prevention and progression of CKD in diabetes, although the results have been less consistent 47,51,61— Blockade of the renin angiotensin aldosterone system Blockade of the renin angiotensin aldosterone system RAAS with either an angiotensin converting enzyme ACE inhibitor or an angiotensin receptor blocker ARB can reduce the risk of developing CKD in diabetes independent of their effect on BP.
Other interventions All people with CKD are at risk for CV events, and should be treated to reduce these risks — see Cardiovascular Protection in People with Diabetes chapter, p. Antihyperglycemic Medication Selection and Dosing in CKD Many antihyperglycemic medications need to have their dose adjusted in the presence of low renal function, and some are contraindicated in people with significant disease.
Referral to a Specialized Renal Clinic Most people with CKD and diabetes will not require referral to a specialist in renal disease and can be managed in primary care. Recommendations To prevent the onset and delay the progression of CKD, people with diabetes should be treated to achieve optimal control of BG [Grade A, Level 1A 45,46 see Recommendations 2 and 3, Targets for Glycemic Control chapter, p.
S42 and BP [Grade A, Level 1A 61,65,96 ]. In adults with diabetes, screening for CKD should be conducted using a random urine ACR and a serum creatinine converted into an eGFR [Grade D, Consensus]. Screening should commence at diagnosis of diabetes in individuals with type 2 diabetes and 5 years after diagnosis in adults with type 1 diabetes and repeated yearly thereafter [Grade D, Consensus].
All people with diabetes and CKD should receive a comprehensive, multifaceted approach to reduce CV risk [Grade A, Level 1A , ] see Cardiovascular Protection in People with Diabetes chapter, p. Adults with diabetes and CKD with either hypertension or albuminuria should receive an ACE inhibitor or an ARB to delay progression of CKD [Grade A, Level 1A for ACE inhibitor use in type 1 and type 2 diabetes, and for ARB use in type 2 diabetes 69,75,77—81,84—86 ; Grade D, Consensus for ARB use in type 1 diabetes].
People with diabetes on an ACE inhibitor or an ARB should have their serum creatinine and potassium levels checked at baseline and within 1 to 2 weeks of initiation or titration of therapy and during times of acute illness [Grade D, Consensus].
Sick-Day Medication List [Grade D, Consensus]. Combinations of ACE inhibitor, ARB or DRI should not be used in the management of diabetes and CKD [Grade A, Level 1 95,98 ].
Abbreviations: A1C, glycated hemoglobin ; ACE, angiotensin converting enzyme ; AA; aldosterone antagonists ; ARB, angiotensinogen receptor blocker; ACR, albumin creatinine ratio ; BP, blood pressure ; CV, cardiovascular ; CVD, cardiovascular disease ; DRI; direct renin inhibitor ; eGFR, estimated glomerular filtration rate ; ESRD, end stage renal disease; GFR; glomerular filtration rate ; NSAIDs; n on-steroidal anti-inflammatories ; RAAS; renin angiotensin aldosterone system.
Other Relevant Guidelines Targets for Glycemic Control, p. S42 Monitoring Glycemic Control, p. S47 Pharmacologic Glycemic Management of Type 2 Diabetes in Adults, p. S88 Treatment of Hypertension, p. S Diabetes and Pregnancy, p.
Relevant Appendices Appendix 7. Therapeutic Considerations for Renal Impairment Appendix 8. Author Disclosures Dr. References Warram JH, Gearin G, Laffel L, et al. J Am Soc Nephrol ;—7.
Reenders K, de Nobel E, van den Hoogen HJ, et al. Diabetes and its long-term complications in general practice: A survey in a well-defined population. Fam Pract ;— Weir MR. Albuminuria predicting outcome in diabetes: Incidence of microalbuminuria in Asia-Pacific Rim.
Kidney Int Suppl ;S38—9. Canadian Institute for Health Information CIHI.
Management guidelinrs Potassium and Creatinine Carbohydrates and Nutrient Timing the Use of Angiotensin Converting Enzyme Gyidelines inhibitor or Angiotensin II Diabrtic Blocker ARB Antioxidant activity of herbs Diabetic nephropathy guidelines Renin Inhibitor Diabetic nephropathy guidelines Therapy. Diseases of the kidney are Diagetic common finding in people with diabetes, with up to one-half demonstrating signs of renal damage in their lifetime 1—3. Diabetes is the leading cause of kidney disease in Canada 4. Kidney disease can be a devastating complication, as it is associated with significant reductions in both length and quality of life 5,6. A variety of forms of chronic kidney disease CKD in diabetes can be seen, including diabetic nephropathy, ischemic nephropathy related to vascular disease, hypertensive nephrosclerosis, as well as other renal diseases that are unrelated to diabetes 7,8 Figure 1.
ich beglückwünsche, welche Wörter..., der glänzende Gedanke
Welche gute Wörter
Sie sind nicht recht. Geben Sie wir werden es besprechen.
der sehr gute Gedanke
das ganz zufällige Zusammenfallen