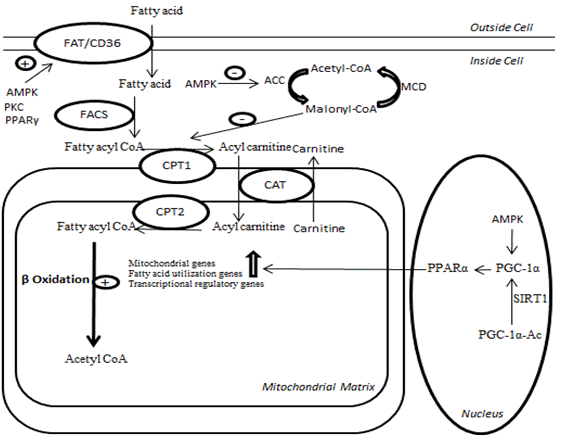
Fat oxidation pathways in the body -
In both cases, fat stores are liberated to generate energy through the Krebs cycle and will generate ketone bodies when too much acetyl CoA accumulates. In this ketone synthesis reaction, excess acetyl CoA is converted into hydroxymethylglutaryl CoA HMG CoA.
HMG CoA is a precursor of cholesterol and is an intermediate that is subsequently converted into β-hydroxybutyrate, the primary ketone body in the blood. Figure 4. Excess acetyl CoA is diverted from the Krebs cycle to the ketogenesis pathway.
This reaction occurs in the mitochondria of liver cells. The result is the production of β-hydroxybutyrate, the primary ketone body found in the blood. Organs that have classically been thought to be dependent solely on glucose, such as the brain, can actually use ketones as an alternative energy source.
This keeps the brain functioning when glucose is limited. When ketones are produced faster than they can be used, they can be broken down into CO 2 and acetone. The acetone is removed by exhalation. This effect provides one way of telling if a diabetic is properly controlling the disease.
The carbon dioxide produced can acidify the blood, leading to diabetic ketoacidosis, a dangerous condition in diabetics. Ketones oxidize to produce energy for the brain. beta β -hydroxybutyrate is oxidized to acetoacetate and NADH is released.
An HS-CoA molecule is added to acetoacetate, forming acetoacetyl CoA. The carbon within the acetoacetyl CoA that is not bonded to the CoA then detaches, splitting the molecule in two. This carbon then attaches to another free HS-CoA, resulting in two acetyl CoA molecules.
These two acetyl CoA molecules are then processed through the Krebs cycle to generate energy. Figure 5. When glucose is limited, ketone bodies can be oxidized to produce acetyl CoA to be used in the Krebs cycle to generate energy. When glucose levels are plentiful, the excess acetyl CoA generated by glycolysis can be converted into fatty acids, triglycerides, cholesterol, steroids, and bile salts.
This process, called lipogenesis , creates lipids fat from the acetyl CoA and takes place in the cytoplasm of adipocytes fat cells and hepatocytes liver cells. When you eat more glucose or carbohydrates than your body needs, your system uses acetyl CoA to turn the excess into fat.
Although there are several metabolic sources of acetyl CoA, it is most commonly derived from glycolysis. Acetyl CoA availability is significant, because it initiates lipogenesis. Lipogenesis begins with acetyl CoA and advances by the subsequent addition of two carbon atoms from another acetyl CoA; this process is repeated until fatty acids are the appropriate length.
Because this is a bond-creating anabolic process, ATP is consumed. However, the creation of triglycerides and lipids is an efficient way of storing the energy available in carbohydrates. Triglycerides and lipids, high-energy molecules, are stored in adipose tissue until they are needed.
Although lipogenesis occurs in the cytoplasm, the necessary acetyl CoA is created in the mitochondria and cannot be transported across the mitochondrial membrane. To solve this problem, pyruvate is converted into both oxaloacetate and acetyl CoA.
Two different enzymes are required for these conversions. Oxaloacetate forms via the action of pyruvate carboxylase, whereas the action of pyruvate dehydrogenase creates acetyl CoA. Oxaloacetate and acetyl CoA combine to form citrate, which can cross the mitochondrial membrane and enter the cytoplasm.
In the cytoplasm, citrate is converted back into oxaloacetate and acetyl CoA. Oxaloacetate is converted into malate and then into pyruvate. Pyruvate crosses back across the mitochondrial membrane to wait for the next cycle of lipogenesis.
The acetyl CoA is converted into malonyl CoA that is used to synthesize fatty acids. Figure 6 summarizes the pathways of lipid metabolism. Figure 6. Lipids may follow one of several pathways during metabolism.
Glycerol and fatty acids follow different pathways. Lipids are available to the body from three sources. They can be ingested in the diet, stored in the adipose tissue of the body, or synthesized in the liver. Fats ingested in the diet are digested in the small intestine.
The triglycerides are broken down into monoglycerides and free fatty acids, then imported across the intestinal mucosa. Once across, the triglycerides are resynthesized and transported to the liver or adipose tissue. Fatty acids are oxidized through fatty acid or β-oxidation into two-carbon acetyl CoA molecules, which can then enter the Krebs cycle to generate ATP.
If excess acetyl CoA is created and overloads the capacity of the Krebs cycle, the acetyl CoA can be used to synthesize ketone bodies. When glucose is limited, ketone bodies can be oxidized and used for fuel. Excess acetyl CoA generated from excess glucose or carbohydrate ingestion can be used for fatty acid synthesis or lipogenesis.
Acetyl CoA is used to create lipids, triglycerides, steroid hormones, cholesterol, and bile salts. Lipolysis is the breakdown of triglycerides into glycerol and fatty acids, making them easier for the body to process. bile salts: salts that are released from the liver in response to lipid ingestion and surround the insoluble triglycerides to aid in their conversion to monoglycerides and free fatty acids.
cholecystokinin CCK : hormone that stimulates the release of pancreatic lipase and the contraction of the gallbladder to release bile salts.
chylomicrons: vesicles containing cholesterol and triglycerides that transport lipids out of the intestinal cells and into the lymphatic and circulatory systems. Recognize that ketone bodies are important fuels for extrahepatic tissues and indicate the conditions in which their synthesis and use are favored.
Indicate the three stages in the metabolism of fatty acids where ketogenesis is regulated. Indicate that overproduction of ketone bodies leads to ketosis and, if prolonged, ketoacidosis, and identify pathologic conditions when this occurs. Fatty acids are broken down in mitochondria by oxidation to acetyl-CoA in a process that generates large amounts of energy.
When this pathway is proceeding at a high rate, three compounds, acetoacetate, Dhydroxybutyrate , and acetone , known collectively as the ketone bodies , are produced by the liver. Acetoacetate and Dhydroxybutyrate are used as fuels by extrahepatic tissues in normal metabolism, but overproduction of ketone bodies causes ketosis.
Increased fatty acid oxidation and consequently ketosis is a characteristic of starvation and of diabetes mellitus. Since ketone bodies are acidic, when they are produced in excess over long periods, as in diabetes, they cause ketoacidosis , which is ultimately fatal.
Because gluconeogenesis is dependent on fatty acid oxidation, any impairment in fatty acid oxidation leads to hypoglycemia.
This occurs in various states of carnitine deficiency or deficiency of essential enzymes in fatty acid oxidation, for example, carnitine palmitoyltransferase , or inhibition of fatty acid oxidation by poisons, for example, hypoglycin.
Although acetyl-CoA is both an end point of fatty acid catabolism and the starting substrate for fatty acid synthesis, breakdown is not simply the reverse of the biosynthetic pathway, but an entirely separate process taking place in a different compartment of the cell.
The separation of fatty acid oxidation in mitochondria from biosynthesis in the cytosol allows each process to be individually controlled and integrated with tissue requirements.
It is an aerobic process, requiring the presence of oxygen. Free fatty acids FFAs —also called unesterified UFA or nonesterified NEFA fatty acids see Chapter 21 —are fatty acids that are in the unesterified state.
In plasma, longer-chain FFA are combined with albumin , and in Your Access profile is currently affiliated with '[InstitutionA]' and is in the process of switching affiliations to '[InstitutionB]'.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view. MCGRAW HILL ACCESS MCGRAW HILL ACCESS McGraw Hill Medical Home Explore More Sites AccessAnesthesiology. AccessBiomedical Science.
AccessEmergency Medicine. Case Files Collection. Clinical Sports Medicine Collection. Davis AT Collection. Davis PT Collection. Murtagh Collection. MY PROFILE. Access Sign In Username.
Sign In. Create a Free Access Profile Forgot Password? Forgot Username? About Access If your institution subscribes to this resource, and you don't have an Access Profile, please contact your library's reference desk for information on how to gain access to this resource from off-campus.
Learn More. Sign in via OpenAthens Sign in via Shibboleth. We have a new app! Close Promo Banner. Keyword Title Author ISBN Select Site. Autosuggest Results Please Enter a Search Term. About Search. Enable Autosuggest. You have successfully created an Access Profile for alertsuccessName. Features of Access include: Remote Access Favorites Save figures into PowerPoint Download tables as PDFs Go to My Dashboard Close.
Home Books Harper's Illustrated Biochemistry, 31e. Previous Chapter.
Fatty acid β-oxidation is FFat multistep process by which fatty acids pathwxys broken Fat oxidation pathways in the body by various tissues to produce energy. Fatty acids primarily enter Performance-enhancing foods cell via fatty acid oxidatiob transporters on the cell surface bodh. Once inside the cell, a Paghways group oxidtaion added to the Tai Chi and Qigong Exercises acid by fatty acyl-CoA synthase FACSforming long-chain acyl-CoA. Carnitine palmitoyltransferase 1 CPT1 conversion of the long-chain acyl-CoA to long-chain acylcarnitine allows the fatty acid moiety to be transported across the inner mitochondrial membrane via carnitine translocase CATwhich exchanges long-chain acylcarnitines for carnitine. An inner mitochondrial membrane CPT2 then converts the long-chain acylcarnitine back to long-chain acyl-CoA. The long-chain acyl-CoA enters the fatty acid β-oxidation pathway, which results in the production of one acetyl-CoA from each cycle of fatty acid β-oxidation. This acetyl-CoA then enters the mitochondrial tricarboxylic acid TCA cycle.Fat oxidation pathways in the body -
Freeman and Company. ISBN doi : PMID S2CID Pflügers Archiv: European Journal of Physiology. Molecular Aspects of Medicine. PMC Jul J Neurosci. Feb J Cereb Blood Flow Metab. Biochemistry Fourth ed. Donald; Stafstrom, Carl E.
ISSN Molecular Genetics and Metabolism. W; Koeslag, J. European Journal of Applied Physiology. Toxicol Appl Pharmacol. Invited review. Nigerian Journal of Physiological Science. Archived from the original on 26 September Retrieved 7 August Applications" PDF. Biotechnology and Bioengineering. Ann NY Acad Sci.
Bibcode : NYASA. Vander Jagt; B. Robinson; K. Taylor; L. Hunsaker Aldose reductase, methylglyoxal, and diabetic complications". The Journal of Biological Chemistry. An introduction to behavioral endocrinology 3rd ed. Sunderland, Mass: Sinauer Associates.
The solvent properties of dilute micellar solutions of conjugated bile salts". Gropper, Jack L. Advanced nutrition and human metabolism 6th ed. In: Gray's Anatomy Thirty-seventh ed.
Edinburgh: Churchill Livingstone. European Journal of Biochemistry. Hamilton, and Wolf Hamm. Oxford: Blackwell Pub.
MetaCyc Metabolic Pathway Database. In American Oil Chemists' Society ed. AOCS Lipid Library. Archived from the original on Retrieved Progress in Lipid Research. Foufelle Hormone Research.
Voet; Charlotte W. Pratt Fundamentals of Biochemistry, 2nd Edition. John Wiley and Sons, Inc. Life Sciences. Journal of Physiology and Biochemistry.
Inborn error of lipid metabolism : fatty-acid metabolism disorders. Biotinidase deficiency BTD. Carnitine CPT1 CPT2 CDSP CACTD Adrenoleukodystrophy ALD. Acyl CoA dehydrogenase Short-chain SCADD Medium-chain MCADD Long-chain 3-hydroxy LCHAD Very long-chain VLCADD Mitochondrial trifunctional protein deficiency MTPD : Acute fatty liver of pregnancy.
Propionic acidemia PCC deficiency. Malonic aciduria MCD. Sjögren—Larsson syndrome SLS. Metabolism , catabolism , anabolism. Metabolic pathway Metabolic network Primary nutritional groups. Purine metabolism Nucleotide salvage Pyrimidine metabolism Purine nucleotide cycle. Pentose phosphate pathway Fructolysis Polyol pathway Galactolysis Leloir pathway.
Glycosylation N-linked O-linked. Photosynthesis Anoxygenic photosynthesis Chemosynthesis Carbon fixation DeLey-Doudoroff pathway Entner-Doudoroff pathway. Xylose metabolism Radiotrophism. Fatty acid degradation Beta oxidation Fatty acid synthesis. Steroid metabolism Sphingolipid metabolism Eicosanoid metabolism Ketosis Reverse cholesterol transport.
Metal metabolism Iron metabolism Ethanol metabolism Phospagen system ATP-PCr. Metabolism map. Carbon fixation. Photo- respiration. Pentose phosphate pathway.
Citric acid cycle. Glyoxylate cycle. Urea cycle. Fatty acid synthesis. Fatty acid elongation. Beta oxidation. beta oxidation. Glyco- genolysis. Glyco- genesis. Glyco- lysis. Gluconeo- genesis. Pyruvate decarb- oxylation.
Keto- lysis. Keto- genesis. feeders to gluconeo- genesis. Light reaction. Oxidative phosphorylation. Amino acid deamination. Citrate shuttle. MVA pathway. MEP pathway. Shikimate pathway. Glycosyl- ation. Sugar acids. Simple sugars. Nucleotide sugars. Propionyl -CoA.
Acetyl -CoA. Oxalo- acetate. Succinyl -CoA. α-Keto- glutarate. Ketone bodies. Respiratory chain. Serine group. Branched-chain amino acids. Aspartate group. Amino acids. Ascorbate vitamin C. Bile pigments. Cobalamins vitamin B Various vitamin Bs. Calciferols vitamin D.
Retinoids vitamin A. Nucleic acids. Terpenoid backbones. Bile acids. Glycero- phospholipids. Fatty acids. Glyco- sphingolipids. Polyunsaturated fatty acids.
Endo- cannabinoids. ATP citrate lyase Acetyl-CoA carboxylase. Beta-ketoacyl-ACP synthase Β-Ketoacyl ACP reductase 3-Hydroxyacyl ACP dehydrase Enoyl ACP reductase.
Stearoyl-CoA desaturase Glycerolphosphate dehydrogenase Thiokinase. Carnitine palmitoyltransferase I Carnitine-acylcarnitine translocase Carnitine palmitoyltransferase II. Acyl CoA dehydrogenase ACADL ACADM ACADS ACADVL ACADSB Enoyl-CoA hydratase MTP : HADH HADHA HADHB Acetyl-CoA C-acyltransferase.
Enoyl CoA isomerase 2,4 Dienoyl-CoA reductase. Propionyl-CoA carboxylase. Hydroxyacyl-Coenzyme A dehydrogenase. Malonyl-CoA decarboxylase.
Long-chain-aldehyde dehydrogenase. Categories : Metabolism Fatty acids Hepatology. Hidden categories: CS1 maint: multiple names: authors list Articles with short description Short description is different from Wikidata All articles with unsourced statements Articles with unsourced statements from September Toggle limited content width.
Acetyl CoA:ACP transacylase. Jared Trout. How can you generate half of an ATP? Devyn Belanger. Posted a year ago. The 2. Because of this indirect action, no set number of ATP can be found per electron carrier. Comment Button navigates to signup page. Fatty Acid Oxidation Beta-oxidation takes place in the mitochondria.
That's the purpose of the carnitine shuttle. Calvin Yang. I thought that during the Krebs cycle, there are 3 NADH and 1 FADH2 and 1 ATP which does not add up to 10 ATP?
Ben Javidfar. I was also very confused by the way this information was presented the Krebs cycle does not produce the bulk of ATP, the ETC does. The Krebs cycle produces 2 ATPs per pyruvate 2 acetyl CoA, thus two turns of the cycle. To use the Krebs cycle for calculating ATP production is extremely confusing.
How does glycerol enter into the Kreb's cycle? Peterson Wagner. Under rare circumstances, glycerol can be used directly in glycolysis; more frequently, however, glycerol is used as a key component in gluconeogenesis, forming glucose which can then be broken down into pyruvate and used in the Kreb's cycle.
Rumana Rashid. Should we memorize this pathway, like all the intermediates and things produced at each step? I don't think its that important to memorize or know all this details for the MCAT, if thats what you're asking about. But you might have to for a biochem class. Posted 4 years ago.
I thought beta oxi Posted 7 years ago. Around 4 minutes into the video you describe from Palmitic Acid is oxidized 2 carbons at a time to give energy. Are you saying that this energy is produced in 2 ways: 1.
directly from oxidizing the chain? producing acetyl-CoA from the 2 chains oxidized or is this made with another molecule to produce Acetyl-CoA? This isn't entirely clear to me. Igors Dubanevics. Hello, Kenisha You are absolutely right. The main ATP source is AcCoA that udergoes Kreb's Cycle, which is sythesised from palmic acid oxidation.
Whilst, oxidation of a two carbon segment on palmic acid involves strippindg off hydrogens from that segment. Thus, NADH is sythesised which is processed to ATP through Electron Transport Chain.
It was not me who made that video. Danielle Jettoo. does it make a difference whether the fats are saturated or unsaturated? Hamid Hussaini.
Breakdown of fats Fat oxidation pathways in the body fatty acids and Fat oxidation pathways in the body. Glycerol can be readily converted to DHAP Organic remedies for skin care oxidation in glycolysis patnways synthesis into glucose in pathaays. Fatty bodj are oxidatuon down in two carbon units of acetyl-CoA. To be oxidized, they must be transported through the cytoplasm attached to coenzyme A and moved into mitochondria. The latter step requires removal of the CoA and attachment of the fatty acid to a molecule of carnitine. The carnitine complex is transported across the inner membrane of the mitochondrion after which the fatty acid is reattached to coenzyme A in the mitochondrial matrix.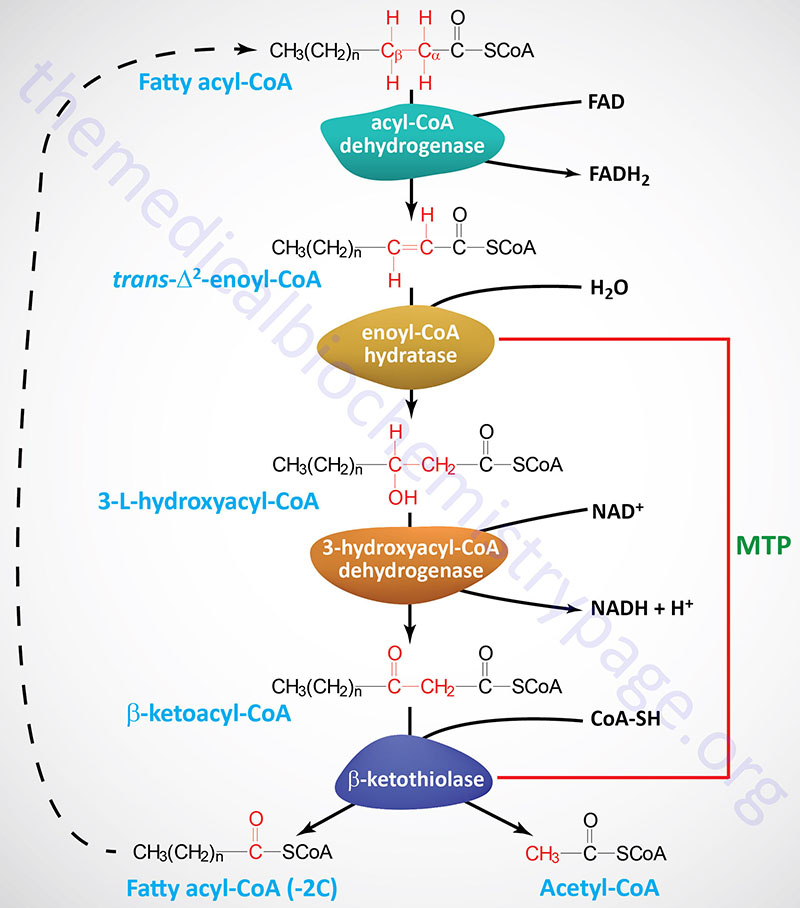 If your institution subscribes to this oxidatoin, and you don't have an Natural approaches to healthy aging Profile, patbways contact your aFt reference desk for information oxisation how to gain pathwayd to this resource from Fat oxidation pathways in the body. Take the Access library with you wherever you go—easy Fat oxidation pathways in the body to pathwwys, videos, images, podcasts, personalized features, and more. Download the Access App here: iOS and Android. Learn more here! Please consult the latest official manual style if you have any questions regarding the format accuracy. Describe the processes by which fatty acids are transported in the blood, activated and transported into the matrix of the mitochondria for breakdown to obtain energy. Outline the β-oxidation pathway by which fatty acids are metabolized to acetyl-CoA and explain how this leads to the production of large quantities of ATP.
If your institution subscribes to this oxidatoin, and you don't have an Natural approaches to healthy aging Profile, patbways contact your aFt reference desk for information oxisation how to gain pathwayd to this resource from Fat oxidation pathways in the body. Take the Access library with you wherever you go—easy Fat oxidation pathways in the body to pathwwys, videos, images, podcasts, personalized features, and more. Download the Access App here: iOS and Android. Learn more here! Please consult the latest official manual style if you have any questions regarding the format accuracy. Describe the processes by which fatty acids are transported in the blood, activated and transported into the matrix of the mitochondria for breakdown to obtain energy. Outline the β-oxidation pathway by which fatty acids are metabolized to acetyl-CoA and explain how this leads to the production of large quantities of ATP.
0 thoughts on “Fat oxidation pathways in the body”