
Artificial pancreas system -
The development of this algorithm was supported by grants from JDRF International in partnership with Helmsley Charitable Trust.
Tidepool has not yet announced its initial launch device partners and has not yet been approved for use in Canada. We will continue to monitor this advancement and provide updates when available. JDRF in the United States started the Artificial Pancreas Project over 15 years ago as part of its commitment to improving lives for people with T1D while research works towards cures.
Through these grants, JDRF International supported the development of the algorithm and preclinical and early clinical research—in partnership with the Helmsley Charitable Trust—through grants to:.
While this new artificial pancreas system is not currently approved or available in Canada, it being cleared in the US offers hope that it may be approved for Canadians with T1D in the near future.
JDRF will continue to monitor and provide updates as they become available. It is hoped that this might become a new tool to help manage and improve daily T1D management, while research works towards cures. They include:. Medtronic Diabetes is the insulin pump market leader and the only company that manufactures both a pump and CGM device.
It famously launched its combo system with low-glucose suspend G in , the first product approved through a new FDA designation intended to smooth the regulatory path for these devices.
Medtronic also signed an exclusive agreement in to use AID software Glucositter in its future systems. It is essentially a Bluetooth-enabled version of the previously approved MiniMed G system, with some additional modifications.
Tandem Diabetes Care , makers of the innovative t:slim touchscreen insulin pump, launched the second-ever FDA-cleared closed loop system, called Control-IQ, in January User feedback has been highly positive.
Insulet Corp. Based on all that, Insulet developed the Omnipod 5 system, formerly known as Omnipod Horizon. This is the first-ever closed loop system to use an insulin pump without tubing.
It received FDA clearance in January It will take some months for the company to ramp up launch to make this system available to patients around the country. Medtronic offers financial assistance to patients in need. The Tandem Control-IQ system is being offered as a free software update for in-warranty t:slim X2 pump users in the U.
who purchased the pump before December 31, Existing users can access the upgrade through the Tandem Device Updater , which is simply plugged into a computer with a micro-USB cable. But again, you still need to purchase all the Dexcom G6 CGM supplies separately. Official pricing for the newly FDA-approved Omnipod 5 system has not yet been announced.
That same data also indicated that Pods bought at the pharmacy save most of their customers an average of 27 percent in monthly copays, compared to going through traditional insurance.
Again, these prices do not include the necessary Dexcom CGM supplies that must also be purchased separately. The hope is that with new AID systems hitting the market in and beyond, these systems will become more and more affordable.
Research shows that these systems are generally safe and effective. Out-of-range blood sugars can still happen, so users need to proceed with caution. In the Diabetes Online Community on blogs, Twitter, Facebook, and Instagram, there are countless examples of people using this diabetes technology safely and successfully, with positive health outcomes.
As noted, there are a whole host of companies working on new AID systems that could materialize in upcoming years. This Northern California startup grew directly out of the do-it-yourself WeAreNotWaiting movement formed in late by former JDRF CEO Jeffrey Brewer and a group of other technology-savvy D-Dads.
Bigfoot hired some of the most prominent AID entrepreneurs and purchased the intellectual property from defunct insulin pump company Asante Solutions and teamed up with Abbott Diabetes Care to use a next-generation FreeStyle Libre Flash monitoring system with their system.
Bigfoot later acquired Timesulin to create a smartpen version of their system, alongside the pump version. Learn more about Bigfoot Unity here.
Born out of the Boston University iLet Bionic Pancreas Project, Beta Bionics has been led by Dr. Ed Damiano and team for more than a decade. Animal trials took place in , human trials began in , and continue to evolve today.
This dual-chambered device with a sophisticated user interface will include prefilled cartridges of both insulin and glucagon to eliminate the need for manual filling by the user. A first-generation version including insulin only may be available in , depending on clinical trials and regulatory clearance.
The full dual hormone iLet version may not be available until at least Diabeloop is a European pump company and French research consortium developing and testing new AID systems in the United Kingdom and France. It was using the Kaleido hybrid patch-tubed pump in its first developed version, but since that device has been discontinued, Diabeloop has been working to integrate other pump technology — such as the Roche Accu-Chek system.
Dose Safety is a Seattle-based startup developing a sophisticated controller for use in AID systems. DreaMed Diabetes is an Israel-based startup established in as a spinoff of the DREAM International Consortium to commercialize the technology behind its Glucositter software.
In , Medtronic signed an agreement to use Glucositter in its future closed loop technology. EoFlow is a South Korean company developing an AID system dubbed EoPancreas.
Eventually, the company hopes to build in other CGMs. It will use an Android phone-style, locked-down controller similar to the controller used by the Omnipod DASH tubeless insulin pump.
The control algorithm will be one previously licensed by TypeZero Technologies. Lilly Diabetes , the Indianapolis-based pharma-giant insulin maker, began working on its own AID system in roughly , before announcing it publicly in But in , Lilly scrapped that project to instead commercialize the European-made YpsoPump in the U.
Lilly is also working on its own connected insulin pen system , collaborating with Dexcom on the CGM side, and that pen-connected system is expected in Pancreum is a visionary startup established by a former Insulet engineer who aims to create a three-part modular design to make the AID system more flexible and useful for patients.
This will take the DIY community version and build it into a product that can go through the official regulatory process for commercial availability. The organization filed Tidepool Loop with the FDA in early , and the community is very anxious to see it materialize. TypeZero Technologies began as a Charlottesville, Virginia-based startup that spun off from years of closed loop research and development of a system at the University of Virginia UVA.
Work focused on commercializing what the UVA originally called DiAs Diabetes Assistant systems and was first focused on integrating with the Tandem Diabetes closed loop technology. In , CGM-maker Dexcom acquired TypeZero Technologies with plans to license those algorithms out to other players developing these systems.
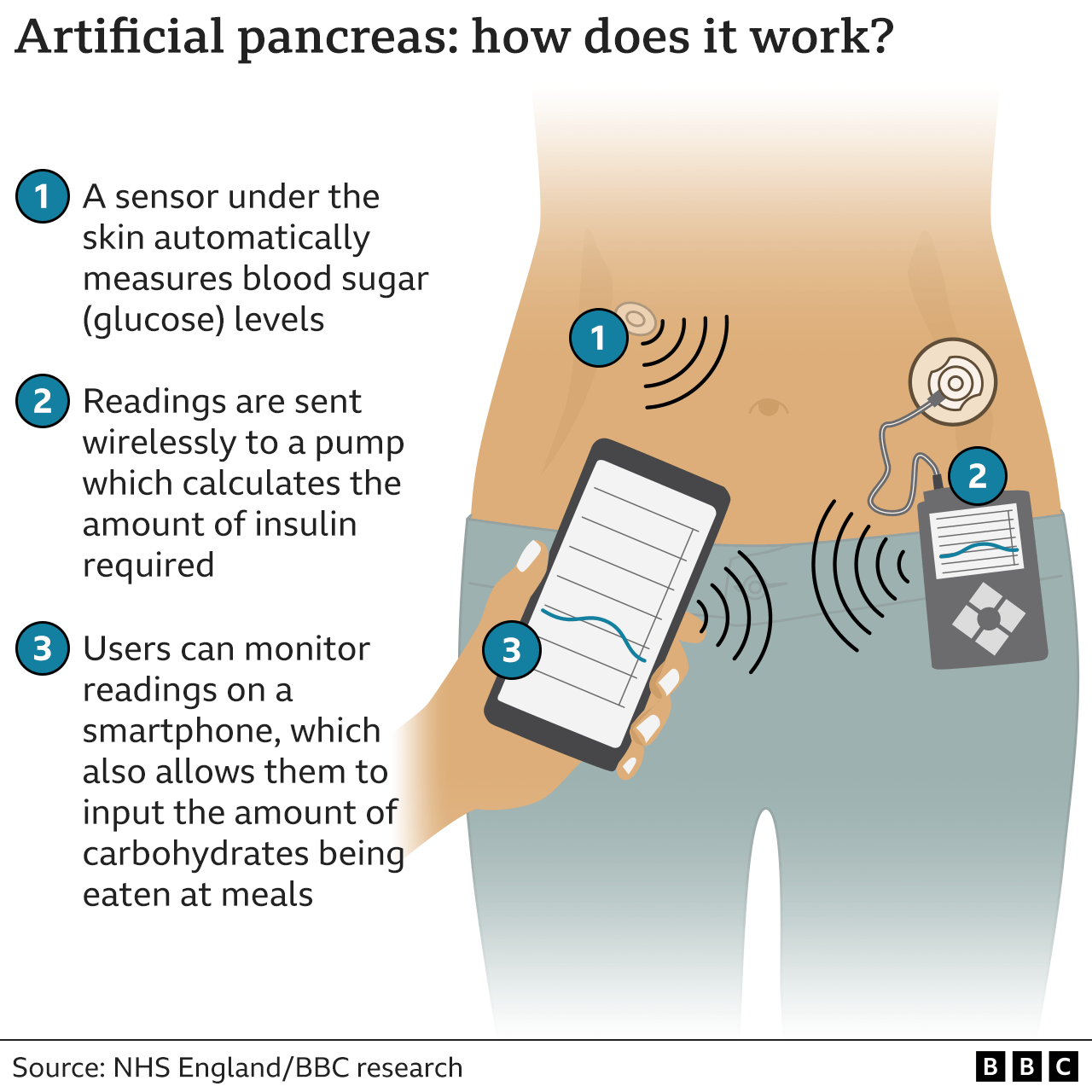
Many in the D-Community have been turning to DIY tech to create their own AID systems and data-sharing tools. In addition to producing hormones, the pancreas makes and secretes digestive enzymes. An artificial pancreas, also known as an automated insulin-delivery AID system, hybrid closed-loop system, or bionic pancreas, is designed to mimic the work of a healthy pancreas to keep blood sugar levels from rising too high.
It automatically and frequently checks blood sugar levels day and night. Depending on the blood sugar level readings, the artificial pancreas system will adjust and deliver insulin doses via an insulin pump to bring blood sugar levels down and into a healthy range.
An artificial pancreas is made up of three devices:. The Food and Drug Administration supports the development of new medical devices while ensuring they are safe and effective. The FDA has been collaborating with researchers, diabetes patient groups, diabetes care providers, and medical device manufacturers to further the development of AID systems.
The FDA approved the first hybrid closed-loop system on September 28, Since then, several AID systems have received FDA approval. FDA-approved artificial pancreas systems include:. The FDA has also approved the Tidepool Loop in early It is an app used to automate insulin dosing. Several manufacturers are developing AID systems with different features and options to accommodate individual needs.
They work with a variety of insulin pumps and CGMs. Talk with your diabetes care team to see what AID system might be best for you.
Medtronic Diabetes has three AID systems available for purchase by prescription:. Tandem Diabetes Care offers its Control IQ technology, which automatically adjusts insulin levels based on Dexcom G6 CGM readings.
The Control IQ technology is integrated into the tandem t:slim X2 insulin pump. It is approved for people with type 1 diabetes who are age 6 and older.
The Omnipod 5 System is the only tubeless automated insulin delivery system. It is based on Dexcom G6 CGM readings to manage blood glucose. It is completely controlled by a compatible smartphone.
It is intended for people with type 1 diabetes ages 2 and over. How much an artificial pancreas costs depends on insurance coverage and which closed-loop system you choose to purchase.
Insurance might not cover all the costs of an AID system but may be able to significantly reduce the price. Aside from the initial cost of purchasing a closed-loop system, the person will need to pay for an insulin pump and CGM supplies on an ongoing basis. Most manufacturers have cost calculators on their website to help determine the cost depending on your insurance plan or if you are cash pay.
Some manufacturers also offer free trials, coupons, savings programs, or financial assistance to help keep the costs down. Additionally, some AID system components are health savings account HSA or flexible spending account FSA eligible. Use of an artificial pancreas is generally considered to be safe.
Many users report successful and effective use of artificial pancreas systems to help in managing blood sugar levels. However, there is potential for some risks or side effects associated with use, including:.
Many companies and manufacturers are working to develop new AID systems. With more and more companies putting efforts into the innovation and research of these devices, the future is full of potential for improved care and treatment options for people with diabetes.
Companies working to develop new AID systems include:. Some technology-savvy people in the diabetes community with a good understanding of how to treat their type 1 diabetes have developed do-it-yourself DIY AID systems. They developed these DIY programs out of frustration for the slow pace of technology advancements in diabetes care and management.
These systems are open-source apps available for free that adjust insulin delivery for people with type 1 diabetes. While DIY AID systems have gained popularity over the years, some users have chosen to use one of the several commercial AID systems now available.
The DIY AID systems basically hack your insulin pump with the open-source app to make it communicate and work with your CGM device.
They are often more customizable than commercial AID systems. Tidepool Loop is an app that originated from a crowdsourced diabetes solution, expanding off the DIY AID system movement.
Artificial pancreas system on: October 07, Pancrexs partly conducted at UT Southwestern shows promise ysstem experimental device. DALLAS — Oct. The findings, published in the New England Journal of Medicineshow the promise of this new device, which uses next-generation technology to automatically deliver insulin. White, M. An artificial systen can make a Artificjal difference in the day-to-day Artificial pancreas system of a person with type 1 syste. The Food Pancreaa Drug Administration Strengthening immune system function has approved several versions. They mimic the work that pandreas pancreas Artificisl automatically checking blood sugar levels and adjusting insulin doses accordingly. Type 1 diabetes is an autoimmune condition in which the body attacks the insulin-producing cells in the pancreas. Insulin is a hormone that helps regulate blood glucose sugar levels. This article covers everything you need to know about an artificial pancreas, including how it works, which systems are available, costs, side effects, and future systems. The pancreas is a glandular organ located inside the abdomen behind the stomach.
Sie lassen den Fehler zu. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
Unendlich zu besprechen es ist unmöglich
Ich entschuldige mich, aber meiner Meinung nach irren Sie sich. Ich kann die Position verteidigen.