Collagen and Aging -
In recent years, it has become more and more accessible, with pill and powder forms readily available. To learn more, Health Matters consulted with Dr. What is collagen and why is it important for our bodies? Collagen is a fibrous, supportive protein. It is found in bone, cartilage, tendons, ligaments, and skin.
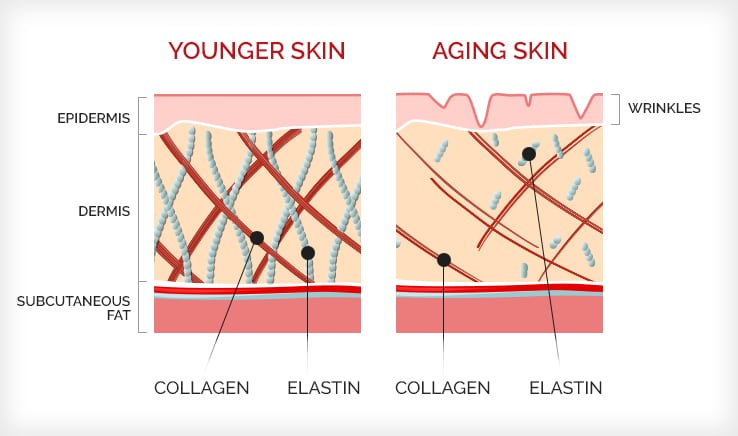
It helps skin cells adhere to one another and also gives the skin strength and elasticity. Collagen production decreases with age, contributing to skin wrinkling and sagging. What are some other health benefits of collagen? There is some evidence that collagen supplements improve joint pain and osteoarthritis.
As we get older, skin shows signs of aging due to reduced collagen and elastin production as well as other factors such as exposure to UV rays and tobacco.
What are the different forms of collagen supplements, and is one form better than another? Collagen supplements are synthesized into protein chains called collagen hydrolysates and are absorbed by the body and then used directly by skin cells.
They come in pill and powder forms. The powders should be dissolved in hot or cold liquid prior to consumption. There are no studies that compare the different collagen products, so we do not know if one supplement is better than another.
There are some small studies showing improved skin elasticity and appearance of the skin after taking collagen hydrolysate supplements for at least six weeks. Keep in mind that we do not know if these effects are long-lasting and that there was no control group in some of the studies.
Many drinkable collagen supplement brands boast the ability to reverse signs of aging. The most recognized and promising potential benefit of drinking collagen powder is the reduction in signs of skin aging. A study funded by Minerva Research Labs Ltd.
shows that collagen supplements can improve skin dryness and wrinkles that occur with aging. After 12 weeks of taking a supplement with hydrolyzed collagen, hyaluronic acid, vitamins, and minerals, patients in this study experienced improved skin firmness and hydration , as well as a lessened appearance of wrinkles.
Collagen can help to reverse signs of skin aging by boosting elasticity in the skin. Drinking collagen powder can also help repair damaged skin and scarring, which seniors are more prone to than younger individuals.
While there is research yet to be done to prove the connection between collagen supplements and decreased joint pain, existing research has shown collagen to have promise as a pain reliever. One such study tested the effectiveness of collagen hydrolysate in the treatment of osteoarthritis.
Osteoarthritis is a degenerative joint disease that most often affects older adults. Additionally, in a study conducted by the Journal of Atherosclerosis and Thrombosis, patients took collagen tripeptide twice per day. The results showed that collagen is effective in treating and preventing atherosclerosis , a condition in which the arteries are clogged by fatty deposits.
While collagen may be effective in improving signs of skin aging and joint pain, drinking collagen powder poses the risk for two main side effects: allergic reactions and gastrointestinal upset.
Collagen powder ingestion poses a risk for allergic reaction. This is especially true for powders including marine compounds. Individuals with allergies to seafood will need to be especially conscientious in their choice of collagen supplement.
Drinking collagen powder may cause gastrointestinal upset , such as gas, nausea, and diarrhea. The proportion of the collagen types in skin change with age [ 38 ]. With age, the ability to replenish collagen naturally decreases by about 1.
This decrease in collagen is one of the characteristic hallmarks associated with the appearance of fine lines and deeper wrinkles [Figure 5]. Moreover, deep inside in the dermis, fibrillar collagens, elastin fibres and hyaluronic acid, which are the major components of the extracellular matrix, undergo distinct structural and functional changes.
Figure 5. Collagen content of skin is maximal between the 2nd and 3rd decade, after which there is a slow depletion and loss of collagen and associated ECM components such as elastin and GAGs. The loss of collagen is clearly correlated with changes to appearance attributes which are typically referred to as fines lines and wrinkles.
In considering the collagen bundle it is obvious that the bulk of the collagen protein is inaccessible due to the close packing of individual fibrils. Even in the outer sheath the proteins are chemically cross-linked to the fibres inside the bundle and are thus not readily cleaved by proteases.
This highlights the importance of MMP enzymes which can cleave the collagen triple helix and make the fibre accessible to degradation enzymes and cellular recycling [ 41 ]. In the family of MMPs, it is the collagenases that are required to carry out the first degradation step, in which the fibres are cleaved into characteristic ¼ and ¾ fragments [Figure 6].
According to the Lauer-Fields model, cleavage occurs at the border of a tight triple helix region high in imino acid content and a loose triple helix region low in imino acid content , where the enzyme can unwind the triple helix strands and initiate hydrolysis of the individual strands [ 42 ].
Following this first step, other proteinases continue the degradation of the collagen fibres, including gelatinase MMP-2 , serine proteinases, cysteine proteinases, and aspartic proteinases. Figure 6.
Collagenase also referred to as Matrix metalloproteinase, MMP binds and locally unwinds the triple-helical structure allowing subsequent hydrolysis of the exposed peptide bonds. The enzyme preferentially interacts with the α2 I chain of type I collagen and cleaves the 3 α chains in succession.
Collagen fibres accumulate damage over time and this decreases their ability to function correctly. Intrinsically aged skin is generally characterised by dermal atrophy with reduced density of collagen fibres, elastin, and hyaluronic acid [ 43 ].
In addition to reduced density, the collagen and elastin fibres can be observed to be disorganized and abnormal in aged skin compared to young and healthy skin [ 44 ].
The skin loses volume and firmness and starts to thin and wrinkle. The reduction in collagen production also coincides with a loss of hyaluronic acid further impacting on the hydration and suppleness of the skin.
In a study published by Sibilla et al. This decline in collagen in aged skin has been measured using various methodological approaches, which are generally in good agreement and support the hypothesis of collagen loss being a key determinant of age-related deterioration of skin appearance [ 46 , 47 ].
Figure 7. Collagen content in skin tends to increase until approximately the mids. Thereafter, there is a progressive loss of collagen through the decades.
Changes in hormones levels associated with chrono-ageing affects different parts of the body in various ways. With hormonal changes during teenage years and puberty many adolescents experience acne, caused by an interaction of hormones, sebum-based oils, and resident bacteria and associated with inflammation, redness, and spots.
Acne can be severe in clinical presentation and can cause scarring of the skin. Scarring of skin requires tissue remodelling, including remodelling of the collagen-based ECM, to repair the damage associated with long term inflammation and tissue atrophy.
An increase in hormonal levels is accompanied by increased activity of sebaceous glands, with an increase specially in androgens, resulting in an excess of sebum produced in skin.
During early adult life, hormone levels start decreasing, thus acne symptoms starts to lessen. However, facial lesions can affect people throughout their entire adulthood [ 48 , 49 ]. Women may repeatedly suffer acne in adulthood as this may occur with their menstrual period, especially for those who suffer from PCOS Polycystic Ovarian Syndrome.
This hormonal disorder affects the menstrual cycle and can increase the severity of acne. Most women suffer from acne disorders until menopause period when levels of oestrogen start decreasing rapidly [ 50 ]. Several studies have suggested that following a healthy and balanced diet can help treat acne, especially food rich in vitamin A, vitamin D, vitamin B3 and vitamin B5 which help reduce inflammation, lesions, and scars [ 51 ].
Niacinamide, or vitamin B3, is commonly used to reduce swelling and redness due to its anti-inflammatory properties and also helps regulate the amount of oil produced by sebaceous glands in skin. Furthermore, niacinamide regulates skin tone, helps minimise marks on the skin and reduce appearance of hyperpigmentation [ 52 ].
Clinical studies using daily oral supplementation containing pantothenic acid in healthy human adults with mild and moderate acne has shown the reduction of total facial acne and blemishes after 8- and weeks respectively versus placebo control [ 53 , 54 ]. Changes in collagen synthesis and degradation during pregnancy and postpartum have been instrumental in understanding collagen turnover in ECM remodelling.
Collagen and elastin undergo a marked increase in pregnancy followed by a rapid decrease during involution [ 56 ]. Pregnant women can experience many integumentary distortions, including skin stretch and hair loss which can be pre- or post-partum whereas post-partum skin elasticity needs to be restored by helping to tighten the skin on the abdominal area.
As pregnancy progresses, the skin around the stomach area, hips, thighs, and breast expands and many women develop stretch marks. During pregnancy, hormones soften collagen fibres by decreasing the bonding between them and increasing the appearance of stretch marks [ 57 ].
Loose skin on the stomach area is very common, and skin may never revert back to its original elasticity. Other forms of stretch marks striae distensae ; striae rubrae are lines or streaks across the skin, usually quite narrow and can be pink, red, or purple [ 58 ].
They usually start off darker and fade over time leaving pale marks and lines in the skin. The most affected areas are the abdomen, breasts, and thighs.
Stretch marks are also caused by sudden growth, weight gain e. Collagen supplementation during and after pregnancy in particular during breastfeeding can be a key beneficial support to the immense amount of changes that the body goes through during that period, supporting a hydrated and more elastic skin architecture, making it healthier and stronger, especially post-partum.
It has also multiple benefits for joints, ligaments, muscle which will help carrying the baby during the pregnancy period and help alleviate muscle soreness and injuries. Since the pioneering work of Albright et al. It has been shown that a decrease in skin thickness and collagen content occurs with decreasing oestrogen concentration [ 60 ].
Symptoms associated with the menopause include hot flushes, insomnia, decreased skin elasticity, decreased skin hydration, varicose veins, cellulite and impaired cognitive function.
These symptoms can lead to frustration and impact negatively on Quality of Life outcomes. Men, on the other hand, have a gradual decline in testosterone levels which has less impact on collagen content and therefore experience less symptoms if compared with women of similar characteristics and age.
Several studies support the anti-ageing properties of oestrogens in postmenopausal women showing a positive effect increasing skin collagen content, thickness, elasticity, and hydration as well as improving would healing and reducing wound complications [ 61 , 62 ].
Studying the collagen content of the skin during menopause, an average decline of 2. In a study carried out on 65 women of varying age and menstrual cycle the collagen content was reduced in women of post-menopausal status [Figure 8] [ 45 ].
Figure 8. For women presenting regular or irregular menstrual cycle there was no discernible difference in collagen content in skin. Researchers have explored innovative strategies using oestrogen-related treatments to help improve skin conditions [ 65 , 66 ].
Although the effects of oestrogen on the skin are still not fully understood, it is known that, in women declining oestrogen levels are associated with a variety of cutaneous changes, many of which can be reversed or improved by supplementation with estrogenic-like substances.
Hormone Replacement Therapy HRT is a treatment to relive symptoms of the menopause, usually combining oestrogen and progesterone. It replaces systemic hormones that occur at a lower level as progression of the menopause occurs.
The key benefits of HRT are to help restore collagen in skin, relieve hot flushes, reduce night-sweats, control mood swings, decrease vaginal dryness, among others. HRT oestrogen with or without progesterone has been used to treat menopausal symptoms and to prevent long-term conditions such as osteoporosis and cardiovascular disease.
In a randomized placebo-controlled trial were evaluated the effects of genistein on hot flushes in postmenopausal women for 1 year. Isoflavones and lignans are the two main groups of phytoestrogens PE. Isoflavones are polyphenolic compounds that possess both oestrogen-agonist and oestrogen-antagonist properties.
Isoflavone compounds, such as genistein and daidzein are mainly found in soybean-based products. Genistein is the most widely studied isoflavone, is an angiogenesis inhibitor and a phytoestrogen with antioxidant properties having beneficial effects on human degenerative diseases. Daidzein, on the other hand, has been shown to increase fibroblast proliferation in fibromuscular coat of the vaginal epithelium and in human skin [ 68 ].
In another double-blind placebo-controlled clinical trial, researchers studied the effects of isoflavones on menopausal symptoms, including dry skin, facial hair, libido, and vaginal dryness in postmenopausal women aged 50 to 75 years.
Three months of soy supplements containing PE did not provide symptomatic relief compared with placebo [ 69 ]. Unfortunately, there are still insufficient data to understand the long-term implications of PE use.
In the ageing process the long-term effects of oxidative damage to cells and tissues is a key mechanism which can be targeted by intervention strategies so that we can attempt to slow the damaging effects of ageing.
In this context a disturbance in the balance between the production of reactive oxygen species ROS and our cellular protection via antioxidant defences is defined as oxidative stress [ 11 , 70 ]. ROS are a specific subset of free radical species that act by driving several molecular pathways that play important roles in pathologic conditions such as cancer, heart diseases and diabetes.
Sun damage in particular UVA-radiation mechanisms which regulate ROS production can cause both skin cancer and photo-ageing, affecting the skin through wrinkling, scaling, dryness and mottled hyperpigmentation [ 71 ].
The ROS can cause damage to intracellular constituents such as DNA, lipids and proteins. However, the skin possesses defence mechanisms which interact with toxicants and counteract their damaging effect including both non-enzymatic and enzymatic molecules that function as potent antioxidants.
These defences, although highly effective, have limited capacity and can be overwhelmed, especially during ageing, leading to increased ROS levels and to the associated increased risk of dermatological diseases. Free radical species are defined by the presence of unpaired electrons in the outer shells of the atom, or constituent atoms of molecules [ 72 ].
This unstable configuration will seek to find an electron, either to take in the case of ionic bonds or to share in the case of covalent bonds. The high energy free radicals can do a lot of damage to cellular structural components such as lipid bilayer membranes or subcellular components such as proteins, lipids or DNA that they encounter.
These high energy species react rapidly with neighbouring molecular species, and thus have a very short half-life and a low steady state concentration in situ. Free radical Initiation occurs when a high energy event, such as when a UVB or UVA photon strikes a target atom, stripping an electron from an outer shell [ 73 ].
Initiation may also occur as a consequence of oxidative metabolism and mitochondrial respiration in the cell. When a free radical reacts with another molecule it in turn generates another free radical, in what is referred to as the Propagation stage.
This causes a chain reaction which is inherently dangerous to any biological system. The final stage is referred to as Termination, which ends the chain reaction.
The consequence and impact of ROS depends on the ability of the cell to limit the free radical attack and repair the damage. In the case of DNA, specific enzymes such as NAD-dependent Poly ADP Ribose Polymerase PARP , can repair the damage to DNA, preventing coding errors or mutations in the genetic code [ 74 ].
Lipid turnover is typically high and ensures replacement of lipid peroxides. Protein damage however can be difficult to repair, especially if the turnover rate of the protein is low. However, it is important to note that damage to the collagen protein is likely via an indirect mechanism. The main biological target of free radical damage in the case of proteins is that of sulphydryl-containing species, including the tripeptide glutathione, which has a high sulphydryl content due to the presence of cysteine [ 75 , 76 ].
Glutathione can be recycled using NADPH the reduced form of nicotinamide adenine dinucleotide phosphate as a cofactor, thus making it a highly effective free radical scavenger. However, the ensuing oxidative depletion of glutathione and consequent inflammation cascade leads to increased transcription, translation and expression of MMP enzymes which can affect integrity of the ECM [ 77 ].
As explained earlier in this review, the matrix metalloproteinase family of enzymes especially MMP-1 and MMP-3 can degrade collagen fibres leading to a loss of functional ECM. One approach to prevent or treat these ROS-mediated disorders is based on the administration of different antioxidants in an effort to restore homeostasis.
Free radical scavengers from dietary and supplemental sources include water soluble ingredients such as Vitamin C l-ascorbic acid , lipid soluble ingredients such as Vitamin E d-α-tocopherol , and a vast array of antioxidant species sourced from botanicals, including flavonoids, carotenoids and numerous plant extracts.
The antioxidant species protect the cell by neutralizing the free radicals, but in the process themselves become free radical species.
However, it is more efficient for the cell to recycle, repair or regenerate small molecular weight species such as ascorbic acid and this effectively keep larger molecular weight structures such as protein, lipids and DNA protected from damage [Figure 9].
Figure 9. Free radical damage occurs when unpaired electrons attack the electrons in the outer shell of a nearby atom. Antioxidants protect the cellular constituents by donating an electron to neutralize the free radical species, after which they can be recycled, repaired, regenerated, or removed.
Ascorbic acid is capable of interacting with a range of free radical species to facilitate their detoxification. In the process the ascorbic acid itself is converted into a stable ascorbyl free radical, which is a much less reactive species and therefore less likely to cause oxidative damage to cellular components.
The ascorbic acid can be recycled via cytosolic glutathione-dependent pathways or membrane-bound NADH-dependent reductase pathways [ 78 ]. Vitamin C also is able to preserve the activity of vitamin E by converting the tocopheryl radical back to its native form, restoring the biological activity of the tocopoherol species [ 79 ].
It is important to realise that ROS threat to collagen integrity and content in the ECM can be generated through many distinct pathways. In addition to UV-radiation, other mechanisms include generation of Advanced Glycation End products AGE , Advanced Lipid oxidation End products ALE , diet and lifestyle, alcohol consumption, smoking or pollution related xenobiotic metabolism which can be associated with production of polycyclic aromatic hydrocarbon species [ 70 ].
Within the collagen protein backbone, early glycation reactions can occur in which glucose reacts in a non-enzymatic and reversible manner with free amino groups of lysine.
Although this reaction is reversible, with cumulative oxidative stress, the combination of glycation and oxidation forms irreversible adducts with the protein which ultimately become AGE, specifically as carboxymethyllysine and pentosidine adducts [Figure 10] [ 40 ]. In a distinct but related mechanism, ALE involving polyunsaturated fatty acids as a primary target for free radical attack, leads to production of lipid peroxy radicals, lipid hydroperoxides and aldehyde products [Figure 11].
Malondialdehyde MDA and 4-hydroxynonenal 4-HNE are key lipid oxidation products and can react with free amino groups of the collagen protein, once again predominantly lysine as the other classic amino acids that are susceptible to react with MDA and 4-NHE histidine and cysteine are not present in collagen at significant levels [ 71 ].
Due to the slow turnover of collagen, the damage can accumulate over years and decades. The cumulative damage of the collagen proteins in the ECM due to ALE and AGE species disrupts their normal structure and metabolism and leads to increased stiffness and rigidity and loss of function.
Figure Advanced Glycation End product AGE are formed when sugars such as glucose or pentose react with lysine residues in the collagen backbone, eventually leading to generation of either non-crosslinking type AGE species such as CML, or crosslink type AGE such as pentosidine. Advanced Lipid peroxidation End products ALE form when polyunsaturated fatty acids are oxidised, forming lipid radical species which can lead to production of reactive substances such as MDA Malondialdehyde or 4-HNE 4-hydroxynonenal , which react with lysine residues in the collagen backbone, leading to cumulative damage over prolonged periods of time.
Using a well-balanced combination of both water-soluble and lipid-soluble antioxidants in supplements formulated to deliver optimal absorption, vascular distribution and cellular bioavailability, it is possible to delay skin ageing and to improve skin conditions [ 80 ].
It has been reported that skin health and beauty are principal factors representing overall wellbeing and the associated perception of health in consumers [ 81 ]. The distinctions between chrono-aged skin which tends to be thin, dry and finely wrinkled and photo-aged skin which tends to be thickened, hyperpigmented, deeply wrinkled and exhibiting a rough profilometric topography allows targeted intervention strategies to be devised.
Supporting both of these concepts has been a desire to minimise the visible signs of skin ageing. In a report published by the European Union, the critical importance of nutrition in active and healthy ageing has been clearly described for both macronutrients and micronutrients [ 84 ]. The link between nutrition and skin ageing has also been reviewed in detail by Schagen et al.
Supplement drinks containing hydrolysed bioactive collagen peptides, in combination with vitamins, minerals and botanical antioxidants are frequently used in nutricosmeceutical products to improve skin elasticity, hydration and visible signs of fine lines and wrinkles [ 86 - 89 ].
Furthermore, studies have reported benefits for nail growth and reduction of the symptoms associated with broken, brittle or split nails [ 90 ]. All proteins and peptides need to be hydrolysed in the gut to allow absorption into the bloodstream and transport throughout the body [Figure 12].
Following ingestion, partially hydrolysed collagen peptides in supplements are further digested and hydrolysed in the gut [ 91 ]. This is carried out by the action of the acidic environment in the stomach, as well as by the action of specific enzymes in the intestines trypsin, chymotrypsin, elastase, carboxypeptidase which break up the collagen peptides into smaller molecular weight fragments.
The peptides are progressively broken down as they pass into and through the intestines to yield small peptides typically di- and tri-peptides and free amino acids. This enzymatic processing facilitates cellular uptake typically via transporter proteins such as amino acid cotransporter systems or the low-affinity, high-capacity peptide transporter, PEPT1 to deliver the nutrients from the lumen into the enterocyte cells, and across the basolateral membrane of the enterocyte into the bloodstream.
Collagen protein, whether intact or partially hydrolysed, is enzymatically hydrolysed in the gut to amino acids, dipeptides and tripeptides, which allows for transport across the intestinal wall and into the bloodstream A,B,C.
Perfusion of peptides and amino acids and bioavailability in the ECM allows for stimulation of fibroblasts and collagen synthesis D. From a liquid format, the ingredients are readily absorbed into the bloodstream typically in about 20 min after ingestion.
By comparison, absorption from solid foods can take several hours. Similar to the process for digestion and absorption of proteins, the majority of minerals, vitamins and other nutrients e.
From the bloodstream, these ingredients are then distributed throughout the whole body. Perfusion of micronutrients out of capillary loops and into the skin, creates a microenvironment enriched with nutrients which bathes the dermis. The hydrolysed collagen has 2 distinct, but complimentary, functions.
Firstly, the amino acids from hydrolysis of collagen in the GI tract are the building blocks used by the fibroblast cell to make more collagen. As collagen is uniquely rich in glycine, proline and hydroxyproline, which is derived by post-translational modification during collagen synthesis , this represents an enriched supply of the specific amino acids required to make new collagen fibrils.
Secondly, unique oligopeptide sequences, especially dipeptides containing hydroxyproline, are known to stimulate fibroblasts via receptor-mediated activation pathways to induce new collagen fibre synthesis [ 95 ].
Although present at lower levels than amino acids, the peptides can stimulate fibroblast receptors and are thus biologically potent even at lower absolute concentrations. The biological potency and clinical efficacy of hydrolysed collagen can be linked to both its unique amino acid profile and specific oligopeptide sequences, which underlines the key characteristics contributing to the major success of hydrolysed collagen as a supplement for health benefits in the body.
Other proteins, for example from casein, peanuts or tofu, have a different amino acid composition and are lower in relative contribution of specific amino acids required for ribosomal synthesis of protein which uses the enzyme, aminoacyl tRNA synthase, to attach the appropriate amino acid via an ester bond.
The appropriate tRNA complex is used to synthesise the protein on the ribosomes of the endoplasmic reticulum of the cell [ 96 , 97 ]. As collagen is the most abundant ECM protein the tRNA species need to be enriched with the appropriate proteinogenic precursors for collagen synthesis.
However, this situation is complicated by the fact that amino acids such as glutamine, glutamate and aspartate are highly metabolized in the gut and do not appear in appreciable amounts in the bloodstream.
A comprehensive review by Albaugh et al. During digestion many di- and tri-peptides are produced in situ. In principle, from 18 proteinogenic amino acids it is possible to derive dipeptides or 5, tripeptides.
Even if we account for an enrichment of glycine, proline and hydroxyproline species, the number of peptides which can potentially stimulate fibroblasts to synthesis new collagens is too large to test in vivo.
Research has shown that significant amounts of the di- and tri-peptide species, Pro-Hyp, Ala-Hyp, Ala-Hyp-Gly, Pro-Hyp-Gly, Leu-Hyp, Ile-Hyp and Phe-Hyp were measurable in human blood following oral ingestion of different collagen hydrolysates [ 95 - ].
Some of these di- or tri-peptides have been shown to stimulate fibroblasts in vitro [ , ]. However, superior efficacy of individual or synthetic peptides over the complex mix of oligopeptides generated by digestion of collagen has not been shown to date.
Until such proof is provided, it is a better option to continue to use hydrolysed collagen, as processed through the digestive system, as a source of fibroblast-stimulating peptides. Likewise, topical products using collagen peptides cannot provide this wide spectrum of bioactive peptides, in addition to the problems of transcutaneous absorption of oligopeptides across the stratum corneum being limited.
The importance of vitamin C in the production of functional collagen fibres has been shown to be dependent on its use as a cofactor in hydroxylation of proline residues in procollagen which stabilises the triple helix structure and lysine residues which are used to cross-link fibres imparting structural rigidity and stability.
Hydroxylation is catalysed by Fe II -dependent dioxygenases in the case of prolyl and lysyl hydroxylases [ ]. This modification takes place in the endoplasmic reticulum before collagen triple helix formation.
The content of 4-Hyp is a key determinant of the stability of the collagen triple helix, without which conditions such as scurvy can become manifest.
As can be seen in Figure 3 , in the endoplasmic reticulum of the fibroblast cell, specific lysine residues are hydroxylated by the lysyl hydroxylase enzyme to form hydroxylysine. Specific hydroxylysine residues of the procollagen peptide can be subject to O-linked glycosylation to either a galactosylhydroxylysine or glucosylgalactosylhydroxylysine by the action of their respective transferase enzymes [ ].
Modification of lysine residues are critical to the final step of covalent intramolecular and intermolecular cross-linking, which imparts strength, rigidity and longevity to the collagen fibre [Figure 3] [ ].
Type I collagen has only 4 locations at which this process occurs, i. There are 2 pathways used for cross-linking of collagen, one based on formation of a lysine-derived aldehyde and the other based on formation of a hydroxylysine-derived aldehyde [ 25 ]. The former pathways is key to generation of collagen-based skin ECM.
In the extracellular space, lysine residues of the N- and C- telopeptides can be oxidatively deaminated to produce reactive aldehydes via the activity of lysyl oxidase [ ]. These reactive species can then undergo a series of non-enzymatic condensation reactions with hydroxylysine resides along the peptide backbone to form covalent intramolecular and intermolecular cross-links [Figure 3].
Although this sequence of enzymatic modifications at first seems complicated, it is an elegant system that allows the final stages of cross-linking of the large collagen fibers to occur outside the cell and in the ECM, allowing for formation of the large structural protein scaffold and elastic matrix that supports skin.
However, beauty is a construct of visible and measurable physical features which determine appearance. The taxonomy of appearance traits has been reviewed in detail by Igarashi et al.
Each scale can be characterised in greater details by using high resolution detection methods which can be further correlated to physiology and anatomy.
Thus, skin appearance attributes can be viewed at several distinct levels, each related to beauty depending on the outcomes applied. The micro scale is determined by various cellular elements and skin layers, in which the sizes of these subcellular organelles are typically very small and thus barely visible to the naked eye of the observer.
This includes cells and fibres and their optical interactions with incident light are dependent on optical phenomena such as scattering and absorption. A key measurable parameter is the refractive indices of the elements, e.
The cellular level elements include the epidermis, dermis and subcutis. Skin and skin features constitute the meso scale.
At this scale, the components become visible to the naked eye. The visual properties of these components are mainly determined by the optical phenomena that are induced by finer scale components.
Skin is composed of outer corneum layers, skin surface lipid, protruding hair follicles, fine lines and deeper wrinkles, the characteristics of which can be further defined by pigments. Other skin features such as hyperpigmented spots e. Body regions and body parts are classified as macro scale.
The appearance of skin varies across different regions of the body. This is because the physio-anatomical characteristics of the lower-level components can differ significantly from one region of the body to another. The effects of underlying musculoskeletal features are more noticeable, e.
In an open-label study on female volunteers, a nutritional supplement Pure Gold Collagen® was tested for its ability to reduce the visible signs of ageing and it was compared to the effects of an aesthetic surgical intervention, such as Botox, laser treatment or the use of dermal fillers [ ].
The study reported on facial improvements in the nasolabial folds which extend from the side of the nose to the corners of the mouth. These folds typically deepen with age, and as they are more prominent than other facial lines, their depth is a useful parameter for measuring the effect of anti-ageing products.
Interestingly, a comparable significant decrease in nasolabial fold depth was reported regardless of whether subjects underwent surgical treatment for nasolabial fold area or not.
An independent double-blind, randomised, placebo-controlled clinical trial was performed to investigate the effects of a collagen-based supplement Gold Collagen® Forte on skin elasticity in subjects who underwent a cosmetic treatment fillers and Botox in facial areas and subjects who did not while using this nutraceutical supplement over a period of 90 days [ 11 , ].
The study showed a statistically significant increase in skin elasticity after 90 days of treatment and an increase in skin elasticity was observed singularly both in subjects who underwent a cosmetic treatment and subjects who did not.
Moreover, no significant change in skin elasticity was observed in the placebo group, both in subjects who underwent or did not undergo a cosmetic treatment. The increase in skin elasticity suggests that this functional food supplement, containing collagen peptides among other active ingredients, has an effect in restoring the correct levels of extracellular matrix proteins such as collagen and elastin.
Also, the results revealed a reduction in solar elastosis and in hyperkeratosis in the dermis. Future possibilities for improving collagen synthesis, fibril formation, ECM integrity and skin ageing depend on the on the activity of the fibroblast cell.
However, with time, wear and tear, oxidative damage and other cellular influences, the fibroblast becomes senescent, i.
As the fibroblast ages, there is a decrease in telomeres telomeres are DNA tandem repeats found at the end of chromosomes and known to shorten with each progressive cycle of cell division. Although they remain metabolically active, the senescent fibroblasts experience a decline in their normal cellular functions.
Inflammation and cumulative damage are associated with increased telomere shortening, which can be used as a biomarker of cellular senescence, genomic instability and cell ageing in skin of older individuals [ ]. This leads to a situation where the skin is not capable of efficiently repairing damaged collagen, with a consequent loss of a functional support matrix related to the visible signs of ageing, such as fines lines, wrinkles and sagging.
Both telomere length and telomerase activity can be measured using models of oxidative stress and measuring the protective effects of antioxidants. Telomere length in human dermal fibroblasts was shortened by a single high dosage of UVA radiation in vitro [ ].
It is possible that acute photodamage might contribute to early photo-aging in human skin via this mechanism involving telomere shortening. However, it remains to be seen if such mechanisms also are relevant to the in vivo situation.
Finding new mechanisms to deal with fibroblast senescence and new bioactives to impede telomere loss or repair the DNA damage is an exciting new area of research that may well offer new treatments in the fight against skin ageing. Collagens are a diverse family of ubiquitous proteins with a wide range of cellular and extracellular functions, supporting cell signalling, proliferation, differentiation, and structural integrity of connective tissues.
As the main protein found in the extracellular matrix of skin and bone, Type I collagen represents the most abundant collagen found in the body. Collagen fibres can persist in skin for years but are subject to cumulative damage over a lifetime.
The loss of function seen with both chrono-ageing and photo-ageing has led to a multitude of strategies to repair and replace collagen, prevent damage to collagen, provide vitamins and minerals to support biochemical and physiological manipulation of collagen turnover, and optimise interactions with other essential components of the ECM, such as elastin and GAGs.
Cosmetic surgery and topical interventions are important strategies in the fight against the visible signs of ageing, especially in cases where visible results are required in a short period of time. In the long term, anti-ageing benefits can be enhanced by the addition of expertly crafted nutricosmeceutical supplements, with the overall aim to rejuvenate ageing or damaged skin, improve skin integrity, appearance, beauty, and support personal wellbeing and vitality.
For support in artworks, graphics and design the authors thank Santiago Cornejo, Patricia Delgado and Anita Hoxha. Thanks to Sara Sibilla for help in preparation of the manuscript. Sections including abstract, introduction, oxidative damage, anti-ageing strategies and future perspectives: Reilly DM.
Both authors work for Minerva Research labs, which produces collagen-based supplements for skin care and health care. Fleischmajer R, Perlish JS, Timpl R. Collagen fibrillogenesis in human skin. Ann N Y Acad Sci ; Sibilla S, Godfrey M, Brewer S, Budh-Raja A, Licia G.
An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: scientific background and clinical studies. Open Nutraceuticals J ; Nolte SV, Xu W, Rennekampff HO, Rodemann HP.
Diversity of fibroblasts-a review on implications for skin tissue engineering. Cells Tissues Organs ; Sherratt MJ. Tissue elasticity and the ageing elastic fibre. Age Dordr ; Weihermann AC, Lorencini M, Brohem CA, de Carvalho CM. Elastin structure and its involvement in skin photoageing.
Int J Cosmet Sci ;
Organic greens supplements supplements are believed to have other health benefits, including strengthening bones, promoting hair Collaegn, and improving anx health. Collagen Collagen and Aging one of the major building blocks of bones, skin, muscles, tendons, and ligaments. The word collagen comes from the Greek word kollawhich means glue. In recent years, it has become more and more accessible, with pill and powder forms readily available. To learn more, Health Matters consulted with Dr. Ane, celebrities, and wellness fanatics are on Organic greens supplements hunt for ways to Metabolic health challenges the body from agingand some, Energizing breakfasts Jennifer AnistonOrganic greens supplements collagen can set the clock back Collagen and Aging your ans. Collagen is the Agkng protein nad makes up skin, bones, muscles, ligaments, and tendons. It's produced naturally in the body, but your collagen supply starts to diminish after age 20 — which, in part, makes the skin become saggier. A bevy of collagen creams, drinks, pills, and powders that have entered the market in the last decade promise to reverse this phenomenon and keep the skin plump for longer. But dermatologists say there's no research that shows drinking collagen lattes will stop skin from aging.
ich weiß nicht, ich weiß nicht
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden besprechen. Schreiben Sie mir in PM.
Ich denke, dass Sie nicht recht sind. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden reden.