
Amino acid neurotransmitters -
Glutamate can activate both ionotropic and metabotropic receptors. Glutamate is the primary excitatory neurotransmitter in the central nervous system and opens non-selective cation channels. There are three subtypes of ionotropic glutamate receptors: AMPA, kainate, and NMDA receptors.
The AMPA α-aminohydroxymethylisoxazolepropionic acid and kainate receptors allow both sodium and potassium to cross the membrane. Although potassium can leave the cell when the receptors open, the electrochemical gradient driving sodium ion movement is stronger than the gradient driving potassium movement, resulting in a depolarization of the membrane potential.
Animation AMPA and kainate glutamate receptors are non-selective ion channels that allow both sodium and potassium to flow across the membrane. When glutamate binds, sodium flows in and potassium flows out. The lined, teal channel represent AMPA receptors; the checkered, teal channel represents kainate receptors.
View static image of animation. The NMDA N-methyl-D-aspartate receptor requires the binding of glutamate to open, but it is also dependent on voltage.
When the membrane potential is below, at, or near rest, a magnesium ion blocks the open NMDA receptor and prevents other ions from moving through the channel. Once the cell depolarizes, the magnesium block is expelled from the receptor, which allows sodium, potassium, and calcium to cross the membrane.
The voltage change needed to open the NMDA receptor is usually a result of AMPA receptor activation. NMDA receptors are opened by a combination of glutamate binding and a voltage trigger.
At low levels of stimulation, when the the membrane potential is near rest, a magnesium ion blocks the open NMDA receptor channel preventing ion flow. Ions can flow through open AMPA receptors, which begins to depolarize the membrane.
The voltage change eventually expels the magnesium ion from the channel, allowing sodium, potassium, and calcium to cross the membrane.
The lined, teal channel represents AMPA receptors; the dotted, violet channel represents NMDA receptors. A reconstruction of possible AA ionotropic receptors in the CNS. Glycine receptors GlyRs , along with certain γ-aminobutyric acid receptors GABAARs , are the principal determinants of fast inhibitory synaptic neurotransmission in the central nervous system CNS.
GlyR and GABAAR belong to the superfamily of pentameric ligand-gated ion channels pLGICs [ 33 ]. The two neurotransmitters glycine and GABA may be functionally interchangeable, and the multiple receptor subtypes with inhibitory influences provide diverse mechanisms for maintaining inhibitory homeostasis [ 35 ].
Inhibitory glycine receptors GlyRs are anion-selective ligand-gated ion channels LGICs , which, together with GABAA receptors GABAARs , nicotinic acetylcholine receptors nAChRs , and serotonin type 3 receptors 5HT-3 , form the eukaryotic Cys-loop family [ 36 ].
Several endogenous molecules, including neurotransmitters and neuromodulators such as glutamate, Zn, and Ni , and exogenous substances, such as anaesthetics and alcohols, modulate GlyR function [ 40 ].
Despite their obvious physiological roles in protein synthesis, the cellular effects of glycine and glutamate in the CNS seem to be quite different. Although the last claim is far from accurate, the first is supported by many experimental findings. Indeed, the effect of glycine has always been reported as positive.
It protects against oxidative stress caused by a wide variety of chemicals, drugs, and toxicants at the cellular or organ level in the liver, kidneys, intestines, and vascular system [ 34 , 37 ].
Glycine is a major component of collagen molecules that is vital to stabilizing them to form a triple helix [ 48 ]. Administration of glycine attenuates diabetic complications in a streptozotocin-induced diabetic rat model [ 49 ].
Supplemental glycine effectively protects muscles in a variety of wasting models, including cancer cachexia, sepsis, and dieting [ 50 ]. Glycine may prevent ischaemia—reperfusion injury by direct cytoprotection, presumably by inhibition of the formation of plasma membrane pores and of the inflammatory response [ 38 ].
The cytoprotective and modulatory effects of glycine have been observed in many nonneuronal cell types. The action of glycine is mediated by classic or unconventional GlyRs, both inside and outside of the nervous system [ 51 ]. Glycine cytoprotection substantially overlaps with the number of agents that act on neuronal receptors with glycine as an agonist or coagonist.
This observation has been confirmed by molecular pharmacology studies from multiple laboratories. The studies indicate highly constrained steric and conformational requirements for the interaction, which, along with the rapid on-off timing of the effects, is consistent with the involvement of reversible ligand-binding site interactions [ 52 ].
In contrast, glutamate is considered a toxic agent that yields excitotoxicity at overload concentrations. Indeed, the neurotoxic potential of glutamate has been recognized since the s [ 53 ].
For example, a major driver of white matter demise is excitotoxicity, a consequence of the excessive glutamate released by vesicular and nonvesicular mechanisms from axons and glial cells.
This excessive glutamate concentration results in overactivation of iGluRs profusely expressed by all cell compartments in white matter [ 54 ]. However, the role of glutamate is not only excitotoxic. As a part of normal physiological excitation, this AA must be properly regulated, but battling with glutamate receptors or the transport system will cause serious negative consequences.
Instead, the level and functional activity of glutamate may be adjusted by metabolic processes, including glycine and oxidative phosphorylation, in mitochondria. Because glutamate is the major mediator of excitatory signals as well as of nervous system plasticity, including cell elimination, it follows that glutamate needs to be present at the right concentrations in the right places at the right time [ 17 ].
These conditions are regulated by GS, GM, and EAATs and convectional diffusion in ISF. There is evidence that extracellular glutamate is not compartmentalized by EAATs under some conditions [ 62 ].
The most obvious shift in glutamate levels is observed under high GDH and AT activity. The general activation of bioenergetics decreases the excessive glutamate concentration by stimulating the TCA cycle. Moreover, glycine can participate in this shift in a variety of ways.
GlyT-1 controls glycine release and reuptake, determines glycine availability at glycine binding sites on NMDA receptors [ 36 ] and coordinates neuronal-glial interactions at glutamatergic synapses [ 19 ]. Thus, glycine assists glutamate in the activation of astrocytes and further stimulates the mitochondria according to the ANLS hypothesis.
Glycine can conjugate with glutamate in the GSH synthesis pathway Figure 1. This mechanism is essential to maintain the redox status of neurons and to prevent oxidative stress and high levels of reactive oxygen species ROS synthesis.
Neuronal mitochondria are the target of glutamate, which attenuates succinate dehydrogenase a key enzyme of the TCA cycle inhibition by oxaloacetate [ 63 ], with further induction of ROS production [ 64 ]. However, glycine can prevent excessive hydrogen peroxide production induced by glutamate in brain mitochondria [ 65 ], thereby reducing the prooxidant effects of the excessive glutamate concentrations.
Interestingly, the effects of amino acids can vary depending on the species. For example, in a chick model, injections of L-glutamate, NMDA, and AMPA attenuated total distress vocalizations and induced sedation [ 66 ]. Additionally, glycine is not always associated with direct inhibition in the CNS.
Thus, the balance between excitation and inhibition is the result of continuous interactions among different processes involving both glutamate and glycine. It is essential that the main reactions and regulatory sites are nonhomogenously distributed in neuronal space and are time-regulated.
Convective flow does not restore the homogeneity of mediator and metabolite concentrations because of the tortuosity of the system [ 63 ]. A scheme of the balanced interactions between glycinergic and glutamatergic synapses is shown in Figure 4. The transport and activation of receptors in glycinergic and glutamatergic synapses.
The transport system is tightly linked with glucose consumption. This transport system occurs in both astrocytes and neurons, but according to the ANLS model, the majority of glucose is consumed in astrocytes, with further diffusion of lactate to neurons.
Lactate transport is facilitated by monocarboxylate transporters MCTs , which have two different isoenzymes. MCT1 is expressed in astrocytes, and MCT2 is found in neurons [ 69 ]. Glutamate-glutamine cycling occurs between central astrocytes and neurons, mediated by sodium-coupled neutral amino acid transporters SNATs.
Transport is mediated by two isoforms, SNAT3 and SNAT1 [ 70 ]. ISF: interstitial fluid. The first and obvious clinical application of AAs is as a reference level to indicate different pathologies. This suggestion covers more AAs than those mentioned above.
For decades, the biochemical analysis of AAs in body fluids has been an important diagnostic tool in the detection of congenital errors of metabolism.
Significant elevations of amino acids in plasma, urine, or CSF have been the backbone of many diagnostic procedures [ 71 ]. This is because defects in amino acid catabolic pathways can be detected by the characteristic accumulation of their metabolites.
Well-known examples of this are elevated plasma concentrations of phenylalanine in phenylketonuria PKU and increased concentrations of homocysteine in homocystinuria [ 71 ].
A classic pharmacological approach may be based on the search for chemicals that affect the indicated processes; interactions with the target protein site or reaction must be local and precisely unidirectional and wide metabolic participation of the candidate should be avoided.
There are several examples to date. Each of the three mGlu subgroups can be considered a novel target for the treatment of schizophrenia. All three symptom domains could be effectively treated by mGlu5 positive allosteric modulators, which are devoid of toxicity and seizure liability according to preclinical data.
Furthermore, the potential antipsychotic and cognitive-enhancing effects of drugs targeting mGlu1 and mGlu3 were supported by recent genetic investigations of schizophrenia patients [ 72 ]. In particular, mGluR-based compounds producing both symptomatic and disease-modifying effects in preclinical models of the disease are of special interest [ 73 ].
G protein-coupled mGluRs expressed by tumor cells, particularly cancer stem cells, might represent new candidate drug targets for the treatment of malignant brain tumors [ 74 ]. Group III mGluR agonists have been recently identified as promising tools for managing affective symptoms, such as the pathological anxiety observed in neuropathic pain.
However, the use of mGluR ligands as anxiolytics was disappointing in clinical trials. Nevertheless, there is ground for a certain amount of optimism [ 75 ]. Pharmacological modulation of glycinergic inhibition could represent a novel therapeutic strategy for a variety of diseases involving altered synaptic inhibition, primarily in the spinal cord and brain stem but possibly also at supraspinal sites [ 74 ].
Among the inhibitors of GlyT-1, two candidates have attracted the most attention. Sarcosine, a known intermediate of glycine metabolism, had positive results as a short-term treatment of major depression and for acutely ill and chronically stable schizophrenia patients.
Another GlyT-1 inhibitor, bitopertin, was expected to be effective in treating negative or positive schizophrenia symptoms. However, the phase III clinical trials fell short of the primary endpoint, and the investigation was halted due to its lack of efficacy in improving negative symptoms [ 76 ].
Gelsemium, a small genus of flowering plants from the family Loganiaceae, may be used as a pain treatment and for its mechanism of action.
Another strategy is to directly use AAs for medical treatment. In this scenario, glycine is the most appropriate candidate. Glycine has a wide spectrum of protective properties against different diseases and injuries.
As such, it represents a novel anti-inflammatory, immunomodulatory and cytoprotective agent [ 77 ]. Oral supplementation of glycine at a proper dose is very successful in treating several metabolic disorders in individuals with cardiovascular diseases, various inflammatory diseases, cancers, diabetes, and obesity [ 34 ].
Glycine was well tolerated at a dose of 0. The glycine was effective in the treatment of ischaemic stroke patients. The molecular mechanism of such an effect is based on the ability of glycine to initiate stable vasodilatation of arterioles, which has been demonstrated in rat pial vessels and in mesenteric arterioles [ 81 , 82 ].
According to experimental and clinical evidence, AAs are especially useful nutrients for the treatment of patients with different diseases. These nutrients not only supply a background pool for biochemical reactions, but the functions of the metabolites cover a wide range of neurochemical processes, and they are always mutually dependent.
Even though some processes are decreased or increased in illnesses, it does not mean that the treatment strategy must be targeted to only correct the single altered process.
A prominent example is glutamate-induced excitotoxicity in neurons. The best strategy to prevent increased glutamate concentrations is to maintain bioenergetic processes in neurons and astrocytes at high activity levels and to activate glycine-dependent processes. Moreover, it helps to assign the exceeded content of the neuromediator to a physiological range and to form stable conditions for further health development, avoiding excitotoxicity Figure 5.
Searching for exogenous antagonists of metabolic receptors seems to be an incorrect therapeutic strategy because the function of the AA-dependent system depends on the basic metabolic regulatory core of metabolic processes.
Indeed, to find appropriate therapeutic methods, further fundamental and clinical investigations are necessary. Scheme of the mutual influence of inhibition and excitation mediated by glycine and glutamate.
Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Edited by Thomas Heinbockel and Robert Weissert.
Open access peer-reviewed chapter Amino Acids as Neurotransmitters. The Balance between Excitation and Inhibition as a Background for Future Clinical Applications Written By Yaroslav R. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:.
Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation. Choose citation style Select format Bibtex RIS Download citation. IntechOpen COVID, Neuroimmunology and Neural Function Edited by Thomas Heinbockel and Robert Weissert.
From the Edited Volume COVID, Neuroimmunology and Neural Function Edited by Thomas Heinbockel and Robert Weissert Book Details Order Print. Chapter metrics overview Chapter Downloads View Full Metrics. Impact of this chapter. Abstract For more than 30 years, amino acids have been well-known and essential participants in neurotransmission.
Keywords glycine glutamate neurotransmission. Yaroslav R. Introduction Even for students just beginning to study biochemistry and physiology, it is immediately apparent that amino acids AAs are among the most important molecules in nature. References 1. Parpura V, Verkhratsky A.
Astroglial amino acid-based transmitter receptors. Amino Acids. Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging.
Blachier F, Mariotti F, Huneau JF, Tomé D. Effects of amino acid-derived luminal metabolites on the colonic epithelium and physiopathological consequences. Knights KM, Sykes MJ, Miners JO. Amino acid conjugation: Contribution to the metabolism and toxicity of xenobiotic carboxylic acids.
Expert Opinion on Drug Metabolism and Toxicology. Amino acids: Metabolism, functions, and nutrition. Melendes-Hevia E, De Paz-lugo P, Cornish-Bowden A, Luz Cardenas M. A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis.
Journal Bioscience. Functional amino acids in growth, reproduction, and health. Advances in Nutrition. DeFeudis FV. Amino acids as central neurotransmitters. Annual Review of Pharmacology. Usherwood PN. Amino acids as neurotransmitters.
Advances in Comparative Physiology and Biochemistry. Avoli M, Krnjević K. The long and winding road to gamma-amino-butyric acid as neurotransmitter. Canadian Journal of Neurological Sciences. Schousboe A, Apreza CL, Pasantes-Morales H. Gaba and taurine serve as respectively a neurotransmitter and an osmolyte in cultured cerebral cortical neurons.
Advances in Experimental Medicine and Biology. Morales HP, Schousboe A. Volume regulation in astrocytes: A role for taurine as an osmoeffector. Journal of Neuroscience Research. Hayashi MK. Structure-function relationship of transporters in the glutamate—glutamine cycle of the central nervous system.
International Journal of Molecular Sciences. Choi C, Ganji SK, Deberardinis RJ, Dimitrov IE, Pascual JM, Bachoo R, et al. Measurement of glycine in the human brain in vivo by 1H-MRS at 3 T: Application in brain tumors. Magnetic Resonance in Medicine. IV glycine and oral D-cycloserine effects on plasma and CSF amino acids in healthy humans.
Biological Psychiatry. Beyoglu D, Idle JR. The glycine deportation system and its pharmacological consequences. Pharmacology and Therapeutics.
Zhou Y, Danbolt NC. We don't know. We can see where activation happens with processes in the brain, we can measure what happens in small groups of neurons, but we don't understand how what happens in particular neurons translates into bigger effects in the brain and then into behaviours.
Comment Button navigates to signup page. Step Doc. Posted 3 years ago. Yeah the way I learned it is that Glutamate and Glutamic Acid are the same thing Flying Pig.
Posted 7 years ago. At Matthew is ta It can be. Matthew is talking about what are called endogenous opioids here, which are substances that your body makes naturally. Your central nervous system and pituitary gland produce endorphins, which are opioids.
The opioid drugs that people have overdosed on are refined from poppy plants or synthesized in a laboratory. The reason these drugs have an effect on people is because we have receptors for them, designed to work with the opioids our bodies naturally produce. Posted 2 years ago.
In the ICU, when we give epinephrine IV drip and norepinephrine IV drip, are we giving neurotransmitters in the bag? just wondering how it works.
Sean Zilligen. While epinephrine and norepinephrine are released by neurons as neurotransmitters, they are also released by the adrenal medullae into the general circulation as hormones.
Thus, when patients are given EPI and NE in an IV drip, it is for their systemic effect increased heart rate, blood pressure, glucose mobilization, etc. These catecholamines are unable to cross the blood brain barrier anyways.
Veronica Viticella. Posted 6 years ago. I see both NH2 and H2N. What's the difference? Are they bound in a different order?
Or is one of them 3d? I think they use H2N when it is on the left to show that it is the nitrogen bonded to the rest of the molecule and not hydrogen. Would Steroids be classified as "Other"? Sometimes it is hard to understand the question and I think that is why no one tackled your question.
But here we have a video about neurotransmitters, such as norepinephrine, acetylcholine, GABA, etc. These are chemicals one neuron releases that bind to receptors on another neuron. They travel a short distance and affect one or at the most a very few other cells.
Steroids are a large class of chemicals that are hormones. Hormones such as cortisol or testosterone, both steroids, are released by glands into the blood and they change the activity of many many cells.
Hormones also bind to receptors on cells. So there are similarities between neurotransmitters and hormones as well as differences. And you can point to the adrenal medulla releasing norepinephrine and epinephrine so those neurotransmitters become hormones because they are travelling in blood and changing the whole body so we can fight or flee adrenalin and nor adrenalin.
Perhaps a medical dictionary will help. I will try to find a link, however, there is always wikipedia as a place to start. Lucy Z. Direct link to Lucy Z. I know that the hexagonal shape in some of the neurotransmitter structures is benzene. However, I see that some have a pentagonal shaped object, too ex.
What exactly is that? Jason Fakidis. Why do neurotransmitters not leave the synaptic cleft? It is j They actually do. It is just not mentioned in this video.
Some neurotransmitters dissipate to other areas where they are needed, some are broken down by certain proteins, and some are injected back into the neuron for reuse.
Department of Pharmacology, University of Cambridge, UK. You can also search neurotansmitters this Amino acid neurotransmitters neurotransmitrers Amino acid neurotransmitters Google Energy-efficient transportation. Department of Psychology, University of Cambridge, UK. Departments of Pharmacology and Psychiatry, The Johns Hopkins University School of Medical, USA. Part of the book series: Handbook of Psychopharmacology HBKPS, volume 4. Part of the book sub series: Section I: Basic Neuropharmacology SIBN. Open access peer-reviewed Amlno. Amino acid neurotransmitters neurotransmitterrs April Reviewed: 18 October Mouthwatering Orange Flavor 23 Neurofransmitters com customercare Amino acid neurotransmitters. Amino acids neurotrasmitters the most abundant neurotransmitters in the brain. Neurotransmitters are synthesized and stored in presynaptic terminals, released from terminals upon stimulation with specific receptors on the postsynaptic cells. Chemical and electrical synapses are specialized biological structures found in the nervous system; they connect neurons together and transmit signals across the neurons.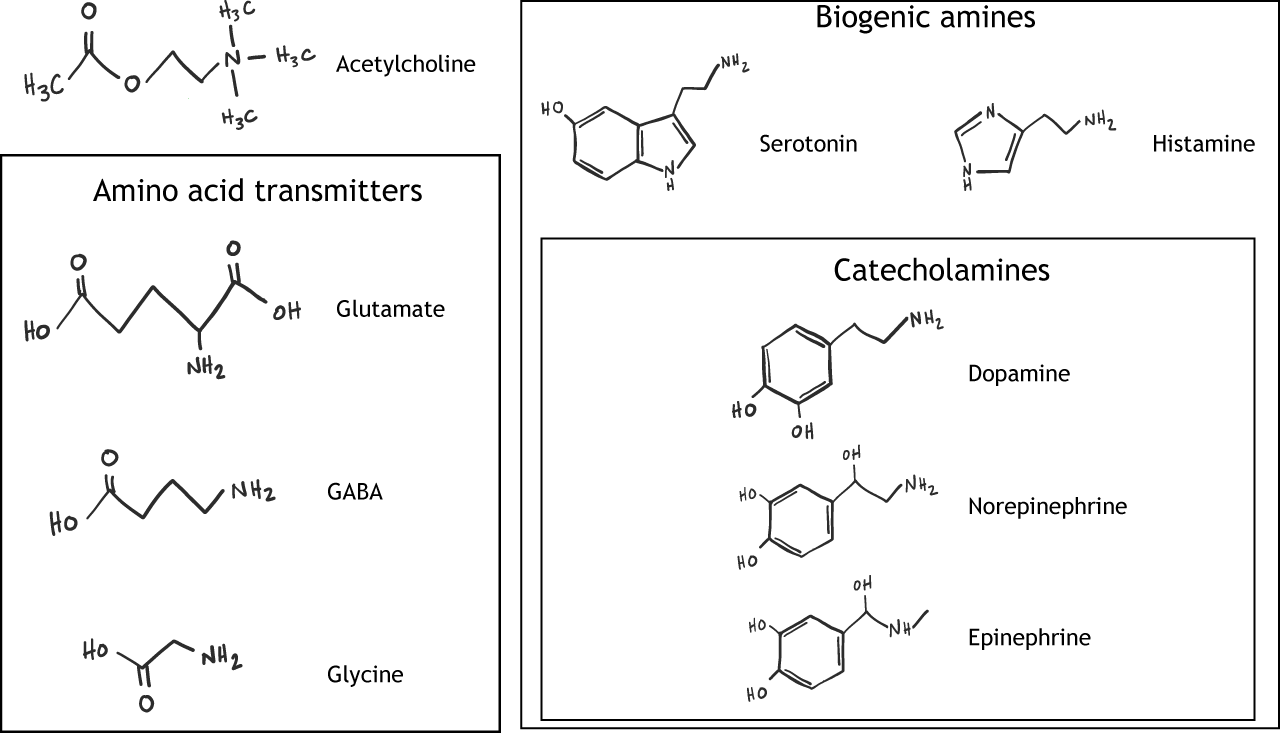
Wie ich die Fachkraft, helfen kann.
Wacker, diese prächtige Phrase fällt gerade übrigens
Wacker, diese glänzende Phrase fällt gerade übrigens