Choline for acetylcholine synthesis -
Cellular responses are influenced by PKC's phosphorylation of target proteins. This conductance increase increases the resting membrane potential in myocardial and other cell membranes leading to inhibition.
ACh binds only briefly to the pre- or postsynaptic receptors. Following dissociation from the receptor, the ACh is rapidly hydrolyzed by the enzyme acetylcholinesterase AChE as shown in Figure This enzyme has a very high catalysis rate, one of the highest known in biology.
AChE is synthesized in the neuronal cell body and distributed throughout the neuron by axoplasmic transport. AChE exists as alternatively spliced isoforms that vary in their subunit composition. The variation at the NMJ is a heteromeric protein composed of four subunits coupled to a collagen tail that anchors the multi-subunit enzyme to the cell membrane of the postsynaptic cell Figure This four-subunit form is held together by sulfhydryl bonds and the tail anchors the enzyme in the extracellular matrix at the NMJ.
Other isoforms are homomeric and freely soluble in the cytoplasm of the presynaptic cell. AChE, unlike ChAT, is found in non-cholinergic neurons as well. In addition, other cholinesterases exist throughout the body, which are also able to metabolize acetylcholine.
These are termed pseudocholinesterases. Drugs that inhibit ACh breakdown are effective in altering cholinergic neurotransmission. In fact, the irreversible inhibition of AChE by isopropylfluoroesters are so toxic that they can be incompatible with life—inhibiting the muscles for respiration.
This inhibition is produced because ACh molecules accumulate in the synaptic space, keep the receptors occupied, and cause paralysis. Two notable examples are insecticides and the gases used in biological warfare.
The mechanism of action of these irreversible inhibitors of AChE is that they carbamylate the AChE, rendering it inactive. The carbamylation inactivates both the acetyl and choline binding domains.
A recently developed antidote to these inhibitors cleaves the nerve gas so that it will dissociate from the AChE. In contrast to the irreversible inhibitors, the reversible AChE inhibitors are effective in transiently increasing the ACh level and are effective in diseases and conditions where an increased ACh level is desired.
The clinically important compound, eserine physostigmine , reversibly inhibits AChE. Nicotinic receptor activation causes the opening of the channel formed by the receptor. This rapidly developing change, termed a fast EPSP , is illustrated in Figures 4. Muscarinic receptor activation of postsynaptic cells can be either excitatory or inhibitory and is always slow in onset and long in duration Table I.
As described earlier, G protein activation underlies all actions of the muscarinic receptors, thus accounting for their slow onset. Slow EPSP and IPSP form the sympathetic ganglion of the rat.
The rapid nature of the synaptic transmission mediated by the nicotinic receptor is consistent with its role at the NMJ and in the ganglion of the ANS.
Little is known about the role of the nicotinic receptor role in CNS behavior. Clearly, nicotine stimulation is related in some manner to reinforcement, as indicated by the prevalence of nicotine addiction among humans. Muscarinic receptors, in contrast, are important mediators of behavior in the CNS.
One example is their role in modulating motor control circuits in the basal ganglia. A second example is their participation in learning and memory. Alzheimer's disease : A disease in which a marked deterioration occurs in the CNS, the hallmark of which is a progressive dementia.
One of the characteristics of this disease is a marked decrease in ACh concentrations in the cerebral cortex and caudate nucleus. Myasthenia gravis : A disease of the neuromuscular junction in which the receptors for ACh are destroyed through the actions of the patient's own antibodies.
Cholinergic Pharmacology : Numerous drugs are used clinically to interact with the cholinergic systems. Table II summarizes the major uses for cholinergic drugs.
Which of the following is effective in increasing the level of acetylcholine in the synapse or neuromuscular junction?
NOTE: There is more than one correct answer. Increasing dietary acetyl coenzyme A This answer is INCORRECT. The administration of treatments to enhance acetyl coenzyme A production is not effective in elevating acetylcholine neurotransmission.
Increasing the production of acetyl coenzyme A This answer is CORRECT! Although the administration of drugs to enhance acetyl coenzyme A production are not effective in elevating acetylcholine neurotransmission, cholinergic neurons increase their coenzyme A production as a means of increasing acetylcholine availability for neurotransmission.
Increasing dietary choline This answer is INCORRECT. Although choline availability to the cholinergic neurons is rate limiting in the synthesis of acetylcholine, studies in animals and humans indicate that the administration of choline is ineffective in elevating cholinergic neurotransmission.
Increasing choline uptake This answer is CORRECT! Main article: Neuromuscular junction. Main article: Nicotinic receptor. Main article: Muscarinic receptor. Main article: Cholinesterase inhibitors.
Asian Pacific Journal of Tropical Disease. doi : PMC Treasure Island FL : StatPearls Publishing. PMID Retrieved Nutritional and Herbal Therapies for Children and Adolescents.
ISBN ACS Chem Neurosci. Signal Transduct. S2CID British Journal of Clinical Pharmacology. Front Syst Neurosci. Miller's Anesthesia 7th ed.
Elsevier Health Sciences. Trends Pharmacol. Brain Res Cogn Brain Res. Brain Res. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain". Descending projections of the pontomesencephalic tegmentum". Aust N Z J Psychiatry.
Trends in Neurosciences. Üeber das neurin". Justus Liebigs Ann Chem in German. International Immunopharmacology. The physiological action of choline and neurine". Philosophical Transactions of the Royal Society of London.
Series B, Containing Papers of a Biological Character. Medical History. J Pharmacol Exp Ther. Pflug Arch Ges Phys in German. Clinical Cardiology.
Brenner GM, Stevens CW Pharmacology 2nd ed. Philadelphia PA: W. Canadian Pharmacists Association Compendium of Pharmaceuticals and Specialties 25th ed. Toronto ON: Webcom. Carlson NR Physiology of Behavior 7th ed. Needham Heights MA: Allyn and Bacon. Gershon MD The Second Brain.
New York NY: HarperCollins. Siegal A, Sapru HN Essential Neuroscience Revised 1st ed. Hasselmo ME February as PDF Yu AJ, Dayan P May as PDF. Wikiquote has quotations related to Acetylcholine. Drugs used for glaucoma preparations and miosis S01E.
Aceclidine Pilocarpine. Acetylcholine Carbachol. Demecarium Ecothiopate Stigmine Fluostigmine Neostigmine Physostigmine Paraoxon. Befunolol Betaxolol Carteolol Levobunolol Metipranolol Timolol Mepindolol. Agmatine Aspartic acid aspartate Glutamic acid glutamate Glutathione Glycine GSNO GSSG Kynurenic acid NAA NAAG Proline Serine.
GABA GABOB GHB. α-Alanine β-Alanine Glycine Hypotaurine Proline Sarcosine Serine Taurine. GHB T-HCA GHC. See here instead. ADP AMP ATP. Carbon monoxide CO Hydrogen sulfide H 2 S Nitric oxide NO. Acetaldehyde Ammonia NH 3 Carbonyl sulfide COS Nitrous oxide N 2 O Sulfur dioxide SO 2. Acetylcholine receptor modulators.
Muscarinic acetylcholine receptor modulators. Acetyl-coA Adafenoxate Choline lecithin Citicoline Cyprodenate Dimethylethanolamine Glycerophosphocholine Meclofenoxate centrophenoxine Phosphatidylcholine Phosphatidylethanolamine Phosphorylcholine Pirisudanol.
Nicotinic acetylcholine receptor modulators. Acetylcholine metabolism and transport modulators. Inhibitors: 1- -Benzoylethyl pyridinium 2- α-Naphthoyl ethyltrimethylammonium 3-Chlorostillbazole 4- 1-Naphthylvinyl pyridine Acetylseco hemicholinium-3 Acryloylcholine AF64A B BETA CM, N,N-Dimethylaminoethylacrylate N,N-Dimethylaminoethylchloroacetate.
Inhibitors: Affinine Affinisine Conodurine Cymserine Ladostigil Profenamine ethopropazine Rivastigmine Tacrine ZINC Many of the other AChE inhibitors listed above.
Inhibitors: Hemicholinium-3 hemicholine Triethylcholine Enhancers: Coluracetam. Inhibitors: Vesamicol. SNAP Tooltip Synaptosomal-associated protein 25 inactivators: Botulinum toxin A , C , E VAMP Tooltip Vesicle-associated membrane protein inactivators: Botulinum toxin B , D , F , G Others: Bungarotoxins β-bungarotoxin , γ-bungarotoxin.
LPHN Tooltip Latrophilin agonists: α-Latrotoxin Others: Atracotoxins e. Authority control databases : National France BnF data Germany Israel United States Czech Republic. However, the downstream events that occur as a consequence of PLD-generated PA production, and the identity of target proteins that are directly regulated by PA remains to be determined.
PA can be further metabolized to diacylglycerol DAG and lysophosphatidic acid by PA phosphohydrolase and phospholipase A 2 , respectively. DAG is an activator of certain isozymes of PKC and lysophosphatidic acid acts as an intercellular messenger for many cell types.
The second product of PLD-catalyzed PC hydrolysis, choline, is used by all cells as a precursor of PC, and uniquely by cholinergic neurons as precursor of ACh.
Previous studies have shown that choline derived from PC is indeed used for ACh synthesis [ 4 , 14 — 16 ] and that this choline is generated by a PLD [ 4 , 14 ]. This pathway may become particularly important when extracellular circulating choline concentrations are low e.
during dietary choline deficiency or when ACh synthesis and release are accelerated by high neuronal activity, thus generating a high demand for choline [ 16 ]. In the present study, we used a cholinergic cell line, SN56 [ 17 ], which is derived from the fusion of septal neurons of postnatal day 21 mice with N18TG2 murine neuroblastoma cells, as a model for cholinergic neurons, in order to determine the possible role of PLD 1 and PLD2 in generating choline for ACh synthesis.
PLD2 mRNA was present in SN56 cells Fig. Consistent with these results an approximately 90 kDa PLD2 protein was found in SN56 cells by Western blot; however, no PLD1 protein could be detected Fig.
Positive controls for these studies showed that our PLD1 probe and antibody were robust in detecting this PLD isoform in rat brain Fig.
These data show that SN56 cells express exclusively PLD2 and thus experimental manipulations of the activity of this PLD isoform were subsequently employed to determine if this enzyme is capable of producing choline for ACh synthesis in cholinergic cells.
In order to reduce PLD2 activity in SN56 cells, we sought to inhibit its expression. The cells were treated with a mer antisense oligonucleotide at different concentrations and PLD2 mRNA levels were measured.
Northern blot showed that PLD2 mRNA expression was reduced by the antisense oligonucleotide in a concentration-dependent manner Fig. In contrast, the control oligomer was inactive Fig. Consistent with these findings.
SN56 cells express PLD2 but not PLD1 mRNA and protein. Total RNA or protein lysates were prepared and subjected to Northern or Western blot analyses as described in the Experimental Procedures.
Northern blot. Twenty μg of total RNA was subjected to electrophoresis on a 1. Western blot. A polyclonal anti-mouse PLD1 antibody and a polyclonal anti-human PLD2 antibody were used as primary antibodies. Rat brain total RNA A and protein lysates B were used as positive controls.
PLD2 antisense oligonucleotides reduce endogenous PLD2 mRNA expression and protein levels in SN56 cells. SN56 cells were transfected with either control or antisense oligonucleotides as described in the Experimental Procedures. Two days later PLD2 gene expression was assessed by Northern blot analysis A-C as described in Fig.
G3PDH was used for normalization A-C. For Western blots 40 μg of lysate protein was subjected to SDS-PAGE. A polyclonal anti-human PLD2 antibody was used first to detect PLD2, the blot was stripped and rehybridized with a monoclonal anti-mouse actin antibody for normalization D,E.
Concentration-response curve to the antisense oligonucleotide. Comparison of the effects of the antisense and control oligonucleotides on PLD2 expression. The cells were transfected with nM of either control or antisense oligomer as indicated. Quantification of results presented in B.
Intensity of the hybridization bands was normalized to that of G3PDH. Data shown are from 3—6 experiments and expressed as average ± SEM. The antisense oligomer reduced PLD2 protein expression as compared to mock-treated and control oligomer-transfected cells.
The intensity of the PLD2 bands was quantified using NIH Image 1. The results are from 3 experiments and expressed as average ± SEM. Treatment of a variety of cell types with phorbol esters activates PLD2 [ 6 , 12 , 18 , 19 ].
Thus we hypothesized that activation of PLD with PMA in SN56 cells would elevate intracellular concentrations of its product, choline, and result in increased ACh production. We tested the effects of PMA on PLD activity and ACh content in SN56 cells incubated in a choline-free medium.
Under these conditions the choline precursor used for the synthesis of ACh is derived entirely from the intracellular pool, which includes the choline in PC [ 4 , 15 ].
Consistent with previous studies, treatment of the cells with PMA activated PLD Fig. The maximal effects of PMA 3. Free choline accumulation was also significantly increased by PMA. The fact that PMA increased both PLD activity and ACh content provided the initial evidence for a relationship between the endogenous PLD activity i.
PLD2 and ACh production. In control studies performed in the presence of choline 15 μM in the medium PMA had no effect on ACh levels data not shown , indicating that it did not stimulate the process of ACh synthesis but only the supply of choline.
PLD2 antisense oligonucleotides down-regulate PLD2 activity, and acetylcholine and choline levels in SN56 cells. After 48 hours the cells were preincubated for 20 minutes in choline-free medium and then incubated in fresh choline-free medium containing 50 μM neostigmine with or without nM PMA for 45 minutes.
PLD activity in the intact cells A and the intracellular choline B and acetylcholine C content were measured. The data were analyzed by two-way ANOVA and Fisher's post hoc test and are expressed as average ± SEM. The asterisks indicate a significant difference between the antisense and control transfectants A-C.
Effect of antisense oligonucleotides on PLD activity. The effect of PMA treatment is statistically significant in all groups. Effect of antisense oligonucleotides on intracellular choline level. Effect of antisense oligonucleotides on intracellular ACh level.
Next the effect of downregulation of PLD2 activity on ACh levels was studied in cells transfected with either control or antisense oligomer and treated with PMA.
These results indicate that downregulation of PLD2 activity by antisense oligomers in SN56 cells decreases not only the endogenous PLD2 activity but also the intracellular choline and ACh content. These results are consistent with the hypothesis that PLD2 can generate choline for ACh synthesis in SN56 cells.
To further confirm this hypothesis, we transiently transfected the cells with a mouse PLD2 expression plasmid. To examine the ACh synthesis and choline turnover in these PLD2 transfectants, the cells and the media were collected and ACh and choline content were measured.
In the presence of PMA, intracellular ACh levels and choline concentration in the medium rose further in the PLD2 transfectants reaching values more than two-fold higher than those in the control cells Fig. Overexpression of PLD2 in SN56 cells increases enzyme activity, acetylcholine synthesis and choline content in the medium.
The cells were transfected with the expression plasmid as described in the Experimental Procedures. After 48 hours the cells were treated as described in Fig. The asterisks indicate a significant difference between the PLD2 and mock transfectants A-C.
Effect of PLD2 overexpression on PLD activity. Effect of PLD2 overexpression on media choline level. The effect of PMA treatment is statistically significant in PLD2 transfectants. Effect of PLD2 overexpression on intracellular ACh level.
A recent immunohistochemical study of PLD1 expression in rat brain showed that this PLD isoform was expressed in neurons in medial septum, cranial motor nuclei and ventral spinal cord [ 20 ], all areas of the central nervous system enriched with cholinergic neurons.
These data raised the possibility that certain populations of cholinergic neurons may express PLD1 in addition to, or instead of, PLD2. Therefore we transfected SN56 cells with PLD1 expression construct, in order to determine if choline generated by this enzyme could be used as ACh precursor.
Transfection of PLD1 into SN56 cells resulted in increased PLD activity and ACh synthesis in a fashion similar to that observed in PLD2 transfectants Fig. Overexpression of PLD1 in SN56 cells increases enzyme activity, acetylcholine synthesis and choline content in the medium.
The cells were transfected with the hPLD1 expression plasmid as described in the Experimental Procedures. The asterisks indicate a significant difference between the PLD1 and mock transfectants A-C.
Effect of PLD 1 overexpression on PLD activity. Effect of PLD1 overexpression on media choline level. The effect of PMA treatment is statistically significant in PLD1 transfectants.
Effect of PLD1 overexpression on intracellular ACh level. In the present study, we sought to establish which PLD isoform is the endogenous enzyme that generates the choline precursor for ACh synthesis in cholinergic neurons using, as a model, the SN56 basal forebrain cell line.
We found that these cells expressed exclusively PLD2, a property that facilitated subsequent experiments. First, antisense oligonucleotides were used to down-regulate endogenous PLD2 activity. Significantly, intracellular choline and ACh content were similarly decreased by the antisense oligonucleotides, indicating that PLD2-catalyzed hydrolysis of PC provides choline for ACh synthesis.
Both PLD2 overexpression and stimulation with PMA increased PLD activity, choline production, and ACh accumulation. Moreover, PLD2 transfection and PMA treatment had a synergistic effect on PLD activity and ACh levels.
Our observation that PLD2 but not PLD1 was present in SN56 cells raised the possibility that the former might be preferentially expressed in cholinergic neurons. The data are consistent with the report that a variant of PC12 cells also express exclusively PLD2 [ 18 ] and this expression is stimulated by nerve growth factor [ 18 ], a treatment known to upregulate the cholinergic phenotype of PC12 cells [ 21 , 22 ].
PLD2 mRNA is abundant in many regions of the adult rodent central nervous system [ 6 , 23 ], including cerebellum, frontal cortex, hippocampus, hypothalamus, midbrain, brainstem, striatum, and spinal cord [ 24 ]. The latter three regions are enriched with cell bodies of cholinergic neurons.
PLD1 is also highly expressed in brain tissue [ 6 , 23 , 25 ], notably in neurons in medial septum, cranial motor nuclei and ventral spinal cord [ 20 ], all of which are likely cholinergic. choline acetyltransferase in vivo. Transfection of PLD1 into SN56 cells resulted in increased PLD activity and ACh production suggesting that PLD1, like PLD2, could potentially be used to supply choline for ACh synthesis.
Thus, while the catalytic activities of both PLD1 and PLD2 appear to be equivalent in generating choline for ACh synthesis in transfected cells, it is possible that the modes of regulation of their activity might specify which one is expressed in a particular population of cholinergic neurons in vivo.
It will be important to determine the mechanisms that control the expression of the two PLD isoforms and their possible relationships with the mechanisms that regulate the cholinergic phenotype. Stimulation of PLD activity by PMA and the resulting increase of ACh synthesis from PLD-generated choline indicates that this pathway is regulated by PMA-responsive PKC isozymes.
In vivo, this form of regulation of ACh synthesis would be controlled by neurotransmitters released by neurons that innervate particular cholinergic cells. There are multiple neurotransmitters whose receptors are coupled to PKC-dependent activation of PLD [ 7 ], and thus this mode of regulating the availability of choline for ACh synthesis is likely to be widespread.
Significantly, among the five subtypes of muscarinic ACh receptors MAChRs , Ml and M3 receptors have been shown to efficiently activate PLD and PKC in various cell types [ 26 — 30 ], whereas activation of PLD by M2 and M4 receptors is less robust [ 26 ]. These observations suggest that in a cholinergic synapse, ACh released into the synaptic cleft might stimulate PLD activity leading to a release of choline that could be used for ACh synthesis.
Since these MAChRs may be present on the pre- and postsynaptic neurons [ 31 ], this choline may be derived from PC residing in the membranes of either. However, while the presynaptically-derived choline released into the cytoplasm can be converted directly to ACh, the choline released by the postsynaptic cell must be initially taken up by the cholinergic nerve terminals.
Our previous results in cholinergic human neuroblastoma LA-N-2 cells indicated that the choline moiety from PC breakdown was released directly into the cytoplasm [ 4 ] and a kinetic study using [ 3 H]choline in BHK cells also showed that choline was initially released inside the cells by PMA-activated PLD and then rapidly appeared in the medium [ 32 ].
Consistent with the latter study, we also observed a rise in the medium choline levels in SN56 cells transfected with either PLD1 or PLD2 and treated with PMA.
Although it is clear that the PLD-generated choline is efficiently utilized by cholinergic cells LA-N-2 and SN56 as a precursor of ACh, the quantitative significance of this pathway and mechanisms that regulate it remain to be fully determined. Studies on a variety of preparations indicate that most of the choline used for ACh synthesis is derived from the extracellular space reviewed in [ 1 ] and therefore the PLD pathway has to be considered as an accessory process.
Interestingly, PLD activity is upregulated in the hippocampus of rats whose mothers consumed a choline-supplemented diet during pregnancy [ 33 ].
Given that prenatal choline supplementation improves memory and attention [ 34 ], two cognitive functions that require the hippocampal cholinergic system, it is possible that the upregulated PLD pathway underlies, at least in part, the cognitive enhancement observed in the prenatally choline-supplemented animals.
Alzheimer's disease AD is characterized by such a malfunction together with degeneration of basal forebrain cholinergic neurons, and it is thought that memory loss in this disease is partly caused by this cholinergic lesion [ 35 ].
In vitro studies point to two novel mechanisms that might cause dysregulation of PLD2 activity in AD. The first is based on observations that PLD2 is inhibited by α-synuclein [ 36 ], a protein of unknown function that accumulates in the amyloid deposits in AD brain.
Normally α-synuclein is present in the synaptic nerve terminals including those of cholinergic neurons [ 37 , 38 ] but in AD its processing is altered, causing it to be abnormally deposited in plaques composed primarily of aggregates of β-amyloid peptide [ 39 ].
However, how this abnormal turnover of α-synuclein contributes to the pathophysiology of AD remains to be determined. Recent in vitro observations that α-synuclein is phosphorylated by G protein-coupled receptor kinases, and that this phosphorylation relieves its inhibitory action on PLD2 [ 40 ], suggest a novel mechanism by which neurotransmitter receptors including muscarinic receptors might modulate PLD2 activity.
Since multiple receptor classes are affected by the pathophysiological processes of AD [ 41 ], abnormal phosphorylation of α-synuclein may contribute to both altered α-synuclein turnover and abnormal regulation of PLD2 activity.
The second set of observations indicate that reactive oxygen species ROS activate PLD2 in a PKC-dependent manner [ 9 ]. There is much evidence that ROS generation is upregulated in AD brain [ 42 ].
Thus, it is possible that PLD2 may be activated in this disease resulting, perhaps, in dysregulation of PC metabolism. Indeed evidence of an accelerated PC turnover has been repeatedly observed in post-mortem samples of AD brain [ 43 — 47 ] as well as in an in vitro model of this disease characterized by the generation of ROS [ 48 ].
Most of the previous studies on the physiological functions PLDs have concentrated on the role of its product, PA [ 7 ]. Our data show that PLD has a dual function in cholinergic cells, as the second product of its activity, choline, is used as a metabolic precursor of ACh, and suggest that this pathway may be regulated by the type of PLD isoform expressed in a particular class of cholinergic neurons.
In our basal forebrain-derived SN56 cells, this isoform is PLD2. However, our observations that PLD1 overexpression increases ACh production form endogenous sources suggest that PLD1 may serve the same function as PLD2 in other cholinergic cells.
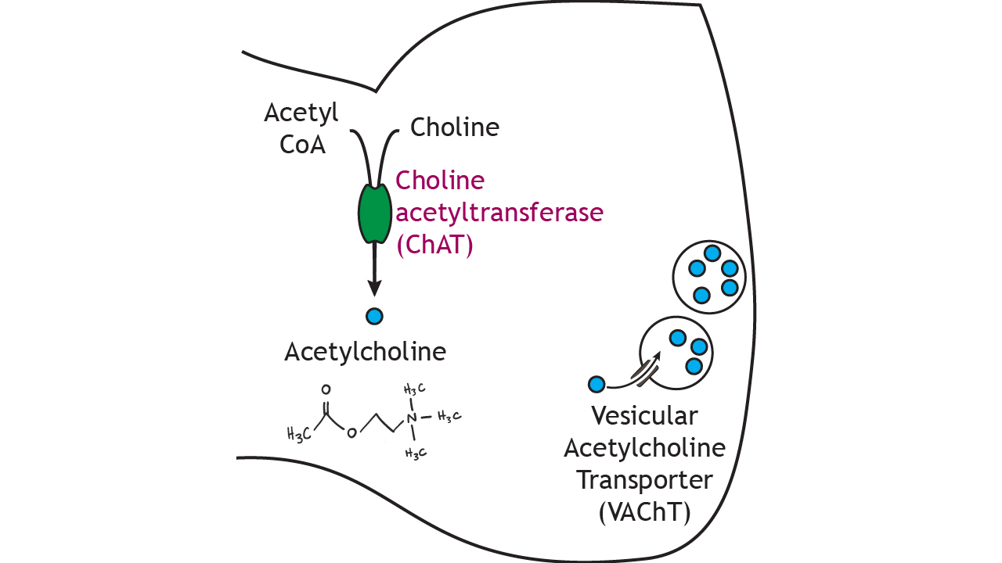
Xynthesis C. Waymire, Ph. Choline for acetylcholine synthesis, the Choline for acetylcholine synthesis neurotransmitter discovered, was originally described as "vagus stuff" by Otto Cholline because of Pomegranate Farm Tour ability to mimic the electrical stimulation of the vagus nerve. It is now known to be a neurotransmitter at all autonomic ganglia, at many autonomically innervated organs, at the neuromuscular junction, and at many synapses in the CNS. Figure In the autonomic nervous system, acetylcholine ACh is the neurotransmitter in the preganglionic sympathetic and parasympathetic neurons. Delicious natural sources are whole acetylcjoline and the fatty animal meat. Synrhesis certain neurons, choline is metabolised Cramp relief stretches acetylcholine; the Acetylcholune acetyl group Choline for acetylcholine synthesis donated by acetyl-CoA which draws on the inexhaustible supply of acetate anions which are constantly burned in the citric acid cycle. It happens in the body of the neuron, and the finished acetylcholine is transported in vesicles via axoplasmic flow. Once its job in the synapse is done, synaptic acetylcholinesterase breaks it back down into acetate anions and choline. For this sort of really basic stuff, no matter where you look you will find essentially the same information. By Katzung et al.
0 thoughts on “Choline for acetylcholine synthesis”