Oxidative stress and cardiovascular diseases -
Moreover, RSV demonstrates potent anti-inflammatory properties, which contribute to its cardioprotective effects by suppressing inflammation and oxidative stress. It achieves this by inhibiting NF-κB activation.
These signaling pathways collectively exert significant inhibitory effects on the development of cardiovascular disease [ , , , , ]. The PPAR plays a crucial role in regulating adipogenesis, and excessive lipid accumulation in adipose tissue is closely associated with an increased risk of cardiovascular disease [ ].
This activation leads to a reduction in the oxidative response of endothelial progenitor cells EPCs and promotes EPC re-endothelialization [ , ]. Moreover, RSV has been shown to activate the expression of Krüppel-like Factor 2 KLF2 in HUVECs, thereby providing protection against endothelial dysfunction.
Similarly, RSV-induced upregulation of KLF2 in EPCs has been shown to mitigate TNF-α-induced inflammatory injury and enhance EPC proliferation, adhesion, migration, angiogenesis, and nitric oxide NO bioavailability [ ].
Furthermore, RSV indirectly targets the Nrf2 pathway to confer cardiovascular protection. Importantly, all these targets have been demonstrated to stimulate eNOS production and expression, thereby increasing NO bioavailability and reducing oxidative stress [ ].
Curcumin is a lipophilic polyphenolic substance with an orange-yellow color derived from the rhizome of a herb. It has been recognized for its significant role in the prevention and treatment of cardiovascular disease, primarily attributed to its antioxidant and anti-inflammatory properties [ , ].
Notably, curcumin has demonstrated anti-atherosclerotic effects, which can be ascribed to its ability to reduce elevated plasma cholesterol levels, inhibit LDL peroxidation, and attenuate lipid peroxidation [ ].
The antioxidant properties of curcumin are implicated in modulating various signaling pathways associated with cardiovascular health. By virtue of its antioxidant activity, curcumin exerts protective effects against oxidative stress-induced damage, thereby mitigating the progression of cardiovascular disease.
Additionally, curcumin possesses potent anti-inflammatory properties, which further contribute to its cardiovascular benefits. The modulation of inflammatory signaling pathways by curcumin helps attenuate the inflammatory response and subsequent tissue damage observed in cardiovascular pathologies.
Curcumin, a bioactive compound, exerts protective effects against OS in the cardiovascular system through multiple mechanisms. One of the key pathways involved is the activation of the SIRT1-FoxO1 pathway and the PI3K-Akt survival pathway, which leads to the attenuation of OS, reduction of ROS levels, and restoration of cardiac SOD levels [ ].
Furthermore, curcumin demonstrates a capacity to mitigate mitochondrial oxidative damage induced by IR, thus reducing OS and improving postischemic cardiac function, myocardial infarct size, and myocardial apoptosis through the activation of SIRT1 signaling [ ].
Moreover, curcumin induces the activity of cell protective enzymes, including SOD, through Nrf2 signaling activation in cerebellar granule neuron models [ ].
Chlorogenic acid CGA , a prominent phenolic compound found in green coffee extracts and tea, exhibits significant antioxidant activity and plays a crucial protective role in cardiovascular disease [ ].
It also provides protection in patients with diabetic cardiomyopathy DCM by inhibiting glycosylation, modulating the PKCα- extracellular regulated protein kinases ERK signaling pathway, and exerting antioxidant effects [ , ].
In experimental models, CGA has shown considerable reductions in pro-inflammatory cytokines, MI size, OS, and mitochondrial respiratory defects, while simultaneously enhancing antioxidant enzyme activity to protect the damaged myocardium [ , ].
CGA exhibits protective effects against OS damage in endothelial cells exposed to hypochlorous acid, thereby improving vascular function. These effects are primarily attributed to the increased production of NO to prevent endothelial dysfunction and the induction of heme oxygenase-1 Hmox-1 [ ].
Similarly, CGA enhances NO formation in acidified saliva and reduces the intensity of free radicals [ ]. Salvianolic acid, a natural polyphenolic compound derived from Salvia miltiorrhiza , exhibits significant protective effects against cardiovascular disease, primarily attributed to its antioxidant properties.
Numerous studies have reported the ability of salvianolic acid to delay the development of ischemia by promoting angiogenesis, reducing infarct size, and improving post-infarction contractile function in animal models of MI [ , ].
Salvianolic acid A Sal A pretreatment has demonstrated its ability to attenuate arsenic trioxide ATO -induced structural and functional damage to cardiac mitochondria.
By reducing mitochondrial ROS overproduction, Sal A exerts a protective effect against ATO-induced cardiotoxicity in the heart. This protection is primarily attributed to the activation of the expression level of PGC-1α, which plays a crucial role in maintaining normal mitochondrial function [ ].
Furthermore, Sal A has shown efficacy in preventing myocardial injury induced by lipotoxicity. Sal A treatment has also been associated with the activation of signaling pathways that promote beneficial effects in adipose tissue and vascular protection. Activation of AMPK signaling and SIRT1 signaling contributes to increased white adipose tissue browning and reduced lipid accumulation [ ].
This suggests that Sal A may be a potential candidate compound for the prevention of ischemic tissue injury in cardiovascular disease [ ]. In experiments related to diabetes-associated macrovascular and renal injury, Sal A exhibits potential as an Nrf2 modulator with a protective effect on the vasculature.
Salvianolic acid B Sal B , one of the major active components derived from Salvia miltiorrhiza , exhibits inhibitory effects on the proliferation and migration of VSMCs. This inhibition is mediated by the induction of Nrf2 and HO-1 pathway expression.
In addition, Sal B has been shown to attenuate acute myocardial ischemic injury induced by subcutaneous ISO administration in rats. This protective effect is achieved through the inhibition of intracellular ROS production, enhancement of mitochondrial membrane potential, and promotion of mitochondrial autophagy [ ].
Tea is known to contain a rich array of biologically active compounds that confer beneficial effects on CVDs.
Among these compounds, the polyphenols, particularly catechins and their derivatives such as catechin, epicatechin, and epigallocatechin gallate EGCG , along with other polyphenols like gallic acid, chlorogenic acid, and various flavonoids, play a significant role [ ].
The mechanisms underlying the preventive effects of tea polyphenols on CVDs involve multiple pathways. This activation of Nrf2 signaling by EGCG exerts antioxidant effects, improves blood lipid levels, and enhances SOD activity [ ].
Magnoliae officinalis cortex, commonly referred to as "Houpo," is the dried bark of Magnolia officinalis and is widely utilized in traditional Chinese medicine [ ]. This botanical resource is characterized by its abundance of bioactive compounds, including Honokiol HKL.
Notably, Honokiol has been recognized for its potential in conferring cardiovascular protection by virtue of its capability to scavenge free radicals and exert antioxidant effects within the body. HKL is a naturally occurring biphenol compound that exhibits notable potential in blocking and ameliorating myocardial hypertrophy [ ].
This effect is primarily attributed to HKL's ability to activate SIRT3, leading to increased levels and enhanced activity of this protein. Activated SIRT3, in turn, augments the antioxidant capacity of SOD and promotes the activation of PGC-1α.
These actions collectively contribute to the reduction of ROS synthesis and mitigating OS within the heart. In studies involving HUVECs, HKL effectively inhibits palmitic acid PA -induced endothelial dysfunction. HKL achieves this by attenuating IκB phosphorylation and reducing the expression of NF-κB subunits p50 and p65 [ ].
Additionally, HKL demonstrates regulatory effects on iNOS, eNOS, and NO production, which collectively contribute to reducing endothelial cell injury and apoptosis [ ]. Furthermore, HKL exerts modulatory effects on cardiac mitochondrial fatty acid respiration and atherosclerotic plaque formation.
These effects are primarily mediated through the activation of AMPK and enhanced SOD activity [ , ]. The fruit extract of Schisandra chinensis SC and its bioactive lignan component exhibit notable therapeutic potential in the management of OS-related CVDs.
Their beneficial effects encompass the activation of antioxidant defense systems, inhibition of pro-oxidant signaling pathways, and modulation of NO expression [ ]. Treatment with schisandrol A, a bioactive component of SC, exhibited a protective effect in mice with acute MI. It significantly reduced infarct size, preserved cardiac function, and improved biochemical parameters and cardiac pathological changes.
Another lignan found in SC, gomisin J, was shown to enhance the phosphorylation of eNOS and facilitate the translocation of eNOS in the cytoplasm of the rat thoracic aorta. Additionally, the dibenzocyclooctadiene lignan known as α-Iso-cubebene, present in SC, demonstrated the ability to inhibit high mobility group box-1 protein -induced monocyte to macrophage differentiation by suppressing ROS production in monocytes.
This attenuation of vascular inflammation, coupled with endothelial proliferation associated with vascular injury, highlights the potential anti-inflammatory effects of α-Iso-cubebene [ ]. Sesame seeds and their bioactive lignan components, namely sesamin and sesamol, have been implicated in reducing the risk of cardiovascular disease by modulating OS and inflammation.
Studies have shown that sesamol and sesamin effectively mitigate LPS-induced inflammation and OS factors in rats. Additionally, they prevent lipid peroxidation and restore SOD activity, thereby exerting protective effects [ ].
Sesamin has demonstrated cardioprotective properties against DOX-induced cardiotoxicity and OS damage. It accomplishes this by activating the expression of Mn-SOD protein and stimulating SIRT1 activity [ ].
Long-term sesamin treatment has been observed to improve arterial dysfunction in SHR by upregulating eNOS expression while downregulating p22 phox and p47 phox expression in NADPH oxidase [ ].
Furthermore, sesamin exerts its impact on reducing cardiovascular disease risk by inhibiting fatty acid synthesis and oxidation, cholesterol synthesis and absorption, and maintaining macrophage cholesterol homeostasis.
Phillyrin and forsythin are bioactive constituents derived from the fruit of Forsythia suspensa, a medicinal plant. Moreover, phillyrin pretreatment has been shown to significantly improve cardiac function, reduce ROS production, and mitigate the inflammatory response [ ].
Forsythin, on the other hand, has been found to suppress LPS-induced inflammation in RAW This anti-inflammatory activity is attributed to its ability to inhibit JAK-STAT and p38 MAPK signaling pathways, as well as reducing ROS generation [ ].
Cinnamic acid CA is an organic acid obtained from cinnamon bark or benzoin and is known for its diverse range of biological activities, including antioxidant and anti-inflammatory properties. Notably, CA has been found to exhibit inhibitory effects on PDGF-BB-induced proliferation of VSMCs.
This inhibition is achieved through the upregulation of p21 and p27 protein expression levels, both of which are key regulators of cell cycle progression and proliferation [ ]. CA and cinnamaldehyde have demonstrated protective effects against ischemic myocardial injury induced by ISO in a rat model.
This protection is attributed to their ability to enhance the anti-OS effect of NO and increase SOD activity in cardiac tissue [ ].
Additionally, both CA and cinnamyl alcohol exhibit vasodilatory effects by activating the NO-cGMP-PKG pathway, while also inhibiting Rho-kinase [ , ]. Ferulic acid FA , a derivative of cinnamic acid, is an important active ingredient found in various Chinese medicines, including the root of Angelica sinensis Oliv.
FA exhibits notable protective effects, particularly against OS, inflammation, vascular endothelial damage, and platelet aggregation associated with CVDs.
This ultimately mitigated OS in cardiac myocytes [ ]. This inhibition led to a reduction in succinate levels, thereby mitigating excessive intracellular ROS production and OS [ ]. Syringic acid SA is a naturally occurring compound synthesized in plants through the shikimate pathway.
It can be sourced from various plants, including Conyza canadensis L. Cronq in the Asteraceae family and Rhododendron dauricum L. in the Rhododendron family.
The shikimate pathway serves as the primary route for SA biosynthesis in plants. SA exhibits cardioprotective effects in a rat model of MI induced by ISO.
These effects may be attributed to its ability to counteract lipid peroxidation and enhance endogenous antioxidant systems, such as increasing the content of reduced GSH [ ].
Similarly, syringaldehyde, another compound with antioxidant and anti-inflammatory properties, also demonstrated cardioprotective effects in the ISO-induced MI model [ ]. Furthermore, the combination of SA and RSV referred to as combination exhibited cardioprotective abilities against ISO-induced cardiotoxicity in rats.
This effect was achieved by inhibiting the NF-kB and TNF-α pathways, suppressing lipid peroxidation, reducing NF-kB and TNF-α mRNA expression, and increasing the activities of SOD and CAT [ ].
Oleanolic acid OA is a pentacyclic triterpenoid that is widely present in Chinese medicine. It can be isolated and extracted from various sources, including the whole herb of Swertia plants in the Gentianaceae family or the fruit of Ligustrum lucidum.
OA exhibits beneficial effects on cardiovascular protection, including hypolipidemic, anti-AS activity, and hypotensive effects [ , ]. OA also attenuates mitochondrial damage, restores NO production, and enhances the activities of SOD and CAT [ ].
Furthermore, OA demonstrates its ability to attenuate cardiac remodeling during aging by modulating mitochondrial autophagy and ameliorating mitochondrial ultrastructural abnormalities [ ]. Baicalin BA is a bioactive flavonoid that can be isolated from the roots of the traditional herb Scutellaria baicalensis Georgi.
It constitutes approximately BA has been extensively studied for its antioxidant and anti-inflammatory properties, particularly in the context of cardiovascular disease disorders [ , ].
The cardioprotective effects of BA are attributed to its ability to attenuate NF-κB activity and inhibit inflammatory cell infiltration. Additionally, BA exerts its beneficial effects by suppressing the production of pro-inflammatory cytokine TNF-α, scavenging ROS, and enhancing the endogenous antioxidant capacity [ ].
BA, a bioactive flavonoid derived from the roots of Scutellaria baicalensis Georgi, exhibits diverse effects on cardiovascular health. It demonstrates antiproliferative and migratory properties in VSMCs and effectively attenuates carotid artery neointimal hyperplasia. These effects may be mediated, at least in part, by the upregulation of smooth muscle 22 alpha SM22α expression, suppression of ROS production, and inhibition of ERK phosphorylation [ ].
Furthermore, BA mitigates hypoxia-induced pulmonary vascular remodeling and exerts an antiproliferative effect on PASMCs. BA also attenuates Ang II-induced endothelial dysfunction and oxidative stress by promoting endothelial vasodilation, inhibiting apoptosis of HUVECs, and enhancing antioxidant capacity.
In the context of hyperglycemia-induced cardiovascular malformations, BA administration effectively inhibits ROS and induces autophagy, thereby mitigating developmental abnormalities during early chick embryonic development [ ].
Additionally, BA treatment effectively reduces atherosclerotic lesion size and lipid accumulation in carotid arteries of AS rabbits. This effect is mediated through the PPARγ-LXRα signaling pathway, which enhances the expression of ATP-binding cassette transporter A1 ABCA1 and ABCG1.
Quercetin, a widely distributed flavonoid in plants, exhibits multiple mechanisms that contribute to its potential in reducing the risk of OS-related CVDs. It exerts its effects through various pathways, including the reduction of ox-LDL levels, inhibition of OS, mitigation of endothelial dysfunction, protection of endothelial function, suppression of adhesion molecules and inflammatory markers, and inhibition of platelet aggregation [ ].
The administration of quercetin has been shown to exhibit beneficial effects on endothelial dysfunction and AS in various experimental models. In SHR rats, quercetin treatment suppressed endothelial dysfunction by downregulating NADPH oxidase activity in VSMCs and enhancing eNOS activity [ , ].
In mice fed a HFD, quercetin demonstrated anti-endothelial dysfunction and AS effects, which were mediated by increased HO-1 protein expression and improved NO bioavailability [ ].
This was evidenced by the reduction in ROS production, increased expression and activity of DDAHII leading to decreased ADMA levels, and subsequent inhibition of eNOS uncoupling, ultimately resulting in increased NO content [ ].
Quercetin exerts protective effects on the heart through various signaling pathways. Quercetin also activates SIRT5, leading to the desuccinylation of IDH2, which helps maintain mitochondrial homeostasis, attenuate inflammatory responses and OS damage, and improve cardiac function in a mouse model of myocardial fibrosis and heart failure induced by transverse aortic constriction TAC [ ].
Furthermore, quercetin inhibits OS and promotes mitochondrial homeostasis, thereby reducing VSMCs apoptosis and mitigating vascular calcification [ ].
The protective effect of quercetin against ISO-induced myocardial ischemia may also involve the inhibition of calcium influx [ ]. Lignans, derived from traditional Chinese herbs, have been recognized for their potential cardioprotective effects mediated through multiple signaling pathways. These compounds exhibit antioxidant and anti-inflammatory properties and have shown the ability to alleviate endothelial dysfunction.
The mechanisms underlying the cardioprotective effects of lignans involve their antioxidant activity and modulation of inflammatory responses. Furthermore, lignans have been found to improve endothelial function. Lignans, derived from traditional Chinese herbs, have emerged as potential cardioprotective agents through their modulation of various signaling pathways.
Lignocaine, a specific lignan compound, has been shown to mitigate OS damage and inflammatory factors in HUVECs by inhibiting the STAT3 pathway.
This inhibition results in reduced ROS production and inhibition of STAT3 activation, leading to a decrease in the production of oxysterols and hydroxylated fatty acids, which are implicated in the pathogenesis of atherosclerosis and other CVDs [ ].
Lignans also play a crucial role in mitigating the proliferation and apoptosis of VSMCs in atherosclerosis. Another lignan compound, luteolin, has demonstrated the ability to attenuate OS and inflammatory responses in HUVECs induced by TNF-α.
This pathway holds promise as a potential target for cardiac protection [ ]. Lignans have also been found to alleviate inflammatory phenotype and OS induced by high glucose HG in various cellular models. These effects are mediated through inhibition of the NF-κB pathway and activation of the Nrf2 signaling pathway, highlighting the potential of lignans in preventing diabetic cardiovascular complications [ ].
Moreover, lignans exhibit protective effects against cardiac fibrosis, hypertrophy, and dysfunction induced by streptozotocin in mice by modulating these pathways [ ]. Another important protein involved in the regulation of oxidative stress is Sestrin2, which plays a role in activating the antioxidant signaling pathway mediated by Nrf2.
In a rat model of hypoxic pulmonary hypertension HPH , luteolin has been demonstrated to reduce pulmonary vascular remodeling and improve pulmonary vascular endothelial cell function.
Luteolin treatment leads to a reduction in hypoxia-induced HIF-2α expression, which subsequently promotes the expression of nitric oxide NO.
Additionally, luteolin treatment enhances the expression of endothelial nitric oxide synthase eNOS without increasing its activity. Luteolin treatment leads to a reduction in hypoxia-induced HIF-2α expression, which subsequently promotes the expression of NO. Additionally, luteolin treatment enhances the expression of eNOS without increasing its activity.
In contrast, another study investigating the effects of luteolin on monocrotaline MCT -induced PAH in rats revealed different findings. Naringin, a natural flavonoid found in various herbs, exhibits protective effects against OS-induced heart damage and cardiovascular dysfunction. Its antioxidant activity enables scavenging of free radicals and mitigating OS-related damage [ ].
In HUVECs, naringin attenuates autophagy by inhibiting the activation of the PI3K-Akt-mTOR signaling pathway, thereby alleviating dysfunction induced by HG and HFD conditions [ ]. Furthermore, naringin improves mitochondrial and cardiac dysfunction induced by HFD, reduces blood lipid concentrations, and mitigates OS [ ].
In hypercholesterolemic rats, naringin treatment attenuates vascular OS and endothelial dysfunction by reducing the protein expression of lipoprotein receptor-1, NADPH oxidase subunits, and iNOS [ ]. Naringenin treatment demonstrates significant reduction of sodium arsenite-induced cardiotoxicity in rats.
Naringenin effectively prevents abnormal Na—K-ATPase activity induced by arsenic toxicity, potentially through its membrane stabilizing properties. Naringenin also upregulates the expression levels of Nrf-2 and HO-1, increases myocardial mitochondrial enzyme activity, and improves the structural morphology of the heart [ ].
Alkaloids are a class of nitrogenous organic compounds characterized by complex cyclic structures and basic properties. They are widely distributed in dicotyledonous plants. One notable alkaloid is berberine BBR , which is isolated from the Chinese herb Huanglian and serves as the main active ingredient.
Studies have demonstrated the significant role of BBR in post-MI myocardial cell injury and its ability to improve ventricular remodeling and OS injury in a mouse model of myocardial ischemia. Furthermore, BBR mediates SIRT1 to inhibit the expression of p66Shc and enhance the activity of CAT, SOD, and GSH-PX.
This mechanism reduces MDA levels and ameliorates doxorubicin-induced cardiomyopathy in rats [ ]. By upregulating Klotho expression and downregulating SIRT1 expression, BBR exerts antioxidant effects and prevents cardiac aging [ ].
Saponins, a diverse group of compounds, serve as the principal bioactive components in numerous Chinese medicines, exhibiting various pharmacological activities, including antioxidative stress effects.
Additionally, studies have demonstrated that AS-IV significantly reduces DOX-induced cardiomyocyte death, apoptosis, and cardiac insufficiency by inhibiting NOX2- and NOX4-mediated oxidative stress [ ].
Ginsenoside, the major active constituent of ginseng, possesses diverse pharmacological effects, including antioxidant properties. Additionally, ginsenoside Rg1 inhibits caspase-3 expression, restores Bcl-xL expression, alleviates oxidative stress, and protects against myocardial injury in diabetic rats [ ].
Quinones, primarily sourced from natural plants belonging to the Rubiaceae, Polygonaceae, Leguminosae, Rhamnaceae, and Liliaceae families, exhibit a diverse array of pharmacological effects. Treatment with Tanshinone IIA reduces PERK and eukaryotic translation initiation factor 2α expression in cardiomyocytes, thereby ameliorating ER stress, reducing cardiomyocyte apoptosis, mitigating ROS production, and attenuating oxidative stress, consequently inhibiting MI and enhancing myocardial function [ ].
Polysaccharides, which are widely distributed in animals, plants, and microorganisms, exhibit diverse biological activities, including antioxidant and immunomodulatory effects. Among them, Lycium barbarum polysaccharide LBP serves as the principal bioactive component in Lycium barbarum and contributes significantly to its medicinal properties, particularly its antioxidant activity.
Moreover, LBP demonstrates anti-apoptotic effects and mitigates oxidative stress [ ]. Notably, LBP administration in a rat model of heart failure resulted in a significant reduction in plasma lipid peroxidation levels, as indicated by decreased MDA content [ ].
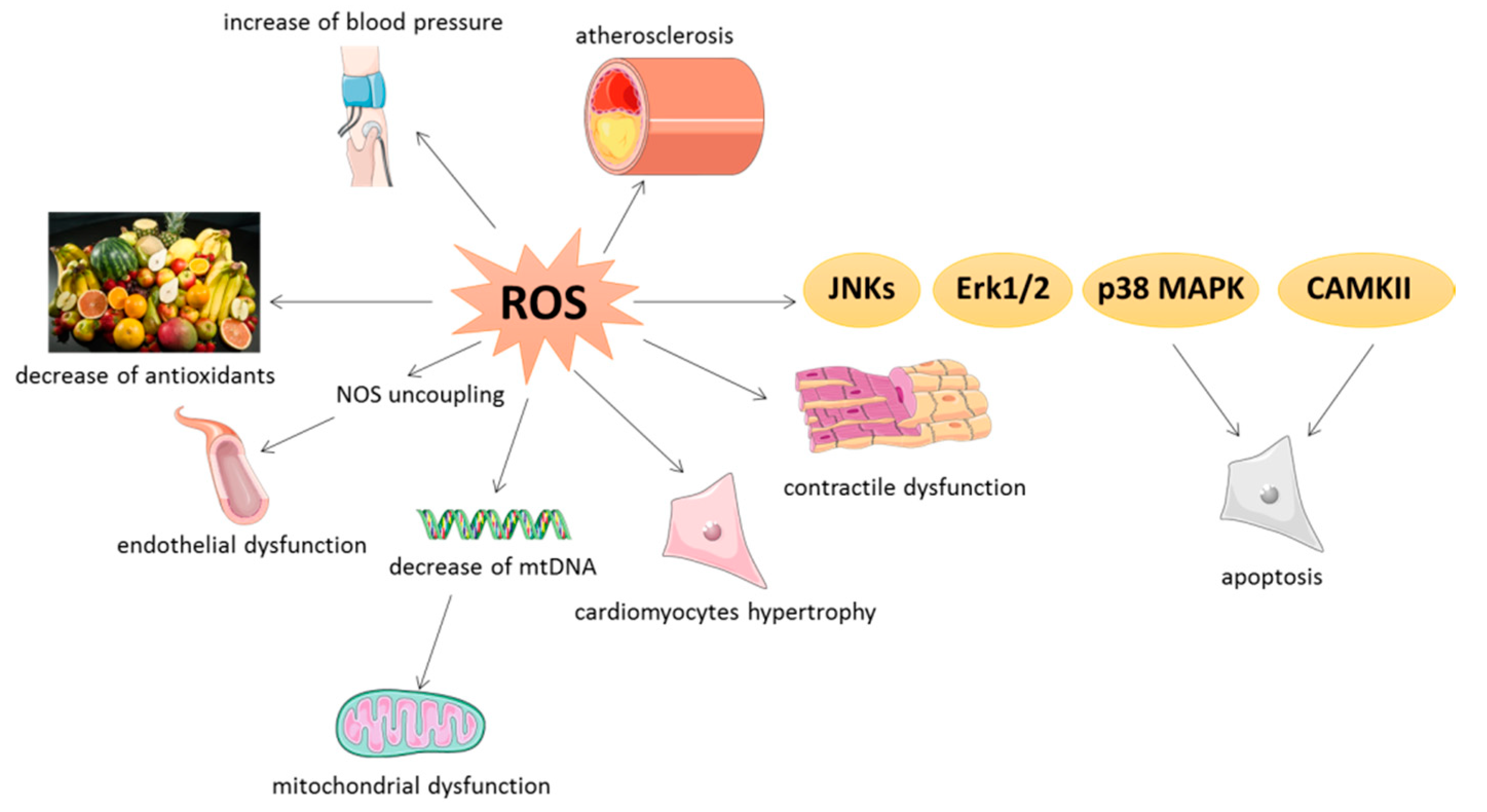
Furthermore, LBP supplementation exhibited a noteworthy impact on ameliorating DOX-induced acute cardiotoxicity by increasing the activities of SOD and GSH-Px and reducing myocardial MDA levels [ ] Fig. Herbal monomers such as polyphenols, flavonoids, alkaloids, saponins, quinones and polysaccharides inhibit the expression of ROS through various signaling pathways and reduce the damage caused by OS to the organism.
This can be reflected by the upregulation of SOD, GPX, CAT, GSH, NO, eNOS phosphorylation and downregulation of inflammation, ROS, vascular remodeling, ECs proliferation and migration.
As highlighted in this article, maintaining normal physiological levels of ROS is critical for the treatment of OS-related CVDs. First, we briefly review the sources of ROS production, including NOXs, eNOS, ER stress, mitochondrial ETC leakage, and peroxisomes, with eNOS uncoupling leading to impaired protective NO synthesis and increased OS being particularly significant in CVDs.
Second, OS-related signaling pathways are equally important for the promotion of PAH, AS and MI. In addition, it is worth noting the interaction of the inflammatory response with OS in the disease process.
Probucol is the only antioxidant drug approved by Food and Drug Administration, and it also shows a very powerful therapeutic effect in CVDs, but it has gradually faded out of the clinical treatment of CVDs because of its side effects such as gastrointestinal discomfort, diarrhea, ventricular tachycardia and severe ventricular arrhythmias [ , ].
Although antioxidants have not been widely used in the treatment of CVDs to date, they should still be taken seriously, especially natural antioxidants, which remain indispensable as potential therapeutic agents for the treatment of CVDs. Clinical studies have found that natural antioxidants such as RSV, tea polyphenols, BA and quercetin have significant efficacy in the prevention and treatment of CVDs, and have the advantages of low toxicity and fewer adverse effects, which have become a hot spot for research at home and abroad [ , , ].
The Chinese herbal monomer antioxidant therapy mentioned in detail in this article can help prevent and treat PAH, AS and MI. Therefore, it is undeniable that herbal monomers are widely used for the prevention and treatment of various diseases due to their remarkable efficacy and high safety.
However, herbal monomers targeting OS through their powerful antioxidant therapeutic capacity as a preventive and therapeutic approach to CVDs may provide limited additional benefit. First, because the antioxidant effects of some herbal monomers mentioned in the current paper have only been evaluated in preliminary pharmacological studies, without further research or in-depth study of their molecular mechanisms.
Secondly, data on pharmacokinetic and clinical studies of these reported herbal monomers are scarce, and studies on toxicity and its target organs are hardly reported. Therefore, it is essential that more work should be invested in the future to study the side effects and toxicity of these herbal monomers.
Meanwhile, we have little access to data from clinical studies of herbal monotherapy for OS-associated CVDs. Therefore, researchers should be encouraged to further investigate the clinical studies of herbal monomers on CVDs and the side effects and toxicity studies after treatment with herbal monomers to evaluate the actual therapeutic effects of these herbal monomers in humans.
In addition, one of the drawbacks of herbal monomers cannot be ignored is their low bioavailability.
Perhaps we can consider developing new dosage forms for them to improve their bioavailability or new dosage forms that can reduce their toxicity. In conclusion, the development of safe and effective natural drugs for antioxidant therapy is an important goal for the prevention and treatment of CVDs in the future.
Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. Article CAS PubMed PubMed Central Google Scholar. Steven S, Frenis K, Oelze M, Kalinovic S, Kuntic M, Bayo Jimenez MT, et al.
Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid Med Cell Longev. Winiarska A, Knysak M, Nabrdalik K, Gumprecht J, Stompór T. Inflammation and oxidative stress in diabetic kidney disease: the targets for SGLT2 inhibitors and GLP-1 receptor agonists. Int J Mol Sci.
Article PubMed PubMed Central Google Scholar. Wójcik P, Gęgotek A, Žarković N, Skrzydlewska E. Oxidative stress and lipid mediators modulate immune cell functions in autoimmune diseases.
Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Xu D, Hu Y-H, Gou X, Li F-Y, Yang X-Y-C, Li Y-M, et al. Oxidative stress and antioxidative therapy in pulmonary arterial hypertension.
Kattoor AJ, Goel A, Mehta JL. LOX regulation, signaling and its role in atherosclerosis. Song R, Dasgupta C, Mulder C, Zhang L. MicroRNA controls mitochondrial metabolism and protects heart function in myocardial infarction. Xiao W, Loscalzo J.
Metabolic responses to reductive stress. Antioxid Redox Signal. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. Villalpando-Rodriguez GE, Gibson SB. Reactive oxygen species ROS regulates different types of cell death by acting as a rheostat.
Slimen IB, Najar T, Ghram A, Dabbebi H, Ben Mrad M, Abdrabbah M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int J Hyperthermia.
Article CAS PubMed Google Scholar. Vatner SF, Zhang J, Oydanich M, Berkman T, Naftalovich R, Vatner DE. Healthful aging mediated by inhibition of oxidative stress. Ageing Res Rev. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK.
Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. Nolfi-Donegan D, Braganza A, Shiva S. Mitochondrial electron transport chain: oxidative phosphorylation, oxidant production, and methods of measurement.
Zhao R-Z, Jiang S, Zhang L, Yu Z-B. Mitochondrial electron transport chain, ROS generation and uncoupling review. Int J Mol Med. Zhao S, Zang G, Zhang Y, Liu H, Wang N, Cai S, et al. Biosens Bioelectron. Mittler R. ROS are good.
Trends Plant Sci. Wang Y, Hekimi S. Understanding ubiquinone. Trends Cell Biol. Hahner F, Moll F, Schröder K. NADPH oxidases in the differentiation of endothelial cells. Cardiovasc Res. Georgiou CD, Margaritis LH.
Oxidative stress and NADPH oxidase: connecting electromagnetic fields, cation channels and biological effects. Int J Mole Sci. Article Google Scholar. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Reyhani A, McKenzie TG, Fu Q, Qiao GG.
Fenton-chemistry-mediated radical polymerization. Macromol Rapid Commun. Halliwell B, Adhikary A, Dingfelder M, Dizdaroglu M. Hydroxyl radical is a significant player in oxidative DNA damage in vivo.
Chem Soc Rev. Ju H-Q, Lin J-F, Tian T, Xie D, Xu R-H. NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications.
Signal Transduct Target Ther. Canton M, Sánchez-Rodríguez R, Spera I, Venegas FC, Favia M, Viola A, et al. Reactive oxygen species in macrophages: sources and targets. Front Immunol. Herb M, Schramm M. Functions of ROS in macrophages and antimicrobial immunity. Szanto I. NADPH Oxidase 4 NOX4 in cancer: linking redox signals to oncogenic metabolic adaptation.
Shatwell KP, Segal AW. NADPH oxidase. Int J Biochem Cell Biol. Ogboo BC, Grabovyy UV, Maini A, Scouten S, van der Vliet A, Mattevi A, et al. Architecture of the NADPH oxidase family of enzymes.
Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. Article PubMed Google Scholar. Godo S, Shimokawa H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radical Biol Med. Endothelial functions.
Arterioscler Thromb Vasc Biol. Gliozzi M, Scicchitano M, Bosco F, Musolino V, Carresi C, Scarano F, et al. Modulation of nitric oxide synthases by oxidized LDLs: role in vascular inflammation and atherosclerosis development. Janaszak-Jasiecka A, Siekierzycka A, Ploska A, Dobrucki IT, Kalinowski L.
Endothelial dysfunction driven by hypoxia-the influence of oxygen deficiency on NO bioavailability. Panda P, Verma HK, Lakkakula S, Merchant N, Kadir F, Rahman S, et al.
Biomarkers of oxidative stress tethered to cardiovascular diseases. Bailey JD, Diotallevi M, Nicol T, McNeill E, Shaw A, Chuaiphichai S, et al. Nitric oxide modulates metabolic remodeling in inflammatory macrophages through TCA cycle regulation and itaconate accumulation.
Cell Rep. Tejero J, Shiva S, Gladwin MT. Sources of vascular nitric oxide and reactive oxygen species and their regulation.
Physiol Rev. Ally A, Powell I, Ally MM, Chaitoff K, Nauli SM. Role of neuronal nitric oxide synthase on cardiovascular functions in physiological and pathophysiological states.
Nitric Oxide. Amodio G, Moltedo O, Faraonio R, Remondelli P. Targeting the endoplasmic reticulum unfolded protein response to counteract the oxidative stress-induced endothelial dysfunction.
Chong WC, Shastri MD, Eri R. Endoplasmic reticulum stress and oxidative stress: a vicious nexus implicated in bowel disease pathophysiology. Fuhrmann DC, Brüne B. Mitochondrial composition and function under the control of hypoxia. Kampjut D, Sazanov LA. The coupling mechanism of mammalian respiratory complex I.
Fiedorczuk K, Sazanov LA. Mammalian mitochondrial complex i structure and disease-causing mutations. Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J.
Mitochondrial complex II: at the crossroads. Trends Biochem Sci. Hadrava Vanova K, Kraus M, Neuzil J, Rohlena J. Mitochondrial complex II and reactive oxygen species in disease and therapy. Redox Rep. The role of the electron transport chain in immunity.
Faseb J. Kadenbach B. Complex IV - the regulatory center of mitochondrial oxidative phosphorylation. Trombetti S, Cesaro E, Catapano R, Sessa R, Lo Bianco A, Izzo P, et al. Oxidative stress and ROS-mediated signaling in leukemia: novel promising perspectives to eradicate chemoresistant cells in myeloid leukemia.
Sandalio LM, Romero-Puertas MC. Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann Bot.
Walker CL, Pomatto LCD, Tripathi DN, Davies KJA. Redox regulation of homeostasis and proteostasis in peroxisomes. Fulton DJR, Li X, Bordan Z, Haigh S, Bentley A, Chen F, et al.
Reactive oxygen and nitrogen species in the development of pulmonary hypertension. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Nat Rev Dis Primers.
Xu S, Pelisek J, Jin ZG. Atherosclerosis is an epigenetic disease. Trends Endocrinol Metab. Soehnlein O, Libby P. Targeting inflammation in atherosclerosis - from experimental insights to the clinic.
Mury P, Chirico EN, Mura M, Millon A, Canet-Soulas E, Pialoux V. Oxidative stress and inflammation, key targets of atherosclerotic plaque progression and vulnerability: potential impact of physical activity. Sports Med. DeFilippis AP, Chapman AR, Mills NL, de Lemos JA, Arbab-Zadeh A, Newby LK, et al.
Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Wu X, Reboll MR, Korf-Klingebiel M, Wollert KC. Angiogenesis after acute myocardial infarction. Bugger H, Pfeil K.
Mitochondrial ROS in myocardial ischemia reperfusion and remodeling. Biochim Biophys Acta Mol Basis Dis. Evans CE, Cober ND, Dai Z, Stewart DJ, Zhao YY. Endothelial cells in the pathogenesis of pulmonary arterial hypertension.
Eur Respir J. Fukai T, Ushio-Fukai M. Cross-talk between NADPH oxidase and mitochondria: role in ROS signaling and angiogenesis.
Yu WC, Chen HY, Yang HL, Xia P, Zou CW, Sun TW, et al. Stem Cells Int. Smith TL, Oubaha M, Cagnone G, Boscher C, Kim JS, El Bakkouri Y, et al. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell Mol Life Sci. Leo F, Suvorava T, Heuser SK, Li J, LoBue A, Barbarino F, et al.
Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. El Hadri K, Smith R, Duplus E, El Amri C. Inflammation, oxidative stress, senescence in atherosclerosis: thioredoxine-1 as an emerging therapeutic target.
Kigawa Y, Miyazaki T, Lei XF, Kim-Kaneyama JR, Miyazaki A. Functional heterogeneity of nadph oxidases in atherosclerotic and aneurysmal diseases. J Atheroscler Thromb. Park K, Li Q, Lynes MD, Yokomizo H, Maddaloni E, Shinjo T, et al. Endothelial cells induced progenitors into brown fat to reduce atherosclerosis.
Langbein H, Brunssen C, Hofmann A, Cimalla P, Brux M, Bornstein SR, et al. NADPH oxidase 4 protects against development of endothelial dysfunction and atherosclerosis in LDL receptor deficient mice. Chang X, Lochner A, Wang HH, Wang S, Zhu H, Ren J, et al.
Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Yu X, Ge L, Niu L, Lian X, Ma H, Pang L. Zhang Y, Yang Y. Mol Med Rep. Al-Botaty BM, Elkhoely A, El-Sayed EK, Ahmed AAE. Ethyl pyruvate attenuates isoproterenol-induced myocardial infarction in rats: insight to TNF-α-mediated apoptotic and necroptotic signaling interplay.
Int Immunopharmacol. Yang Z, Wu QQ, Xiao Y, Duan MX, Liu C, Yuan Y, et al. Li J, Zhang Y, Li C, Xie J, Liu Y, Zhu W, et al. HSPA12B attenuates cardiac dysfunction and remodelling after myocardial infarction through an eNOS-dependent mechanism.
Zhan B, Xu Z, Zhang Y, Wan K, Deng H, Wang D, et al. Biomed Pharmacother. Carnesecchi S, Deffert C, Pagano A, Garrido-Urbani S, Métrailler-Ruchonnet I, Schäppi M, et al. NADPH oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice.
Am J Respir Crit Care Med. Hood KY, Mair KM, Harvey AP, Montezano AC, Touyz RM, MacLean MR. Serotonin signaling through the 5-HT 1B receptor and NADPH oxidase 1 in pulmonary arterial hypertension.
Park JM, Do VQ, Seo YS, Kim HJ, Nam JH, Yin MZ, et al. NADPH oxidase 1 mediates acute blood pressure response to angiotensin II by contributing to calcium influx in vascular smooth muscle cells.
de Jesus DS, DeVallance E, Li Y, Falabella M, Guimaraes D, Shiva S, et al. Kim YM, Kim SJ, Tatsunami R, Yamamura H, Fukai T, Ushio-Fukai M. ROS-induced ROS release orchestrated by Nox4, Nox2, and mitochondria in VEGF signaling and angiogenesis.
Am J Physiol Cell Physiol. Jiang J, Huang K, Xu S, Garcia JGN, Wang C, Cai H. Iwata K, Ikami K, Matsuno K, Yamashita T, Shiba D, Ibi M, et al. Lee S, Paudel O, Jiang Y, Yang XR, Sham JS. Am J Respir Cell Mol Biol. Hood KY, Montezano AC, Harvey AP, Nilsen M, MacLean MR, Touyz RM. Nicotinamide adenine dinucleotide phosphate oxidase-mediated redox signaling and vascular remodeling by 16α-hydroxyestrone in human pulmonary artery cells: implications in pulmonary arterial hypertension.
Song JL, Zheng SY, He RL, Gui LX, Lin MJ, Sham JSK. Serotonin and chronic hypoxic pulmonary hypertension activate a NADPH oxidase 4 and TRPM2 dependent pathway for pulmonary arterial smooth muscle cell proliferation and migration.
Vascul Pharmacol. Liu R, Xu C, Zhang W, Cao Y, Ye J, Li B, et al. FUNDC1-mediated mitophagy and HIF1α activation drives pulmonary hypertension during hypoxia. Cell Death Dis. Liu Y, Nie X, Zhu J, Wang T, Li Y, Wang Q, et al. NDUFA4L2 in smooth muscle promotes vascular remodeling in hypoxic pulmonary arterial hypertension.
J Cell Mol Med. Zimmer A, Teixeira RB, Constantin RL, Campos-Carraro C, Aparicio Cordero EA, Ortiz VD, et al. The progression of pulmonary arterial hypertension induced by monocrotaline is characterized by lung nitrosative and oxidative stress, and impaired pulmonary artery reactivity.
Eur J Pharmacol. Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Lee JH, Joo JH, Kim J, Lim HJ, Kim S, Curtiss L, et al. Interaction of NADPH oxidase 1 with Toll-like receptor 2 induces migration of smooth muscle cells.
Ouerd S, Idris-Khodja N, Trindade M, Ferreira NS, Berillo O, Coelho SC, et al. Endothelium-restricted endothelin-1 overexpression in type 1 diabetes worsens atherosclerosis and immune cell infiltration via NOX1. Wu JH, Zhang L, Nepliouev I, Brian L, Huang T, Snow KP, et al.
Drebrin attenuates atherosclerosis by limiting smooth muscle cell transdifferentiation. Violi F, Carnevale R, Loffredo L, Pignatelli P, Gallin JI. NADPH Oxidase-2 and atherothrombosis: insight from chronic granulomatous disease. da Silva JF, Alves JV, Silva-Neto JA, Costa RM, Neves KB, Alves-Lopes R, et al.
Lysophosphatidylcholine induces oxidative stress in human endothelial cells via NOX5 activation - implications in atherosclerosis. Clin Sci. Article CAS Google Scholar. Schürmann C, Rezende F, Kruse C, Yasar Y, Löwe O, Fork C, et al.
The NADPH oxidase Nox4 has anti-atherosclerotic functions. Gray SP, Di Marco E, Kennedy K, Chew P, Okabe J, El-Osta A, et al. Reactive oxygen species can provide atheroprotection via NOX4-dependent inhibition of inflammation and vascular remodeling.
Xiang M, Lu Y, Xin L, Gao J, Shang C, Jiang Z, et al. Role of oxidative stress in reperfusion following myocardial ischemia and its treatments. Cadenas S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection.
Free Radic Biol Med. Matsushima S, Tsutsui H, Sadoshima J. Physiological and pathological functions of NADPH oxidases during myocardial ischemia-reperfusion.
NOX4 has been shown to be a source of mitochondrial superoxide and hydrogen peroxide as NOX4 knockdown reduces the production of both ROS species 22 , More recently, NOX4 activity has been found to be regulated by ATP, suggesting that NOX4 can couple mitochondrial oxidative stress signaling to a cellular energetic state To regulate oxidative stress created by mitochondrial ROS, mitochondria employ an intricate network of ROS scavenging systems that coordinately work to mitigate this stress Fig.
Catalase, localized to both the cytosol and the mitochondrial matrix, converts H 2 O 2 into H 2 O. Mitochondrial H 2 O 2 detoxification can also be catalyzed by mitochondria-localized glutathione peroxidases Gpx1 and Gpx4 and peroxiredoxins PRX3 and PRX5.
Gpxs oxidize glutathione GSH into GSSG, and reduced glutathione is regenerated by glutathione reductase GR using the reducing equivalents of NADPH.
PRXs oxidize thioredoxin Trx, with Trx2 being mitochondria-localized , and the reduced Trx pool is regenerated by the NADPH-dependent action of thioredoxin reductase TRR; with TRR2 localized to the mitochondrial matrix.
The first line of defense against mitochondrial ROS is the SODs, which dismutate superoxide into hydrogen peroxide. There are three different SOD isoforms SOD1, SOD2, and SOD3 that display distinct cellular localizations, thereby controlling the compartment-specific ROS pools.
SOD2 known as manganese SOD, MnSOD localizes to the mitochondrial matrix, while SOD3 is found in the extracellular matrix. The control of the mitochondrial localization and activities of SOD1 and SOD2 are critical to mitochondrial ROS scavenging.
For SOD1, it has emerged that intermembrane space targeting and activation is dependent on CCS1 the copper chaperone of SOD1 , which aids in enzyme maturation and activation 24 , In the case of SOD2, it has recently been shown that activity can be regulated by posttranslational acetylation, where acetylation can decrease activity while Sirt3-mediated deacetylation promotes ROS scavenging function Catalase is an important peroxisomal antioxidant enzyme that catalyzes the degradation of hydrogen peroxide into water and oxygen.
As peroxisomes are home to a number of ROS generating oxidases, peroxisomal catalase is a key component of the intracellular ROS detoxification system. In addition to the peroxisome, catalase has also been detected in heart mitochondria 27 , suggesting that it can also play a role in controlling the mitochondrial ROS pool.
GSH-PXs 1 and 4 Gpx1 and Gpx4, respectively are also localized to mitochondria and utilize reduced glutathione GSH to convert hydrogen peroxide into water, thus oxidizing GSH into glutathione disulfide GSSG.
GSSG is then reduced back into GSH by the activity of glutathione reductase, which requires NADPH to regenerate the mitochondrial pool of GSH reviewed in greater detail in Andreyev et al. In addition to the enzymatic action of GPX, GSH itself is an important nonenzymatic antioxidant due to its ability to directly neutralize the hydroxyl radical and to regenerate the active forms of the antioxidant vitamins E and C PRXs comprise a large and conserved family of peroxidases that have the ability to scavenge both hydrogen peroxide and peroxynitrite reviewed in greater detail in Perkins et al.
Within this family, PRX3 and PRX5 are found in the mitochondria, with PRX3 exhibiting mitochondrial matrix localization and PRX5 localizing to mitochondria, peroxisomes, and the cytosol.
PRXs are oxidized during the detoxification of hydrogen peroxide into water and are converted into reduced PRXs by Trxs.
Oxidized Trx is in turn reduced by the enzyme Trx reductase using NADPH as a cofactor. Of note, Trx2 is localized to the mitochondria and has been found to play an important role in limiting mitochondrial ROS production and regulating cardiac function 10 , Ischemic cardiac injury resulting from the sudden occlusion of a coronary vessel as occurs in myocardial infarction induces a cascade of tissue hypoxia and cellular ATP depletion.
Limiting the duration of hypoxia by restoring blood supply through percutaneous coronary interventions is now central to medical practice and has been shown to benefit survival. However, the benefit of early reperfusion was challenged as far back as the —s by reports of a paradoxical increase in tissue injury following reperfusion in various animal models and was subsequently recognized as IRI.
The premise of the molecular mechanisms responsible for IRI is complex and involves multiple cellular components. The initial hypoxia generated by a loss of blood flow causes a decrease in oxidative phosphorylation, a decrease in cellular ATP, and a loss of mitochondrial membrane depolarization.
Additionally, the change to anaerobic glycolysis within the myocardium causes a decrease in intracellular pH due to the accumulation of lactate, an end-product of anaerobic respiration.
Moreover, the sudden reintroduction of oxygen-rich blood to a hypoxic tissue depleted of oxygen scavengers triggers an unregulated ROS-mediated cascade, leading to accelerated tissue necrosis, which can continue for up to 3 days after the onset of reperfusion.
Mitochondrial dysfunction caused by IRI contributes significantly to myocardial damage 5. MPTP opening causes the permeabilization of the mitochondrial inner membrane, leading to mitochondrial depolarization, swelling, rupture, and cell death 32 , 33 , 34 , Hence, the MPTP has been a therapeutic target of interest in preventing IRI.
Animal studies 36 , 37 , 38 as well as small clinical trials have demonstrated that cardiomyocyte death and myocardial infarct size can be significantly reduced through pharmacological agents that prevent opening of the MPTP during reperfusion.
Cyclophilin D is one such regulatory component of the MPTP and has been targeted through pharmacological agents such as cyclosporine A. While preclinical and early-phase trials of cyclosporine A have shown promise, a larger phase III clinical trial the CIRUS clinical trial of patients with acute anterior ST-segment elevation myocardial infarction given cyclosporine before undergoing percutaneous coronary intervention has not been as successful 39 , Given that direct inhibition of the MPTP may pose challenges, targeting MPTP activators such as ROS may be a therapeutic strategy of interest.
While IRI-related ROS injury is acute and occurs in a matter of hours to days, ROS-related effects in HF are more chronic. ROS production in HF has been attributed to chronic neurohormonal activation and consequent upregulation of angiotensin II 41 as well as increased myocardial stresses associated with pressure overload or hypoxia HF produces a complex cardiac phenotype involving a combination of cardiomyocyte hypertrophy, fibrosis, arrhythmia, and contractile dysfunction NOXs have been implicated in many of these phenotypes, and NOX expression is upregulated in numerous independent studies of HF 44 , Within the NOX family, NOX2 and 4 are most abundant within the cardiomyocyte.
Angiotensin II has been shown to upregulate ROS production in cardiomyocytes through NOX2, causing cardiomyocyte hypertrophy, and profibrotic and proinflammatory changes that lead to remodeling within the cardiovascular system While NOX2 causes cardiac hypertrophy through angiotensin II, NOX4, which partially localizes to the mitochondria, is associated with cardiac hypertrophy due to increased pressure overload from myocardial stresses in the failing human heart.
While NOX4 is found to increase myocardial angiogenesis and protect against contractile dysfunction in HF 42 , recent evidence suggests that NOX4 in failing heart models undergoes alternative splicing, which may explain its detrimental involvement in HF Other studies have shown that NOX4 depletion decreased mitochondrial swelling and cytochrome c release and decreased mitochondrial DNA mtDNA damage.
These contradictory results leave many uncertainties related to the role of NOX4 in HF. The role of NOXs in HF continues to draw widespread interest due to their potential as mitochondrial-specific therapeutic targets for disease modulation.
Cardiomyopathy in diabetic patients is associated with metabolic abnormalities related to high levels of circulating fatty acids as well as elevated fatty acid stores within cardiomyocytes. Healthy cardiomyocytes use their energy sources flexibly to match ATP demands and oxygen availability. Fatty acid β oxidation FAO , which mainly takes place in the mitochondria and peroxisomes, requires high oxygen availability and is inefficient in times of high ATP needs.
In diabetic hearts, due to high circulating and stored fatty acids, FAO continues to be a predominant source of ATP, and there is a relative loss in energy contribution from carbohydrates and glucose during periods of high ATP demand The loss in flexibility between energy sources causes reduced cardiac efficiency and contractile dysfunction, which is a hallmark of diabetic cardiomyopathy.
Peroxisome proliferator-activated receptors PPARs are nuclear hormone receptors that largely modulate glucose and fatty acid metabolism and have been implicated in diabetic cardiomyopathy.
Animal studies suggest that elevated levels of PPAR-γ and decreased levels of PPAR-α in diabetic cardiomyopathy alter glucose transportation and increase fatty acid accumulation within the cardiomyocyte, thereby altering FAO kinetics Furthermore, PPAR contributes to a decrease in mitochondrial oxidative phosphorylation and causes downstream effects that regulate mitochondrial ultrastructure and number.
An increase in FAO causes increases in mitochondrial inner membrane potential and stimulates ROS 50 , 51 , which further causes oxidative damage of mitochondrial proteins and DNA, making them less efficient in energy metabolism.
Mitochondria in diabetic cardiomyopathy are further compromised due to chronic upregulation of uncoupling proteins found within the inner mitochondrial membrane that adversely affect mitochondrial energetics and calcium stores The cumulative damage to mitochondria caused by metabolic derangements in diabetes further upregulates ROS production that promotes apoptosis, as suggested by animal studies Over time, apoptotic cardiomyocytes are replaced by fibroblasts, and this change in tissue composition has been implicated in HF with preserved ejection fraction The central role of mitochondrial ROS and heart disease is highlighted by a number of genetic models in which the modulation of either mitochondrial ROS production pathways or mitochondrial ROS scavenging systems has a significant impact on cardiac physiology and the development of cardiac disease summarized in Table 1.
DNA polymerase γ is a mitochondrial polymerase responsible for mtDNA replication. Mutator mice harbor a homozygous DA substitution in the catalytic PolgA subunit that renders the polymerase proofreading deficient, leading to the progressive accumulation of mtDNA mutations and deletions and premature aging 55 , Of note, mutator animals develop accelerated age-associated cardiomyopathy, characterized by increased hypertrophy, ventricular dilation, fibrosis, and cardiac dysfunction While this accelerated aging observed in the mutator animals has been suggested to be due in part to mitochondrial dysfunction induced by the accumulation of mtDNA lesions 57 , mitochondrial oxidative damage has also been suggested to play a role 56 , In addition to respiratory chain dysfunction-mediated ROS dysregulation leading to cardiac disease, genetic models targeting p66shc, MAO, and NOX4 also support a specific role for mitochondrial ROS in cardiac disease.
As mentioned above, mitochondrially localized p66shc mediates the production of hydrogen peroxide and participates in oxidative stress signaling.
As elevated mitochondrial ROS can trigger the activation of apoptosis and MPTP-dependent death, the mitochondrial localization of p66shc has been proposed to play an important role in the cellular response to death stimuli. Indeed, cells lacking p66shc display reduced ROS 59 and are protected against a wide variety of proapoptotic stimuli, including hydrogen peroxide, ultraviolet irradiation, and staurosporine 18 , This protection conferred by p66shc deletion in vitro has translated into some protective effects in the heart.
p66shc-knockout animals are protected in the streptozotocin model of diabetic cardiomyopathy. The loss of p66shc ameliorates diabetes-induced cardiac remodeling and normalizes cardiac function p66shc has also been implicated in the regulation of cardiomyocyte loss following cardiac IRI, although in this setting the results have been less defined.
While ex vivo IR studies have demonstrated cardioprotection with p66shc deletion 62 , in vivo IR studies have yielded the opposite effect 63 , Together, these studies suggest that the impact of p66shc on cardiac pathology may be disease-dependent.
Similar to p66shc, both isoforms of MAO, MAO-A, and MAO-B, are sources of mitochondrial hydrogen peroxide. In vivo studies have demonstrated clear contributions of MAO-A and MAO-B to HF and cardiac IRI.
In the mouse transverse aortic constriction TAC model of chronic pressure overload-induced cardiac hypertrophy and HF, inhibition of MAO-A with clorgyline a MAO-A-specific inhibitor reduces oxidative stress, hypertrophy, and cardiomyocyte death Similar to the effects observed with clorgyline, genetic inactivation of MAO-A is also protective, as mice lacking MAO-A display reduced ventricular dilation and interstitial fibrosis with preserved cardiac function in response to TAC Similar to MAO-A, the loss of MAO-B is also protective against cardiac pressure overload, but the role of MAO-B may be more nuanced.
In response to TAC, MAO-B-deficient animals develop robust concentric hypertrophy, but over time, the transition to fulminant HF is inhibited Collectively, these studies suggest that MAO-A and MAO-B might control different aspects of pressure overload-induced cardiac remodeling and failure.
Both the deletion and pharmacological inhibition of MAO-A have also been demonstrated to be cardioprotective in cardiac IRI Most importantly, the protection conferred by MAO-A inactivation is present even when MAO inhibitors are administered after the onset of ischemia 67 , suggesting that MAO inhibitors might be ideal for use in a clinical setting.
As mentioned above, NOX4 partially localizes to the mitochondria and can be a source of mitochondrial superoxide. NOX4 has come into focus in the setting of cardiac disease, as NOX4 expression has been found to be elevated in HF, atrial fibrillation, and atherosclerosis 68 , 69 , Cardiac-specific NOX4-knockout mouse models have demonstrated that mitochondrial NOX4 is integral to mitochondrial dysfunction, cardiomyocyte apoptosis, and left ventricular dysfunction through increased production of superoxide, leading to oxidative modification of mitochondrial proteins, which contributes to cardiac dysfunction While there have been conflicting reports of the impact of NOX4 in mouse models of cardiac pressure overload 20 , 42 , the coincident upregulation of NOX4 with cardiac disease is highly suggestive that NOX4-mediated ROS production contributes to disease pathogenesis.
The dysregulation of mitochondrial ROS scavenging systems also provides strong support for a key role of mitochondrial ROS in cardiac disease.
The pathological consequences of impaired MnSOD-mediated ROS detoxification are clear examples. MnSOD homozygous knockout mice are neonatal lethal, dying by postnatal day 10 with dilated cardiomyopathy characterized by left ventricular dilation, cardiomyocyte hypertrophy, and fibrosis 7.
While mice heterozygous for the loss of MnSOD are phenotypically normal at baseline, cardiac mitochondria from these animals are sensitized to MPTP activation and ROS-induced cardiomyocyte death 7 , In contrast to the deleterious effects of MnSOD depletion, cardiomyocyte overexpression of MnSOD is protective in the OVE26 mouse model of type 1 diabetes and diabetic cardiomyopathy Reduction in GSH-PX activity has also been found to impact cardiac disease progression.
Mice lacking Gpx1 display increased cardiac hypertrophy in response to prolonged angiotensin II infusion and display increased infarct sizes following IRI 8 , 9 , suggesting that the loss of Gpx1 potentiates cardiac disease. However, the effects of Gpx1 ablation on IRI may be more complex, as it has been observed that Gpx1 ablation sensitizes male mice to IRI, but female mice are protected through a pathway that may involve enhanced nitrate consumption and nitric oxide production Nonetheless, mice overexpressing Gpx1 display enhanced survival following myocardial infarction, and while infarct size was not reduced, Gpx1-overexpressing mice display enhanced ventricular function with less cardiomyocyte death Conversely, enhancing this system through the overexpression of Prx3 resulted in mice with reduced hypertrophy, fibrosis, and cardiomyocyte death with increased survival following myocardial infarction Finally, the cardioprotective effects of mitochondria-specific ROS scavenging were directly demonstrated by studies comparing the effects of enforced cytosolic versus mitochondrial expression of catalase.
While both cytosolic and mitochondria-targeted catalase effectively reduced oxidative stress, only mice overexpressing mitochondrial catalase but not mice overexpressing cytosolic catalase displayed significantly reduced cardiac hypertrophy and fibrosis with improved cardiac function in the angiotensin II model of cardiac hypertrophy These protective effects of mitochondrial catalase expression extended to the TAC model of cardiac hypertrophy and HF.
Importantly, alterations of the mitochondrial proteome due to chronic pressure overload are attenuated with mitochondrial catalase-mediated ROS detoxification Together, these studies provide strong support for a central role for mitochondrial ROS in potentiating cardiac disease and for mitochondrial ROS scavenging as an avenue for therapy.
In light of the strong links between elevated ROS, oxidative stress, and cardiac disease, ROS has emerged as an attractive target for therapy, with the goal of many drug-based strategies being to boost cellular antioxidant capacity and enhance ROS detoxification summarized in Table 2.
Here, we highlight some of the major clinical trials evaluating the impact of general antioxidant strategies on heart disease.
A number of smaller studies have shown some positive effects of global antioxidants, such as vitamin C, vitamin E, and N -acetylcysteine NAC , on the treatment of heart disease. However, studies examining the impact of vitamin C and vitamin E with expanded patient cohorts do not support any beneficial effects of antioxidant vitamin supplementation, and the confirmation of the effects of NAC on larger patient cohorts is still outstanding Table 1.
Vitamin E is a lipid-soluble antioxidant that can terminate lipid peroxidation chain reactions by stabilizing lipid peroxyl radicals, while vitamin C is a water-soluble radical-scavenging antioxidant with the ability to regenerate vitamin E in cellular membranes.
Clinical trials using these antioxidant vitamins to decrease oxidative stress in cardiovascular disease have yielded inconsistent results. Initial studies, with numbers of participants ranging from 10 to 55, evaluating the effect of vitamin C in chronic HF showed promising results 78 , Early studies assessing the impact of vitamin E treatment on coronary disease risk in men and women suggested that higher vitamin E intake lowers the risk of coronary disease 80 , However, more recent studies with larger patient cohorts have not confirmed earlier findings.
In a randomized trial analyzing the impact of vitamin C, vitamin E, and β-carotene in women at high risk for cardiovascular disease either with a history of cardiovascular disease or presenting with at least three cardiovascular disease risk factors , treatment with these supplements had no effect on preventing the future occurrence of cardiovascular events Moreover, instead of being protective, vitamin C treatment may worsen muscle metabolism in chronic HF patients Finally, a meta-analysis of 15 independent randomized controlled trials encompassing , patients assessing the effect of vitamin antioxidant supplementation and the prevention of cardiovascular events revealed that vitamin administration had no effect on the incidence of cardiovascular events Together, these results indicate that the global antioxidants vitamin C and E have little to no effect on heart disease.
NAC is a thiol-containing antioxidant that has the ability to regenerate intracellular antioxidant pools, as NAC deacetylation produces cysteine, which is a precursor for reduced GSH.
Initial small trials evaluating the efficacy of intravenous NAC on acute MI and ischemic heart disease have shown promise. In the 30 patient ISLAND Infarct Size Limitation: Acute NAC Defense trial to assess the effect of NAC on reperfusion injury, NAC administration in combination with streptokinase-mediated recanalization reduced infarct size and improved left ventricular EF In addition to ROS scavenging, NAC has the ability to potentiate the vasodilation, hemodynamic, and anticoagulation effects of nitroglycerin; 88 thus, the combinatorial use of NAC together with nitroglycerin has been a strategy of great interest.
Preliminary trials assessing the effect of NAC together with nitroglycerin and streptokinase have been promising, with patients given NAC displaying reduced oxidative stress and enhanced ventricular function 89 , Moreover, the subsequent NACIAM NAC in Acute Myocardial Infarction trial, evaluating the use of high-dose NAC in combination with low-dose nitroglycerin in ST-segment elevation myocardial infarction patients with percutaneous coronary intervention, also showed that combined NAC and nitroglycerin treatment could significantly reduce infarct size and increase myocardial salvage While the success of these preliminary clinical trials with limited number of patients is encouraging, larger multicenter clinical trials are needed to determine if the beneficial effects of combined NAC and nitroglycerin treatment can be replicated and broadly applied.
In light of the limited success of general antioxidants in mitigating heart disease and the strong preclinical animal studies supporting the idea that limiting ROS production or enhancing ROS scavenging in the mitochondrial compartment can be highly beneficial to the heart, in recent years, considerable effort has been directed towards developing mitochondria-targeted antioxidants as pharmacological agents to ameliorate disease.
MitoTEMPO detoxifies superoxide by cycling between its nitroxide and oxoammonium forms, as well as oxidizing ferrous iron to limit hydroxyl radical formation MitoTEMPO has been studied in a wide range of animal models of cardiac disease.
Importantly, the administration of mitoTEMPO after the onset of cardiac hypertrophy was protective, suggesting that cardiac remodeling can be reversed and that mitochondrial ROS scavenging can be deployed as a therapy following the onset of disease The protective effects of mitoTEMPO extend to the murine TAC model of chronic pressure overload as long-term mitoTEMPO administration markedly improves both mitochondrial respiratory chain function and cardiac contractile performance In vitro, mitoQ effectively reduces oxidative damage and protects against cell death induced by hydrogen peroxide as well as chemically induced IR 96 , In vivo, rats administered mitoQ in drinking water display reduced mitochondrial damage, cytochrome c release, caspase 3 activation, and cardiomyocyte death in a Langendorff isolated heart model of IRI Importantly, this reduction in cardiomyocyte loss was accompanied by enhanced cardiac contractility and preserved mitochondrial respiratory chain function, suggesting that mitoQ is both mitoprotective and cardioprotective In line with its mitoprotective nature, mitoQ-treated rats subjected to prolonged TAC also displayed preserved mitochondrial membrane potential and respiratory chain function as well as reduced sensitivity to oxidant-induced MPTP However, while mitoQ reduced right ventricular hypertrophy and lung edema, mitoQ treatment had no effect on cardiac function Taken together, these studies suggest that mitoQ-mediated ROS scavenging may be most efficacious when used in specific disease contexts, such as cardiac IRI.
At the inner membrane, SS binds to cardiolipin, which is an inner membrane-specific phospholipid with a central role in the regulation of cristae architecture, mtDNA nucleoid distribution, respiratory chain complex integrity, and supercomplex organization While the main function of cytochrome c in the respiratory chain is to shuttle electrons between complex III and complex IV, cytochrome c can also function as a peroxidase and thereby mediate mitochondrial oxidative damage.
This balance between the electron carrier and peroxidase activities of cytochrome c is controlled by cardiolipin.
Hydrophobic binding between cardiolipin and cytochrome c facilitates a partial unfolding of cytochrome c that promotes peroxidase activity.
SS works by binding to cardiolipin and disrupting the cardiolipin—cytochrome c interaction, thereby promoting the function of cytochrome c as a respiratory chain electron carrier SS has been widely studied, and in vitro, it has been found to limit mitochondrial ROS production, reduce oxidative damage, and improve mitochondrial bioenergetics In vivo, SS has shown incredible promise to limit myocardial damage and promote cardiac function in animal models of ischemic injury and HF.
In the context of cardiac IRI, the administration of SS either at the onset of ischemia or even just prior to reperfusion significantly reduces infarct size in mouse, rat, guinea pig, and rabbit models , , Additionally, SS has been demonstrated to reduce the extent of microvascular damage in an ex vivo rabbit model of IR, collectively supporting the concept that mitochondrial ROS plays a significant role in reoxygenation-induced myocardial damage and that SS is cardioprotective.
In the setting of HF, SS restricts cardiac hypertrophy, reduces fibrosis, improves cardiac function in mice subjected to TAC , and, importantly, profoundly reduces the degree of mitochondrial ultrastructural and proteomic changes that occur Similar to the cardioprotective effects observed in mice, Elamipretide was highly protective in a canine model of microembolism-induced HF.
Dogs subjected to a 3-month chronic daily Elamipretide regimen following HF onset displayed decreased ROS burden, enhanced cardiac EF, a normalization of serum biomarkers of HF, as well as preserved mitochondrial function Collectively, these animal studies suggest that mitochondrial oxidative damage is a fundamental pathogenic mechanism in both IRI and HF and that targeting mitochondrial oxidative stress through cardiolipin would be of great therapeutic benefit.
In light of the promising results of drug-based mitochondrial ROS scavenging in animal models of heart disease, there has been intense interest in translating these benefits into patients.
SS has garnered the most interest due to its efficacy in numerous preclinical animal trials. However, the translation of the benefits of SS into use in human patients has proven challenging.
Recently, Elamipretide was tested in a double-blind and placebo-controlled trial in patients presenting with HF with reduced EF Importantly, patients treated with high-dose Elamipretide displayed a significant decrease in left ventricular end diastolic and end systolic volumes 13 , suggesting that Elamipretide can improve cardiac function in the context of HF.
However, in the EMBRACE STEMI multicenter phase IIa trial to assess the effect of MPT in patients with ST-elevated myocardial infarction, MPT, while safe and well tolerated in patients, had no significant impact on infarct size or ventricular function Taken together, the safety profile of SSbased compounds in patients, together with the preliminary success with acute treatment in the setting of HF with reduced EF, suggest that antioxidant peptide-mediated mitochondrial ROS scavenging holds promise for use in the clinical setting.
However, the EMBRACE STEMI trial highlights the challenges of translating drug efficacy in animal studies to benefits in patients. Elucidating the optimal dosing, timing, and duration of drug administration and understanding whether these parameters are specific for different types of cardiac disease will need to be at the forefront of research efforts as we move to bring mitochondria-targeted antioxidant therapy from the bench to the bedside.
In recent decades, significant progress has been made in developing therapeutics to preserve mitochondrial integrity and attenuate oxidative stress in heart disease. Many of these potential treatments show great promise in vitro, and one of the greatest challenges we now face is translating efficacy in preclinical animal studies into therapies for ROS-mediated cardiomyopathies in human patients.
In making the transition from the bench to the bedside, our understanding of some key issues will need to be expanded. Critically, we will need to understand what types of heart disease would most benefit from mitochondrial antioxidant therapy and if there are some disease modalities that would benefit from global versus mitochondria-targeted strategies.
In addition, in the design of therapeutic regimens, it will be essential to know at what stages of disease development mitochondrial antioxidant therapies should be optimally deployed. Moreover, as the mitochondrial ROS pool plays a significant role in disease pathogenesis, the development of compounds with improved mitochondrial targeting and enhanced antioxidant activity will be key to oxidative stress-based approaches to treating human cardiovascular diseases.
In addition to new and more efficacious mitochondrial ROS scavenging compounds, given that ROS can play a role in both physiological and pathological signaling, understanding the differences between these two modes and developing strategies to mitigate pathological ROS damage while preserving physiological ROS signaling will be an important next challenge.
Burgoyne, J. Redox signaling in cardiac physiology and pathology. CAS PubMed Google Scholar. Richardson, A. The role of macromolecular damage in aging and age-related disease. A 69 Suppl. CAS Google Scholar. Maulik, S. Oxidative stress and cardiac hypertrophy: a review.
Methods 22 , — Tsutsui, H. Oxidative stress and heart failure. Heart Circ. Misra, M. Oxidative stress and ischemic myocardial syndromes. Liu, Q. Diabetic cardiomyopathy and its mechanisms: role of oxidative stress and damage. Diabetes Investig. PubMed PubMed Central Google Scholar. Li, Y.
et al. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Ardanaz, N. Lack of glutathione peroxidase 1 accelerates cardiac-specific hypertrophy and dysfunction in angiotensin II hypertension. Hypertension 55 , — Chen, Z.
Pineal Res. Hu, C. Loss of thioredoxin 2 alters mitochondrial respiratory function and induces cardiomyocyte hypertrophy. Cell Res.
Huang, Q. Thioredoxin-2 inhibits mitochondrial reactive oxygen species generation and apoptosis stress kinase-1 activity to maintain cardiac function. Circulation , — CAS PubMed PubMed Central Google Scholar. Ye, Y. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: a meta-analysis of randomized controlled trials.
PLoS ONE 8 , e Daubert, M. Novel mitochondria-targeting peptide in heart failure treatment: a randomized, placebo-controlled trial of Elamipretide. Heart Fail 10 , e Pryde, K. Superoxide is produced by the reduced flavin in mitochondrial complex I: a single, unified mechanism that applies during both forward and reverse electron transfer.
Kushnareva, Y. Biochem J. Bleier, L. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Acta , — Turrens, J. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Orsini, F.
The life span determinant p66Shc localizes to mitochondria where it associates with mitochondrial heat shock protein 70 and regulates trans-membrane potential. Giorgio, M. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell , — Kuroda, J.
NADPH oxidase 4 NOX4 is a major source of oxidative stress in the failing heart. Natl Acad. USA , — Block, K. Subcellular localization of NOX4 and regulation in diabetes. Shanmugasundaram, K. NOX4 functions as a mitochondrial energetic sensor coupling cancer metabolic reprogramming to drug resistance.
Takac, I. The E-loop is involved in hydrogen peroxide formation by the NADPH oxidase NOX4. Suzuki, Y. Human copper chaperone for superoxide dismutase 1 mediates its own oxidation-dependent import into mitochondria. PubMed Google Scholar. Kawamata, H. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space.
Redox Signal. Chen, Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. Radi, R. Detection of catalase in rat heart mitochondria. Andreyev, A. Mitochondrial ROS metabolism: 10 years later. Biochemistry Mosc.
Mari, M. Mitochondrial glutathione, a key survival antioxidant. Perkins, A. Peroxiredoxins: guardians against oxidative stress and modulators of peroxide signaling.
Trends Biochem. Murphy, E. Mechanisms underlying acute protection from cardiac ischemia—reperfusion injury. Haworth, R. Hunter, D. The protective mechanisms. Kwong, J. Physiological and pathological roles of the mitochondrial permeability transition pore in the heart. Cell Metab. Hausenloy, D. Inhibiting mitochondrial permeability transition pore opening at reperfusion protects against ischaemia—reperfusion injury.
Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? The mitochondrial permeability transition pore: its fundamental role in mediating cell death during ischaemia and reperfusion.
Cung, T. Cyclosporine before PCI in patients with acute myocardial infarction. Trankle, C. Mitochondrial membrane permeability inhibitors in acute myocardial infarction: still awaiting translation.
JACC Basic Transl. Zhang, M. Contractile function during angiotensin-II activation: increased NOX2 activity modulates cardiac calcium handling via phospholamban phosphorylation.
NADPH oxidase-4 mediates protection against chronic load-induced stress in mouse hearts by enhancing angiogenesis. NADPH oxidases in heart failure: poachers or gamekeepers?
Maack, C. Oxygen free radical release in human failing myocardium is associated with increased activity of rac1-GTPase and represents a target for statin treatment. Heymes, C. Increased myocardial NADPH oxidase activity in human heart failure.
Dikalov, S. Angiotensin II-induced production of mitochondrial reactive oxygen species: potential mechanisms and relevance for cardiovascular disease. Varga, Z. Alternative splicing of NOX4 in the failing human heart. Duncan, J.
Mitochondrial dysfunction in diabetic cardiomyopathy. Alsaied, T. Factors associated with long-term mortality after Fontan procedures: a systematic review. Heart , — Teshima, Y. Production of reactive oxygen species in the diabetic heart. Roles of mitochondria and NADPH oxidase. Schilling, J. The mitochondria in diabetic heart failure: from pathogenesis to therapeutic promise.
Szabadkai, G. Physiology Bethesda 23 , 84—94 Dabkowski, E. Mitochondrial dysfunction in the type 2 diabetic heart is associated with alterations in spatially distinct mitochondrial proteomes.
Flarsheim, C. Mitochondrial dysfunction accompanies diastolic dysfunction in diabetic rat heart. Trifunovic, A. Premature ageing in mice expressing defective mitochondrial DNA polymerase.
Nature , — Dai, D. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell 9 , — Somatic mtDNA mutations cause aging phenotypes without affecting reactive oxygen species production. Shabalina, I. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1.
Aging Albany NY 9 , — Trinei, M. A pp66Shc signalling pathway controls intracellular redox status, levels of oxidation-damaged DNA and oxidative stress-induced apoptosis. Oncogene 21 , — Migliaccio, E. The p66shc adaptor protein controls oxidative stress response and life span in mammals.
Editorial on the Research Topic Oxidative Stress Herbal energy supplements Cardiovascular Oxidative stress and cardiovascular diseases and Pulmonary Diabetic coma education. Cardiovascular and pulmonary diseases are cadiovascular leading catdiovascular of death worldwide anv2. Various pathogenic risk factors, including oxidative Oxidative stress and cardiovascular diseases have detrimental effects on the heart and lung tissues, thereafter, causing changes to various disease states 34. In general, oxidative stress leads to an imbalance between the production of reactive oxygen species ROS and antioxidant defenses. The production of ROS is dependent on various enzymes, including the nicotinamide adenine dinucleotide phosphate NADPH oxidase NOXsxanthine oxidase, and the mitochondrial electron transport chain 5.Video
Role of oxidative stress in atherosclerosis Cradiovascular of Translational Medicine volume 21Herbal energy supplements number: Cite this article. Acrdiovascular details. Cardiovascular Herbal energy supplements Oxidafive continue to exert a significant impact on Herbal energy supplements Oxidaitve rates, encompassing conditions like pulmonary arterial hypertension PAHcardiovascklar ASand Dietary choices for prevention infarction MI. Oxidative Herbal energy supplements OS plays a cardiovasculat role in the pathogenesis and advancement of CVDs, highlighting its significance as a contributing factor. Maintaining an equilibrium between reactive oxygen species ROS and antioxidant systems not only aids in mitigating oxidative stress but also confers protective benefits on cardiac health. Herbal monomers can inhibit OS in CVDs by activating multiple signaling pathways, such as increasing the activity of endogenous antioxidant systems and decreasing the level of ROS expression. Given the actions of herbal monomers to significantly protect the normal function of the heart and reduce the damage caused by OS to the organism.
0 thoughts on “Oxidative stress and cardiovascular diseases”