Video
How to treat a snake bite - First Aid - St John WA Thank you for Sake nature. Energy-boosting antioxidant supplements are using a methdos version with limited support for CSS. Snake envenomation diagnosis methods obtain diagnosi best experience, we recommend diaghosis use a more up Snake envenomation diagnosis methods date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Diagnosis of snake envenomation is challenging but critical for deciding on antivenom use. Phospholipase A 2 enzymes occur commonly in snake venoms and we hypothesized that phospholipase activity detected in human blood post-bite may be indicative of envenomation.Snake envenomation diagnosis methods -
This topic will discuss the management of snakebites that occur worldwide, other than those by snakes found in the United States. FIRST AID Initial first aid of snake envenomation is directed at reducing the spread of venom and expediting transfer to an appropriate medical center.
To continue reading this article, you must sign in with your personal, hospital, or group practice subscription. Subscribe Sign in. It does NOT include all information about conditions, treatments, medications, side effects, or risks that may apply to a specific patient.
It is not intended to be medical advice or a substitute for the medical advice, diagnosis, or treatment of a health care provider based on the health care provider's examination and assessment of a patient's specific and unique circumstances. Patients must speak with a health care provider for complete information about their health, medical questions, and treatment options, including any risks or benefits regarding use of medications.
This information does not endorse any treatments or medications as safe, effective, or approved for treating a specific patient.
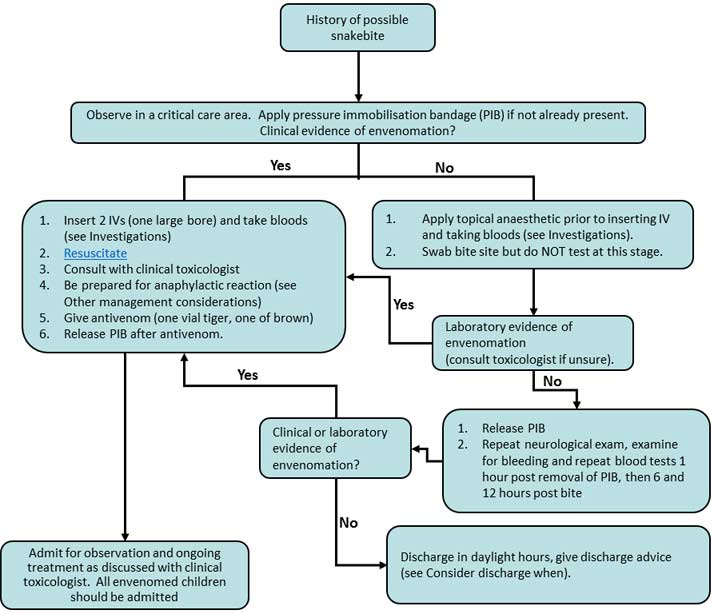
UpToDate, Inc. and its affiliates disclaim any warranty or liability relating to this information or the use thereof. All rights reserved. Topic Feedback. Algorithm for diagnosis of the snakebite in Sri Lanka Diagnostic algorithm for Australian snakebites based upon local effects Diagnostic algorithm for Australian snakebites based upon systemic bite effects.
Algorithm for diagnosis of the snakebite in Sri Lanka. Diagnostic algorithm for Australian snakebites based upon local effects. Diagnostic algorithm for Australian snakebites based upon systemic bite effects.
Positions of function for selected joints. Venomous snake groups each cause a characteristic clinical syndrome, which can be used in combination with local geographical distribution information to determine the probable snake involved and appropriate antivenom to use.
The Snake Venom Detection Kit may assist in regions where the range of possible snakes is too broad to allow the use of monovalent antivenoms.
When the snake identification remains unclear, two monovalent antivenoms eg, brown snake and tiger snake antivenom that cover possible snakes, or a polyvalent antivenom, can be used.
One vial of the relevant antivenom is sufficient to bind all circulating venom. However, recovery may be delayed as many clinical and laboratory effects of venom are not immediately reversible. For expert advice on envenoming, contact the National Poisons Information Centre on 13 11 A ustralian snake envenoming is rare but potentially life-threatening and challenging to manage.
Most snakebites do not result in clinical envenoming 1 , 2 , 4 because insufficient venom is injected ie, a dry bite or because the snake is non-venomous.
Clinical envenoming may include local effects, systemic symptoms and major toxin syndromes — venom-induced consumption coagulopathy VICC , neurotoxicity and myotoxicity Box 1. Neurotoxicity and myotoxicity are uncommon and evolve over hours, making the timing of decisions to use antivenom problematic.
Clinical assessment of patients with snakebite includes details of the bite and symptoms or signs occurring in the first hour. A clinically important feature of severe envenoming is early collapse, most common with brown snake envenoming Box 2. Bite site: fang marks, bruising or local necrosis; draining lymph nodes may be painful and support a diagnosis of systemic envenoming.
Neurological: cranial nerves ptosis, ophthalmoplegia, bulbar weakness , limb weakness and respiratory muscle weakness. Haematological: evidence of abnormal coagulation bleeding from bite site, cannula site, oral cavity or occult sites, including gastrointestinal, urinary and intracranial sites.
Box 2 provides a summary of major clinical effects of the clinically important groups of Australian snakes. Investigations for diagnosis and treatment of snake envenoming as well as excluding envenoming in suspected snakebites include coagulation studies, a full blood count and biochemical tests.
Laboratory measurement of the international normalised ratio INR and aPTT is imperative. Measurement of fibrinogen and D-dimer levels may help with diagnosis of VICC eg, to differentiate between VICC and anticoagulant effect but is not essential.
The D-dimer level is usually elevated by — times the assay cut-off in VICC, and modest increases, in the absence of other indications of envenoming, must be interpreted with caution.
Point-of-care devices for measuring INR or D-dimer have been found to give false negative results in VICC and should not be used. Thrombocytopenia and red cell fragmentation on a blood film indicate a diagnosis of thrombotic microangiopathy. Serial measurements of electrolyte, urea and creatinine levels may assist in assessing renal function.
The Snake Venom Detection Kit SVDK; CSL Ltd is designed to assist in determining the appropriate antivenom to use in envenomed patients.
However, due to its potential for inaccuracy, the result must only be used in light of the clinical syndrome and knowledge of local snake geographical distributions except for snakes that are captive outside their distribution.
Clinical and laboratory features will often indicate which snake group is involved 1 , 12 and, in turn, the appropriate antivenom, without the need for an SVDK. To ensure test procedures are properly followed, the SVDK should be used by laboratory staff wherever possible. The best specimen is a bite-site swab.
A pressure bandage with immobilisation PBI is recommended first aid for suspected or definite snakebite. The PBI can be removed when the initial clinical and laboratory assessment shows no evidence of envenoming and the patient is in a facility where antivenom is available Box 3.
As there are reports of cases where envenoming appears to be delayed by early application of a PBI but becomes evident soon after its removal, 26 careful observation of the patient in the hour after PBI removal is essential. Most patients present with a report of a definite or suspected snakebite, but it is often unclear whether a patient is envenomed and what type of snake is involved.
Investigation and treatment should follow a logical process to ensure envenoming is correctly diagnosed and the correct antivenom is used:. Patients with no clinical or laboratory evidence of envenoming on arrival can be observed for 12 hours, with appropriate repeat laboratory testing INR, aPTT, CK and neurological examination to exclude envenoming 1.
In patients with envenoming, determine which snake or group of snakes is most likely involved. Administer an appropriate antivenom monovalent, combination of monovalent antivenoms, or polyvalent that covers the likely snake s.
Admit the patient for observation or adjunctive treatment eg, mechanical ventilation if required. This process is summarised in Box 3.
All patients presenting with a suspected snakebite require an initial full set of investigations INR, aPTT, full blood count, biochemistry, CK level. Most patients will have no clinical or laboratory features of envenoming when first assessed. Once this initial assessment is complete, these patients can have their PBI if present removed in a critical care area.
If no clinical features of envenoming occur within an hour of PBI removal, patients should be moved to a general clinical area and observed. Evidence of envenoming exists if neurotoxicity develops or the INR, aPTT or CK level becomes abnormal, and antivenom treatment should be considered based on the timing, severity and specific abnormality.
Investigations conducted 1 hour after removal of the PBI may coincide approximately with those required 6 hours after the bite; in this instance, only one set of blood tests are required. If results of 6-hour laboratory investigations are normal, hour investigations can be delayed a few hours if necessary to avoid recall of overnight laboratory staff.
For the rare circumstances in which a PBI is left on for more than 6 hours, a final set of blood tests and a neurological examination should be done 6 hours after removal of the PBI. Accumulating data suggest that antivenom might prevent certain envenoming syndromes if used early, 5 , 13 , 27 but may have little, if any, effect once major envenoming syndromes are established.
However, there is no high-level evidence to guide us in this regard. In the absence of evidence from properly conducted trials, clinicians have to balance the risks of giving antivenom anaphylaxis and serum sickness against the potential benefit of giving it early without waiting for confirmation of envenoming.
Any early evidence of envenoming, such as non-specific systemic symptoms or mild coagulopathy, may be an indication for antivenom. Discussion with a clinical toxicologist may be beneficial in these circumstances. Absolute and relative indications for antivenom are listed in Box 4.
Determination of the snake or snake group involved and therefore the appropriate antivenom to be administered requires:. Observation of the specific clinical syndrome characterised by the clinical and laboratory features at presentation or subsequently Box 2.
In some cases, an expert may be available for snake identification, or the person bitten may be a snake handler who can identify the snake. Snake identification should only be performed by experts such as professional herpetologists and museum curators. Some professional snake handlers may be able to provide accurate identification of the snakes in their possession, which should be used rather than the SVDK.
If there is any doubt about the snake involved, it may be safer to administer polyvalent antivenom or two monovalent antivenoms according to species endemic to the region. In most parts of southern and central—eastern Australia, one vial each of brown snake and tiger snake antivenom will cover the clinically important snakes in the local area, based on the clinical envenoming syndrome.
One vial of relevant snake monovalent antivenom is required to treat both children and adults for all snake types. Recovery of most clinical syndromes of snake envenoming takes time because of their irreversibility or slow reversibility eg, synthesis of new clotting factors required for VICC to resolve.
Some guidelines or previous studies have suggested more than one vial of antivenom should be used for some snakes or in some situations. Antivenom must be given in a critical care area, and staff must be prepared to treat anaphylaxis.
It is advisable to use a small-bore cannula 18—20 G in adults for antivenom infusion and to have a second, large-bore cannula 16—14 G in adults inserted ready for emergency resuscitation Box 5. Antivenom is given diluted in — mL of isotonic saline smaller dilutions should be used for children over 15—30 minutes.
The rate of antivenom administration does not appear to be associated with increased reactions. Reactions appear to be more common with larger-volume antivenoms. Serum sickness can be treated with prednisolone 25 mg daily for 5—7 days. Patients who are given antivenom must be admitted for repeat laboratory testing and observation to determine when envenoming has resolved and to identify complications.
Measurement of INR, aPTT, creatinine level and CK level for myotoxic snakes and a full blood count should be done 6 and 12 hours after administration of antivenom, and then once to twice daily until there is sustained improvement.
Thrombotic microangiopathy should be excluded in all patients with VICC by observing no change in creatinine level and platelet count over the first 24 hours.
The role of clotting factor replacement in treating VICC remains contentious. A recent randomised controlled trial of fresh frozen plasma FFP versus no additional treatment within 4 hours of administration of antivenom for VICC found that FFP results in more rapid restoration of clotting function in most patients, but with no decrease in time to discharge.
In the meantime, it is reasonable to administer FFP after giving antivenom to patients who have active bleeding and an imminent threat to their life. FFP is the best factor replacement rather than cryoprecipitate or Prothrombinex-VF [CSL Bioplasma] because patients are deficient in fibrinogen, factor V and factor VIII.
Complications of snake envenoming are rare and generally occur in patients presenting late with severe neuromuscular paralysis, rhabdomyolysis or thrombotic microangiopathy with acute renal failure.
Major bleeding may require clotting factor replacement, such as FFP, and supportive care. Thrombotic microangiopathy may require haemodialysis, but there is no evidence to support the use of plasmapheresis.
Significant rhabdomyolysis with acute renal failure is rare but should be treated with generous fluid therapy and close monitoring for electrolyte imbalances eg, hyperkalaemia. Severe neuromuscular paralysis may require intubation and mechanical ventilation for days or weeks.
However, they may develop hypersensitivity reactions to venom, which must be considered in the differential diagnosis. Uncomplicated snakebite can be managed in a hospital with basic laboratory facilities, appropriate antivenom stocks, a critical care area in which to monitor for and treat anaphylaxis, and a clinician capable of treating complications, including anaphylaxis.
Primary retrieval or early interhospital transfer to large centres is not routinely required. However, patients with a suspected snakebite or definite envenoming should be transferred immediately to a hospital with a laboratory that can do a formal INR test, unless it can be done locally with the result available within 2 hours.
Patients with definite systemic envenoming can be admitted to any hospital with close nursing observation, critical care resources and after-hours medical support after antivenom administration — an emergency department observation or short-stay unit is ideal in larger hospitals.
Venom components, particularly those of viperids, are known to contain distinctive active sites that usually depend on three amino acid residues. The ability therefore, of compounds to block such an active site makes it possible to functionally disable the venom component.
Lately, various small molecule inhibitors have caused excitement, with the potential to expedite the utility of such compounds through molecular docking approaches given the already encouraging results available [ 15 ].
One of the most interesting small molecule therapeutics SMTs to have recently emerged is varespladib and its orally available prodrug format, methyl-varespladib. As a treatment drug initially meant for acute coronary syndrome, varespladib has been shown to successfully inhibit phospholipase A2 activity of various snake venoms obtained from different parts of the world.
The effect of varespladib against venom-derived PLA2s is relevant given their poor immunogenicity, which may elicit a poor immune response during immunization of animals as part of the development of conventional antivenoms.
Consequently, such antivenom products obtained from poorly immunogenic components may not be very effective against PLA2s. When varespladib was given following a little delay in the injection of V. belus venom, the same result was produced. belus venom. In yet another experiment, rats injected with M.
fulvius venom subcutaneously were entirely rescued following the intravenous administration of varespladib within 5 minutes of the challenge. In addition, varespladib was shown to suppress the increase in PLA2 activity induced by the venom, as well as haemolysis of the venom [ 44 ].
In another study conducted quite recently, varespladib was reported to inhibit the in vitro PLA2-induced toxic effects of Agkistrodon halys , Deinagkistrodon acutus, Naja atra, and Bungarus multicinctus.
acutus and A. Again, signs of muscle damage induced by the venoms including desmin degradation were reduced by varespladib. At median effective doses ED50s of 0. halys and D. acutus, respectively, as opposed to those of elapids; atra and B. multicinctus, respectively [ 45 ]. Venoms from most snakes contain toxic components, especially PLA2s that act synergistically with other toxins.
To this end, it could be conjectured that the administration of varespladib could potentially obstruct the synergistic effect of key toxins, resulting in the complete inhibition of venom toxicity.
Nonetheless, the usefulness of varespladib may be limited because the extensive reliance on PLA2s does not apply to all snake venoms. For instance, venom from the genus Dendroapsis is nearly exclusively devoid of phospholipases A2; thus, it is not probable that varespladib would be beneficial against snakebites from this genus [ 46 , 47 ].
However, whereas varespladib on its own may possess exciting applications, methyl-varespladib, its corresponding prodrug format, is available orally for administration, rendering it a potential first-choice candidate drug for protection.
Therefore, when methyl-varespladib is used alone or combined with other drugs, it may be capable of providing some respite to victims, whiles efforts are made to access further antivenom treatment at appropriate health facilities [ 48 ].
Another example of a SMT that has shown great promise is the matrix metalloproteinase batimastat and its orally available prodrug format marimastat. The incubation of these SMTs with a challenge dose of E.
ocellatus venom 4 LD50 and subsequent co-injection into the tail vein of mice resulted in prolonged survival, although it was not fully protective.
However, administering batimastat abrogated the hemorrhagic, in vitro coagulant, defibrinogenating, and proteinase activities induced by E. ocellatus venom sourced from Cameroon. When E. ocellatus venom samples from Ghana were used, an increased abrogation in hemorrhagic activity was observed when batimastat was quickly administered.
Conversely, the delayed administration of batimastat resulted in better abrogation of defibrinogenating activity, with the possibility of completely abrogating the effect following a min delay in administration of the molecule.
While batimastat proved to be more efficacious in abrogating hemorrhagic activity, defibrinogenating activity was more effectively abrogated by marimastat [ 49 ].
Other SMTs including acetylcholinesterase inhibitors e. In addition, nanoparticles and C60 fullerene are also being investigated, with the latter showing antivenom features in an insect model [ 27 ].
Apart from antivenom, protein, peptide, and oligomer-based therapies are being explored. Human monoclonal-based single-chain variable fragments and fully humanized monoclonal IgGs have been developed. There are indications that these technologies may possibly be crucial in inhibiting various snake venom components in the future given that they are associated with relatively less adverse reactions, as well as the potential to be competitive in terms of cost [ 27 ].
According to El-Aziz et al. In exploring the neutralization action of αC-conotoxin PrXA found in Conus parius , a species of cone snail by oligonucleotides, a sample of oligonucleotide tested demonstrated capacity to neutralize the in vitro activity of αC-conotoxin PrXA.
Although at the doses tested, the oligonucleotide was not fully protective, it, however, provided extended survival. Nonetheless, at much higher concentrations, the oligonucleotide provided full protection. There is the need, however, for more studies to evaluate the cost of manufacturing oligonucleotides on a large scale before its evaluation for clinical-based application.
In addition, a large number of alternative binding scaffolds AbScaffs are being investigated for their therapeutic prospects in snakebite envenoming because they cost low to produce, highly stable, and easy to engineer.
Advances in basic research culminated in the development of the breakthrough hybridoma technology in by Kohler and Milstein in which hybrid cells producing rodent-derived monoclonal antibodies mAbs were generated in unlimited quantities [ 51 ].
These hybrid cells were achieved by fusing B cells obtained from an immunized animal with myeloma cells and the resulting selected cell secreting one specific antibody.
Thus, the interest in the use of antibodies in therapeutics was again awoken by the hybridoma technology following its discovery. Subsequently, techniques were developed to overcome the safety, efficacy, and immunogenicity problems associated with the use of rodent-derived antibodies by transforming same into structures akin to human antibodies, ensuring that the binding properties to the target are retained.
Consequently, the first humanization method resulted in the development of chimeric antibodies through combining sequences of human constant region domain and murine variable domain, with the resulting antibody preserving the specificity and reducing its immunogenicity [ 52 ].
In a previous study [ 53 ], the possibility of antivenom based on monoclonal antibodies was investigated. In this study, neutralizing mAbs were developed and tested against three key toxic constituents of Bothrops atrox , the main snake group causing accidents in the Northern region of Brazil.
Like the venom of other Bothrops species, B. atrox venom contains a complex mixture of venom proteins including proteases whose substrates include blood clotting system components such as factors X, XII, and fibrinogen.
The venom of B. atrox is also made up of proteins that are zinc-dependent metalloproteinases, majority of which are hemorrhagic and phospholipase A2, which has been demonstrated to play a role in myonecrosis and inflammation.
atrox were cultured, expanded and cells injected i. Ascitic fluid was subsequently collected via abdominal puncture and mAbs purified via caprylic acid followed by ammonium sulfate precipitation. The results obtained showed that purified mAbs specific to these three venom proteins were successfully generated.
When submitted to immunochemical analysis via SDS-PAGE, all three mAbs showed two main protein bands, one around 55 kDa and the other approximately 29 kDa, indicative of immunoglobulin heavy and light chains, together with several minor contaminant bands.
On performing western blot analysis with anti-mouse IgG as the primary antibody, the two major bands were confirmed to correspond to mouse IgG heavy and light chains. In another study by Laustsen et al. The approach to discovering the suite of human IgGs combined among other things toxicovenomics, antibody phage display technology and engineering, and mammalian cell expression.
A human antibody phage display library of clones and single-chain variable fragments scFvs antibodies were constructed from B-lymphocytes obtained from nonimmunized human donors.
ScFv antibodies that produced the highest binding signals from an expression-normalized capture ENC assay were selected and converted to IgG format. The results of the study demonstrate the possibility of exploiting oligoclonal cocktails of monoclonal human IgGs to treat black mamba envenoming.
The findings further showed that individual monoclonal IgGs targeting neutralization of dendrotoxins cannot do that alone and for which reason it could be beneficial to employ antibody cocktails that neutralize multiple black mamba venom toxins in order to achieve full protection.
In a recent study by Manson et al. ashei venom in mice. Preliminary activity of the purified mAbs against 3FTx antigen was assessed by testing the ability of the mAbs to identify and bind to the target antigen in an ELISA titration.
All three mAbs demonstrated capacity to recognize 3FTxs, and thus were able to bind to the antigen at the lowest concentration tested 0.
When compared with the control sample, all three clones were found to bind to the target antigen with comparatively higher efficacy as assessed by optical density. Further confirmation of the recognition, binding, and activity of the mAbs was evaluated using an inhibition ELISA assay previously described [ 43 ].
Both the mAbs and polyclonal antibodies induced comparable inhibition. Similarly, there was a significant difference in the inhibition induced by the polyclonal antibodies relative to both antivenoms. Thus, the results demonstrate that the immunoaffinity-purified test mAbs have higher binding efficacy, and hence higher specificity for the target antigen relative to both the negative control and the two leading commercial brands of antivenoms on the Kenyan market.
The results further demonstrate the prospects of developing toxin-specific monoclonal-based antivenoms for snakebite immunotherapy.
Making available low-cost and heat-stable SMTs at the local level to high-risk areas could allow the administration of pre-hospital treatments after establishing envenomation that could lower the risks of tissue damage and paralysis.
Subsequently, surveillance and treatment at a hospital may be procured using improved treatment approaches such as more specific monoclonal antibodies or AbScaffs [ 15 , 56 ]. The authors acknowledge the support of Pan African University Institute of Basic Sciences, Technology and Innovation PAUSTI and the Japan International Cooperation Agency Africa-ai-Japan Project.
Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3. Open access peer-reviewed chapter - ONLINE FIRST Diagnostic and Antivenom Immunotherapeutic Approaches in the Management of Snakebites Written By Ernest Ziem Manson, Joseph K.
Gikunju and Mutinda Cleophas Kyama. DOWNLOAD FOR FREE Share Cite Cite this chapter There are two ways to cite this chapter:. Choose citation style Select style Vancouver APA Harvard IEEE MLA Chicago Copy to clipboard Get citation.
Choose citation style Select format Bibtex RIS Download citation. IntechOpen Poisoning - Prevention, Diagnosis, Treatment and Poison Repurposi Edited by Farid A.
From the Edited Volume Poisoning - Prevention, Diagnosis, Treatment and Poison Repurposing [Working Title] Prof. Farid A. Badria and Dr. Kavitha Palaniappan. Chapter metrics overview 37 Chapter Downloads View Full Metrics. Impact of this chapter.
Keywords snakebites antivenom diagnostics immunotherapy envenoming neglected tropical diseases. Introduction Snakebite envenoming is a potentially fatal disease that normally occurs as a result of the injection of venom following the bite of a venomous snake. ashei N. nigricollis N. haje B. arietans D.
Table 1. References 1. Gutierrez J, Calvete J, Habib A, Harrison R, Williams D, Warrell D. Snakebite envenoming. Nature Reviews. Disease Primers. DOI: Geneva; 3.
Harrison RA, Oluoch GO, Ainsworth S, Alsolaiss J, Bolton F, Arias AS, et al. Preclinical antivenom-efficacy testing reveals potentially disturbing deficiencies of snakebite treatment capability in East Africa. PLoS Neglected Tropical Diseases. Nkwescheu A, Mbasso LCD, Pouth FBB, Dzudie A, Billong SC, Ngouakam H, et al.
Snakebite in bedroom kills a physician in Cameroon: A case report. The Pan African Medical Journal. Gutiérrez JM, Warrell DA, Williams DJ, Jensen S, Brown N, Calvete JJ, et al. The need for full integration of snakebite envenoming within a global strategy to combat the neglected tropical diseases: The way forward.
Gutiérrez JM, Solano G, Pla D, Herrera M, Segura Á, Villalta M, et al. Assessing the preclinical efficacy of antivenoms : From the lethality neutralization assay to antivenomics. Williams DJ, Faiz MA, Abela-Ridder B, Ainsworth S, Bulfone TC, Nickerson AD, et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming.
Alirol E, Sharma SK, Bawaskar HS, Kuch U, Chappuis F. Snake bite in south asia: A review. Leon G, Vargas M, Segura A, Herrera M, Villalta M, Sanchez A. Current technology for the industrial manufacture of snake antivenoms. Habib A, Brown N.
The snakebite problem and antivenom crisis from a health-economic perspective. Alirol E, Lechevalier P, Zamatto F, Chappuis F, Alcoba G, Potet J. Antivenoms for snakebite envenoming: What is in the research pipeline?
Harrison RA. Development of venom toxin-specific antibodies by DNA immunisation: Rationale and strategies to improve therapy of viper envenoming.
Casewell NR, Cook DAN, Wagstaff SC, Nasidi A, Durfa N, Harrison RA, et al. Pre-clinical assays predict pan-African Echis viper efficacy for a species-specific Antivenom.
Theakston RDG, Laing GD. Diagnosis of snakebite and the importance of immunological tests in venom research. Toxins Basel. Williams HF, Layfield HJ, Vallance T, Patel K, Bicknell AB, Trim SA, et al.
The urgent need to develop novel strategies for the diagnosis and treatment of snakebites. Pucca MB, Cerni FA, Janke R, Bermúdez-Méndez E, Ledsgaard L, Barbosa JE, et al. History of envenoming therapy and current perspectives. Frontiers in Immunology. Chong HP, Tan KY, Tan NH, Tan CH.
Exploring the diversity and novelty of toxin genes in Naja sumatrana, the equatorial spitting cobra from Malaysia through De novo venom-gland transcriptomics.
Sanhajariya S, Duffull SB, Isbister GK. Pharmacokinetics of snake venom. Omara T, Kagoya S, Openy A, Omute T, Ssebulime S, Kiplagat KM, et al. Antivenin plants used for treatment of snakebites in Uganda: Ethnobotanical reports and pharmacological evidences.
Tropical Medicine and Health. Kasturiratne A, Wickremasinghe AR, De Silva N, Gunawardena NK, Pathmeswaran A, Premaratna R, et al. The global burden of snakebite: A literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Medicine.
Warrell DA. Snake bite. WHO Regional Office for Africa. Guidelines for the Prevention and Clinical Management of Snakebite in Africa.
Open access peer-reviewed chapter - ONLINE Wnvenomation. Submitted: 27 April Reviewed: 09 June Snake envenomation diagnosis methods 07 October According Snakke World Emvenomation Organization, snakebite is mfthods to have high mortality among the neglected tropical diseases. The administration of toxin-specific therapy in snake envenoming is predicated on improving diagnostic techniques capable of detecting specific venom toxins. Various serological tests have been used in detecting snakebite envenoming. Comparatively, enzyme-linked immunosorbent assay has been shown to offer a wider practical application.
0 thoughts on “Snake envenomation diagnosis methods”