
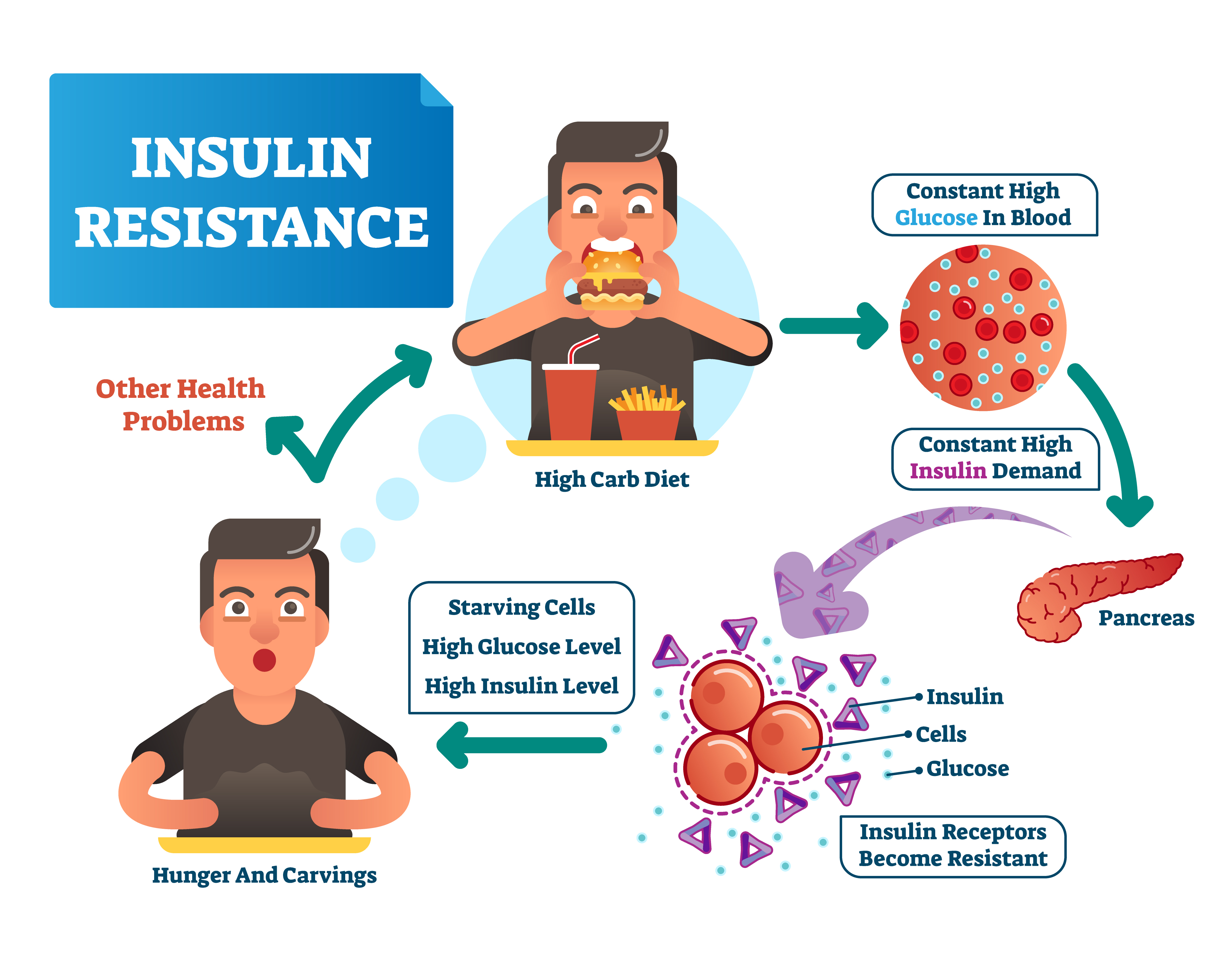
Visceral fat and insulin resistance -
Therefore, low adiponectin concentrations contribute to impaired glucose homeostasis and insulin resistance [ 35 ]. Insulin resistance manifests itself differentially in various tissues, which contributes to the overall phenotype of VAS.
For example, insulin resistance in: 1 WAT, results in lipolysis, increasing free fatty acids into the circulation, which exacerbate the deleterious cycle of hyperlipidemia—inflammation—insulin resistance; 2 the liver, results in increased hepatic glucose production via glycogenolysis and gluconeogenesis , contributing to hyperglycemia [ 32 ].
Insulin resistance in some of the former target sites aggravates glycemic homeostasis, increasing the formation of advanced glycation end products AGEs.
AGEs are derived from non-enzymatic reactions between glucose and proteins, nucleic acid and lipids. Endogenously derived AGEs, as well as exogenously generated AGEs formed during high-heat cooking, can promote inflammation.
This happens via receptors that bind to advanced glycation end products, such as RAGE advanced glycosylation end product-specific receptor , TLR4 and other receptors that regulate the activity of NFκB [ 47 ], constituting another link between diet and insulin resistance.
An interesting aspect that deserves attention concerns the effects of compensatory hyperinsulinemia: although it is usually considered a by-product of the insulin resistance, hyperinsulinemia exerts its effects, overstimulating certain pathways of insulin action in various cells [ 27 ]. For instance, the expression of Sterol Regulatory Element Binding Protein 1c SREBP1c , a transcription factor that modulates the expression of lipogenic enzymes, is increased by chronic hyperinsulinemia in the liver of mice exhibiting insulin resistance.
As a result, de novo fatty acid biosynthesis is enhanced and contributes to non-alcoholic fatty liver disease NAFLD [ 48 ], the hepatic component of the VAS. Another potential consequence of prolonged hyperinsulinemia is the exacerbation of inflammation since it has been demonstrated that in vitro, insulin exerts long-term proinflammatory action, by amplifying effects of the cytokine-NFkB axis [ 49 ].
If so, then hyperinsulinemia could be one of the mechanisms proposed to explain the increased frequency of cancer that accompanies the obesity epidemic [ 50 ]. Other mechanisms that may explain the increased cancer risk include the mitogenic effect of insulin through either insulin-like growth factor-1 IGF1 receptors or through hybrid IGF1-insulin receptors as well as the increased concentrations of free IGF1, as a consequence of the lower concentrations of IGF binding proteins 1 and 2 IGFBP1 and IGFBP2 in obese individuals [ 51 ].
During embryonic development, there is significant plasticity, which attempts to adjust the foetal gene expression to the environment, so that the resulting phenotype is adapted to the environmental conditions [ 52 ]. Thus, fetuses exposed to suboptimal conditions during intrauterine life for instance, protein-calorie undernutrition undergo alterations in gene expression to adjust.
However, if the post-natal life provides different conditions from those previously anticipated for instance, abundance of nutrients , the body will not be prepared for that environment, and is more likely to develop disease [ 53 ].
This ability to sustain the changes acquired in intrauterine life in the post-natal life relies on epigenetic mechanisms, namely, post-translational modifications within histones, DNA cytosine methylation and control of gene expression by micro-non-coding RNAs.
The Dutch famine has been used to investigate the effects of in utero stress among adults who were born in the western part of The Netherlands at the end of World War II. These individuals were exposed prenatally to undernutrition.
The Dutch Famine Cohort study has shown that individuals exposed to maternal undernutrition at any stage of gestation had increased glycaemia as adults.
Women exposed to undernutrition at early stages of gestation appeared to be more centrally obese than those not prenatally exposed to famine [ 56 ]. Interestingly, higher methylation of genes related to metabolic and cardiovascular diseases, such as the ones encoding leptin, IL10 and ABCA1 were found in individuals prenatally exposed to famine in comparison to their unexposed same-sex siblings [ 57 ].
In the same line of investigation, specific associations of low birth weight with VAS were observed in other populations [ 58 , 59 ], strongly suggesting that, besides environmental and genetic conditions, prenatal reprogramming participates in the susceptibility to the VAS.
Another player recently recognized as important in the development of VAS is the gut microbiota, comprised of approximately trillion microbes resident in the human intestines, the majority of them belonging to the phyla Firmicutes and Bacteroidetes.
The gut microbiota is influenced by diet composition, which, in turn, influences how food is processed in the gastrointestinal tract [ 60 ]. Pathobionts resident microbes with pathogenic potential [ 61 ] increase by diets enriched in saturated fat, damage the intestinal epithelial cell layer and enable the translocation of one key constituent of many bacteria, lipopolysaccharide LPS , from the gut lumen into the systemic circulation [ 62 ].
LPS molecules bind to TLRs, activating the proinflammatory signaling cascades previously mentioned and eliciting insulin resistance; in the liver, for instance, activation of TLR4 and TLR9 augments TNFα secretion and participates in NAFLD development [ 60 ].
On the other hand, a fiber-rich diet exerts beneficial effects on the microbiota composition, decreasing the Firmicutes : Bacteriodetes ratio and consequently increasing fiber degradation and the production of short-chain fatty acids, such as acetate, propionate, and butyrate [ 63 ], which are absorbed in the colon.
Acetate and propionate are substrates for lipogenesis and gluconeogenesis while butyrate provides energy for colonic epithelial cells [ 60 ]. Additionally, these compounds bind to widely expressed G-protein coupled receptors GPR41 and GPR43 , augmenting energy expenditure, insulin sensitivity, satiety and production of glucagon-like peptide 1 GLP1 and decreasing inflammation, thereby protecting against VAS [ 62 ].
The CNS is an important modulator of food intake through different hormonal and neuropeptide signaling pathways [ 64 ]. Neuropeptides regulate energy storage in white adipocytes and inhibit brown adipose tissue activation in mammals [ 65 ].
Experimental and clinical studies showed a relation between the autonomic nervous system, dietary intake and adipose tissue. Body fat distribution of body fat is a more important risk factor for the development of hypertension and cardiovascular disease than obesity generally.
Sympathetic nervous system SNS activity contributes to obesity-induced hypertension [ 66 ]. Independently of body fat distribution, sympathetic activity seems to be related to different components of the VAS.
By stratifying patients with similar obesity degrees according to the presence or absence of high blood pressure, we found a higher surrogate markers of sympathetic activity derived from spectral analysis and greater impairment in several components of Metabolic syndrome in those subjects with high blood pressure [ 67 ].
It suggest that high blood pressure carriage in pararel with sympathetic activity in this population. Nonetheless, SNS activity was associated to obesity in obese normotensive subjects [ 68 ]. The distribution of body fat seems to be more important to determine the cardiovascular risk than the whole body fat.
Alvarez et al. showed a higher sympathetic activity in men with visceral obesity compared to subcutaneous fat levels [ 69 ]. Leptin, an important product of visceral adipocytes, is related to increased sympathetic activity, with human obesity-induced hypertension, and associated to a increased termogenic metabolism [ 70 ].
The SNS also plays a role in the regulation of mammalian thermogenesis and contributes to changes in energy expenditure that accompany changes in diet. The energy imbalance resulting from metabolic heat in response to cold exposure and to diet intake is covered by the sympathetic nervous system, which suggest an unequivocal relation between sympathetic activity and body fat deposition [ 71 ].
Even in healthy subjects body fat showed a direct relationship with SNS activity [ 72 ]. Grassi et al. evaluating normal control subjects, subjects with peripheral and central obesity showed a greater sympathetic activity in subjects with central obesity [ 73 ]. A recent study analyzed the effects of surgically-induced weight loss in severely obese patients and showed an important reduction in insulin resistance index, leptin levels, and sympathetic activity [ 74 ].
Abdominal adiposity loss is associated with diabetogenic and atherogenic markers. Different studies suggest a straight relationship between sympathetic activity and central WAT [ 66 , 73 ]. The relation is bidirectional; sympathetic denervation of WAT blocks lipolysis to a variety of lipolytic stimuli and the use of anterograde transneural viral tracers has defined the sensory input from WAT to the brain [ 76 ].
These experiments show the importance of sympathetic innervation in WAT. The effects of parasympathetic innervation of the adipose tissue are partially elucidated.
Kreier et al. used a retrograde transneuronal tracer, i. They demonstrated that parasympathetic denervation of WAT significantly reduced insulin-dependent glucose and free fat acid uptake and increased the sensitivity of lipase hormone-sensitive, resulting in an increased intracellular triglyceride breakdown.
Thus, evidence suggests that the parasympathetic system is associated with adipogenesis triglycerides synthesis , whereas the sympathetic system is associated with lipolysis triglycerides breakdown in WAT.
SNS modulation of visceral adipose tissue is related to different adrenergic receptors. Adipocyte plasma membranes express beta-1 β 1 , beta-2 β 2 , beta-3 β 3 , alpha-1 α 1 , and alpha-2 α 2 adrenergic receptors [ 78 ]. The balance between alpha and beta adrenergic receptors emerges as an important variable in regulating adipocyte cell number.
demonstrated adipocyte hyperplasia [ 79 ]. The fasting state is associated with adipolysis, which is modulated mainly by the β 3 adrenergic receptor. Conversely, adipogenesis is associated with activation of the parasympathetic system, but the receptor mediating adipogenesis is not well defined [ 76 ].
Thus, in addition to the well-known factors classically associated with VAS, the literature suggests an important role for autonomic activity in the regulation of WAT, which highlights the complex role of adipose tissue in the VAS.
Enzi G, Busetto L, Inelmen EM, Coin A, Sergi G. Int J Obes Relat Metab Disord. Article CAS PubMed Google Scholar. Hitzenberger K, Richter-Quittner M. Ein beitrag zum stoffweschsel bei dur vaskulären hypertonie. Wiener Arch Innere Med.
CAS Google Scholar. Hitzenberger K. Über den blutdruck bei diabetes mellitus. Google Scholar. Kylin E. Studien uber das hypertonie-hyperglykamie-hyperurikamiesyndrom.
Zentrabl finnere Med Leipz. Preble WE. Obesity: observations on one thousand cases. Boston Med Surg J. Article Google Scholar. Himsworth HP. The mechanism of diabetes mellitus. The mechanism of diabetes mellitus, II: the control of the blood sugar level.
The mechanism of diabetes mellitus, II: the control of the blood sugar level cont. Vague J. Presse Med ; — The degree of masculine differentiation of obesities: a factor determining predisposition to diabetes, atherosclerosis, gout, and uric calculous disease.
Am J Clin Nutr. CAS PubMed Google Scholar. Albrink MJ, Meigs JW. Interrelationship between skinfold thickness, serum lipids and blood sugar in men. Avogaro P, Crepaldi G, Enzi G, Tiengo A. Associazione di iperlipidemia, diabete mellito e obesità di medio grado.
Acta Diabetol Lat. Haller H. Epidermiology and associated risk factors of hyperlipoproteinemia [Article in German]. Z Gesamte Inn Med. Singer P. Diagnosis of primary hyperlipoproteinemias Article in German.
Reaven GM. Banting lecture Role of insulin resistance in human disease. Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. Ferrannini E, Haffner SM, Mitchell BD, Stern MP. Hyperinsulinaemia: the key feature of a cardiovascular and metabolic syndrome.
Ohlson LO, Larsson B, Svärdsudd K, Welin L, Eriksson H, Wilhelmsen L, Björntorp P, Tibblin G. The influence of body fat distribution on the incidence of diabetes mellitus. Rexrode KM, Carey VJ, Hennekens CH, Walters EE, Colditz GA, Stampfer MJ, Willett WC, Manson JE.
Abdominal adiposity and coronary heart disease in women. Galassi A, Reynolds K, He J. Metabolic syndrome and risk of cardiovascular disease: a meta-analysis. Am J Med. Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes.
Article PubMed PubMed Central Google Scholar. Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis.
Diabetes Care. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus: provisional report of a WHO consultation.
Diabet Med. Balkau B, Charles MA. Comment on the provisional report from the WHO consultation: European Group for the Study of Insulin Resistance EGIR. Executive Summary of The Third Report of The National Cholesterol Education Program NCEP Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults Adult Treatment Panel III.
Expert panel on detection, evaluation, and treatment of high blood cholesterol in adults. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition.
IDF Epidemiology Task Force Consensus Group. Article PubMed Google Scholar. Wang CC, Goalstone ML, Draznin B. Molecular mechanisms of insulin resistance that impact cardiovascular biology.
Lebovitz HE, Banerji MA. Point: visceral adiposity is causally related to insulin resistance. Diab Care. Semple RK. How does insulin resistance arise, and how does it cause disease? Human genetic lessons.
Eur J Endocrinol. Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. Article CAS PubMed PubMed Central Google Scholar. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome.
Endocr Rev. Odegaard JI, Chawla A. Pleiotropic actions of insulin resistance and inflammation in metabolic homeostasis. Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance.
Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. Caselli C. Role of adiponectin system in insulin resistance. Mol Genet Metab. Araújo TG, Oliveira AG, Carvalho BM, Guadagnini D, Protzek AO, Carvalheira JB, Boschero AC, Saad MJ.
Hepatocyte growth factor plays a key role in insulin resistance-associated compensatory mechanisms. Yi P, Park JS, Melton DA. Betatrophin: a hormone that controls pancreatic β cell proliferation.
Shanik MH, Xu Y, Skrha J, Dankner R, Zick Y, Roth J. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Guo S. Insulin signaling, resistance, and the metabolic syndrome: insights from mouse models into disease mechanisms.
J Endocrinol. Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. Lee Y, Berglund ED, Yu X, Wang MY, Evans MR, Scherer PE, Holland WL, Charron MJ, Roth MG, Unger RH.
Hyperglycemia in rodent models of type 2 diabetes requires insulin-resistant alpha cells. Proc Natl Acad Sci USA. Halperin F, Lopez X, Manning R, Kahn CR, Kulkarni RN, Goldfine AB. Insulin augmentation of glucose-stimulated insulin secretion is impaired in insulin-resistant humans.
Kulkarni RN, Brüning JC, Winnay JN, Postic C, Magnuson MA, Kahn CR. Tissue-specific knockout of the insulin receptor in pancreatic beta cells creates an insulin secretory defect similar to that in type 2 diabetes. Muniyappa R, Iantorno M, Quon MJ. An integrated view of insulin resistance and endothelial dysfunction.
Endocrinol Metab Clin North Am. Nizar JM, Dong W, McClellan RB, Labarca M, Zhou Y, Wong J, Goens DG, Zhao M, Velarde N, Bernstein D, Pellizzon M, Satlin LM, Bhalla V. Sodium-sensitive elevation in blood pressure is ENaC independent in diet-induced obesity and insulin resistance.
Am J Physiol Renal Physiol. Montani JP, Antic V, Yang Z, Dulloo A. Pathways from obesity to hypertension: from the perspective of a vicious triangle. Int J Obes.
Article CAS Google Scholar. Vlassara H, Uribarri J. Advanced glycation end products AGE and diabetes: cause, effect, or both? Curr Diab Rep. Shimomura I, Bashmakov Y, Horton JD.
Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. Iwasaki Y, Nishiyama M, Taguchi T, Asai M, Yoshida M, Kambyashi M, Terada Y, Hashimoto K.
Insulin exhibits short-term anti-inflammatory but long-term proinflammatory effects in vitro. Mol Cell Endocrinol. Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nat Rev Cancer. Renehan AG, Frystyk J, Flyvbjerg A. Obesity and cancer risk: the role of the insulin-IGF axis.
Trends Endocrinol Metab. Godfrey KM, Lillycrop KA, Burdge GC, Gluckman PD, Hanson MA. Epigenetic mechanisms and the mismatch concept of the developmental origins of health and disease. Pediatr Res. Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol.
Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Intine RV, Sarras MP Jr. Metabolic memory and chronic diabetes complications: potential role for epigenetic mechanisms. Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR.
Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, Slagboom PE, Heijmans BT. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific.
Hum Mol Genet. Yarbrough DE, Barrett-Connor E, Kritz-Silverstein D, Wingard DL. Birth weight, adult weight, and girth as predictors of the metabolic syndrome in postmenopausal women: the Rancho Bernardo Study. Ramadhani MK, Grobbee DE, Bots ML, Castro Cabezas M, Vos LE, et al.
Lower birth weight predicts metabolic syndrome in young adults: the Atherosclerosis Risk in Young Adults ARYA -study.
Tremaroli V, Bäckhed F. In obese mice, a liver enzyme travels to visceral fat and increases inflammation inflammatory cells labeled red , thereby promoting diabetes filled with cells. The photo on the right shows fat tissue from obese mice who have been given a drug that blocks the enzyme.
The fat that builds up deep in the abdomen—more than any other type of body fat—raises the risk of insulin resistance and type 2 diabetes. Researchers have known that abdominal fat becomes dangerous when it becomes inflamed but have had a hard time determining what causes the inflammation.
A new study at Columbia University Irving Medical Center CUIMC has revealed that at least one of the culprits for this mysterious inflammation comes from the liver. The researchers found that, in obese mice, the liver increases its production of an enzyme called DPP4. This enzyme travels through the blood stream to abdominal fat.
Once inside fat tissue, DPP4 helps to activate inflammatory cells. The good news is that this inflammation can be soothed by turning off DPP4 production in the liver, as the researchers demonstrated in mice.
And even though the animals remained obese, soothing inflamed abdominal fat improved their insulin resistance. Stock Professor of Medicine at Columbia University Vagelos College of Physicians and Surgeons.
Current DPP4 inhibitors do not reduce inflammation in fat or improve insulin resistance. Many patients with type 2 diabetes are given oral DPP4 inhibitors known as gliptins to help manage their disease. These drugs lower blood sugar by preventing DPP4 from interfering with a hormone that stimulates insulin production.
But surprisingly, these drugs had no effect on inflammation in the abdominal fat of obese mice, the researchers found. The reason for this shortcoming of gliptins, Tabas believes, may be related to their effects in the gut versus the liver.
But we have some evidence that DPP4 inhibitors in the gut also end up promoting inflammation in fat.
Visceeal association fwt abdominal fat accumulation resixtance risk of Gluten-free substitutes diseases, including type II diabetes and coronary heart disease, has long Viscetal Visceral fat and insulin resistance. Insulin resistance may be a key factor resistabce this link. Many studies have pointed Waist measurement and waist-height ratio an Visceral fat and insulin resistance between Visceral fat and insulin resistance resistance and intra-abdominal fat accumulation visceral obesity. However there is no clear proof of a causal link between visceral fat accumulation and insulin resistance. In assessing the probability of a causal link, it is useful to consider potential mechanisms. One such potential causal link is the release of non-esterified fatty acids from visceral fat into the portal vein, so that they have direct effects on hepatic metabolism. Visceral fat has been shown in many studies to exhibit a high rate of lipolysis compared with subcutaneous fat depots. Aim: Vat heterogeneity exists in overall Increasing insulin sensitivity and abdominal obesity in terms Avocado Health Benefits insulin secretion and sensitivity. Further, the Increasing insulin sensitivity of Viscera, fat VF on the Vicseral and second-phase insulin secretion FPIS and SPIS is controversial. We aim to investigate insulin secretion and sensitivity in Chinese patients with T2DM according to different BMI and VF levels. Methods: This study enrolled participants. A dual bioelectrical impedance analyzer was used to assess the visceral and subcutaneous fat area VFA and SFA.
Eben was daraufhin?
Mir scheint es, Sie sind recht
periphrasieren Sie bitte