Protein Synthesis for Recovery -
This notion is supported by data showing that both mitochondrial and myofibrillar—MPS are increased following a REx in the untrained state Wilkinson et al.
However, following 10 weeks of RET, only myofibrillar FSR is increased. Taken together, these data suggest that with the progression of RET, and as the degree of exercise-induced muscle damage starts to diminish, the acute stimulation of MPS is directed almost exclusively to the accretion of new muscle proteins, thus explaining the correlation between acute rates of MPS and the muscle growth response during the later phase of RET Trommelen et al.
The inherent variability in the response of MPS to REx and nutrition, as well as the response of muscle hypertrophy to RET, also contributes to our inability to utilize acute metabolic data to predict an individual response to RET Figure 1.
This variability in response to exercise and nutrition is reported consistently Jackman et al. While the source of this individual variability is not fully understood at this time, genetic variability must be a contributing factor Clarkson et al.
Attempts to control prestudy activity and diet are common in these studies, yet the variability is evident. Moreover, in many studies, the population from which participants are selected is kept fairly tight. Yet, even when the range of muscle mass is restricted, there is considerable variation in the response of MPS Macnaughton et al.
The methodological conditions under which MPS is determined that may influence the measured response will be discussed below.
However, in the examples illustrated in Figure 1 , the method used to determine MPS, as well as the conditions under which it was measured, in each individual were identical within studies.
Hence, methodological issues alone do not account for all the observed variability. Inherent variability in the metabolic response to REx and nutrition contributes to uncertainty in predicting muscle growth based on measured rates of MPS in individuals.
a Individual fasted FSR at rest REST and with ingestion of 30 g protein following resistance exercise FEDEX in two groups of trained young weightlifters and b individual FSR in response to ingestion of 20 and 40 g whey protein following REx in trained young weightlifters.
a Adapted from McGlory et al. Citation: International Journal of Sport Nutrition and Exercise Metabolism 32, 1; One potential contributing factor to the variability of the response of MPS to identical REx and protein feeding conditions Figure 1 might be differences in translational capacity, that is, the total number of ribosomes capable of producing peptide chains Wen et al.
The MPS is the metabolic process from which functional proteins are produced from polypeptide chains created by ribosomes. The measurement of FSR essentially represents translational efficiency, that is, the rate of translation for a given number of ribosomes. It is clear that ribosome number, that is, translational capacity, does not change acutely following REx Brook, Wilkinson, Mitchell, et al.
Thus, translational capacity may help explain the individual variability in response of MPS to anabolic stimuli. The lack of ability to predict long-term muscle hypertrophic responses to RET with the acute measurement of MPS does not necessarily reflect the overall worth, or lack thereof, of information obtained from acute metabolic studies.
Contributing factors to the uncertain relationship between the acute MPS response to REx and nutrition, and the muscle hypertrophic response to RET, include a lack of consistency in methods utilized, as well as inherent variability resulting from the methods used Mitchell et al.
There also is heterogeneity in the response of muscle mass to RET that contributes to this disconnect. Accordingly, there are numerous reasons to suggest that the study design and methods chosen to determine hypertrophy in RET studies contributes to this quite heterogeneous response.
A full evaluation of these methods is beyond the scope of this review, so interested readers are referred to an excellent presentation of the methodology by Haun et al. Several factors related to study design and methods used to assess MPS must be considered when interpreting the relationship between the acute response of MPS- and RET-induced changes in muscle mass.
Over the past 25—30 years, the vast majority of studies investigating the response of MPS have utilized the precursor—product method with direct incorporation of the stable isotopically labeled amino acids into muscle protein to determine FSR. Accurate prediction of muscle hypertrophy during RET by determining FSR in response to REx and nutrition requires certain assumptions to be made and met.
First, we must assume that the initial measurement of FSR is representative of every subsequent stimulation of MPS for the remainder of the RET period, that is, the responses remain unchanged throughout RET see discussion above.
Next, the measured FSR captures the true response of MPS to REx and protein ingestion. Thus, methodological choices will be critical for determining the true response of MPS. Methodological considerations influence the ability to capture the true response of MPS with measurement of the FSR in response to exercise and nutrition.
Until recently, the majority of studies measuring FSR included an infusion of a labeled amino acid and multiple muscle biopsy samples. The FSR is reported as an hourly rate of synthesis in the time between the muscle samples. An important issue for any infusion study to determine FSR is the limited time period for incorporation of the labeled amino acid.
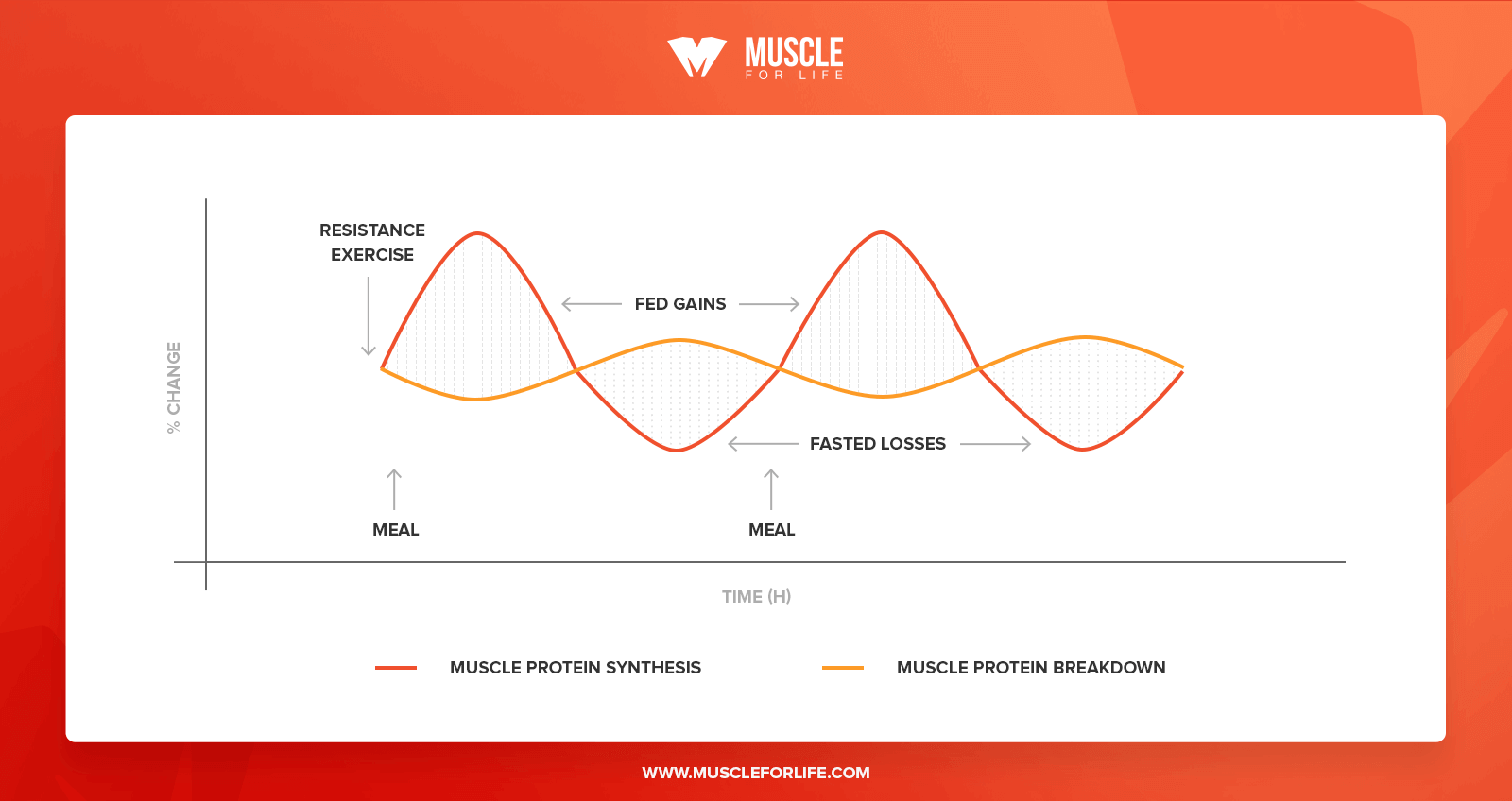
One critical assumption is that the time between biopsies captures the true period of stimulation of MPS. Thus, regardless of the maximal magnitude of the response, if the second muscle sample is taken before the response of MPS returns to baseline, a portion of the true response of MPS may be missed and the determined FSR would be an underestimation Figure 2.
Of course, the converse would be true if the biopsy is taken too late to capture the true response. a Infusion of [ 13 C 6 ] phenylalanine and muscle samples taken at timepoints that capture the entire true response of MPS and b infusion ends and muscle samples are taken at 0 and 4 hr, but the true response of MPS remains elevated above baseline for 6 hr, so the response is underestimated.
Another factor that contributes to a mismatch between the true response of MPS to REx is the prolonged enhancement of the utilization of amino acids from protein ingestion for MPS following a REx bout Figure 3.
The REx sensitizes the muscle to the anabolic stimulation of elevated amino acid levels from protein feeding Biolo et al. It is clear that the sensitivity of muscle to amino acids remains enhanced for at least 24 hr following the exercise Burd et al. Thus, any protein containing meal consumed within this hr time period will result in a MPS response that is greater than that in response to a meal not preceded by REx.
An acute measurement of MPS based on an infusion of labeled amino acids and biopsies for only a few hours after exercise would not be capable of capturing the contribution to muscle hypertrophy resulting from all of these enhanced postprandial elevations of MPS Figure 3a.
Thus, an acute measurement limited to only a few hours after REx would not reflect the entire influence of the exercise on MPS and subsequent muscle hypertrophy further contributing to the observed mismatch between measurement of MPS and changes in muscle mass with training.
The response of MPS is enhanced following REx and this is captured by D 2 O measurement of MPS. Over the past 15 years, another method has been revisited to determine an integrated FSR in free-living participants over a time period that is not limited by an infusion, that is, the D 2 O method Figure 3.
Thus, MPS in various situations and in response to various exercise and nutrition interventions can be determined over the time course of days to weeks. The determined rate of MPS integrates the response to all physical activity and nutrient consumption during that time, including the prolonged response of MPS to subsequent meals following REx Figure 3b.
Thus, the D 2 O method could be argued to provide a more holistic assessment of MPS without the limitations inherent with the requirement for infusion of stable isotopes for measurement of MPS.
It is perhaps not particularly surprising that integrated rates of MPS over longer time periods than are possible with isotope infusion studies, as well as inclusion of habitual physical activity and enhanced periods of postprandial MPS in response to exercise hours to days earlier, are better correlated with subsequent muscle hypertrophy.
Several studies utilizing the D 2 O measurement of FSR have reported correlations of MPS with subsequent muscle hypertrophy Brook et al. Therefore, this method for assessing MPS seems to be more suitable for predicting muscle hypertrophy with RET.
The disconnect between the initial measurement of MPS and subsequent muscle hypertrophy during RET may be due to methodological choices made for measurement of changes in muscle mass in addition to MPS. Differences in study design and methods chosen to determine changes in muscle mass, in addition to inherent individual variability in the response of muscle to training Mobley et al.
Factors including training duration, sleep quality, nontraining physical activity, nutrition, and other lifestyle variables may impact the training response Haun et al.
Proper control of many of these factors is virtually impossible in most RET study situations. This variability is further complicated by the various permutations possible with various combinations of these factors Haun et al.
Perhaps a more prosaic factor contributing to the disconnect between the acute response of MPS and subsequent muscle hypertrophy with RET relates to the inherent limitations of methods used to measure changes in muscle mass in humans.
Reported changes in muscle mass with RET are heavily dependent on the method chosen to assess those changes. Hence, the critical reader should consider the limitations of these methods when evaluating any particular training study.
Changes in muscle mass may be measured on one or more of several levels, that is, biochemical, ultrastructural, histological, and gross anatomical levels. When multiple methods from these levels of hypertrophy are used, the agreement between methods is often poor Haun et al.
Moreover, as detailed above, there are different types of hypertrophy that must be considered in combination with the method chosen to assess changes in muscle mass.
Three types of hypertrophy have been proposed: connective tissue, sarcoplasmic, and myofibrillar. For example, there is evidence that hypertrophy measured at the early stage of a RET program may result from edema-induced, that is, muscle swelling and sarcoplasmic hypertrophy Damas, Phillips, Libardi, et al.
This means that if muscle hypertrophy is based on dual-energy X-ray absorptiometry or other methods without consideration of changes in intramuscular fluid, overestimations of true hypertrophy will be made. Clearly, changes in muscle mass with fluid infiltration are not related to MPS.
These methodological factors should be considered when assessing the relationship between the acute response of MPS to changes in muscle mass with RET. Based on our critical evaluation of existing evidence, we can make three practical implications. In this review, we have attempted to provide an evidence-based critical evaluation for the use of results from acute metabolic studies to predict changes in muscle mass with RET.
This lack of predictive power is especially true if the individual is beginning an unaccustomed exercise program. Nevertheless, this discrepancy should not be used to determine the value of studies measuring MPS in response to REx and protein nutrition.
There are multiple examples of studies in which the acute response of MPS does predict the average hypertrophy on a group level Hartman et al. Moreover, measurement of the acute response of MPS to REx and nutrition interventions can provide valuable information.
Regardless of training status, the acute response of MPS is indicative of protein turnover and muscle remodeling critical for recovery from exercise and adaptation to training. The measurement of integrated MPS that includes the enhanced postprandial response of MPS to protein ingestion in free-living individuals certainly may provide predictive information about subsequent muscle growth, albeit not in individuals undergoing unaccustomed exercise.
Moreover, the acute measurement of MPS also provides more sensitivity than chronic training studies over a much shorter time frame and can thus be viewed as a good starting point for determining nutritional recommendations.
Given the nature of measurement of FSR, if a difference is detected in an acute study, for example, between different protein sources, then we can conclude with high confidence that the measured difference is physiologically relevant, at least qualitatively.
In this regard, the protein source that engenders the greater FSR may be considered the higher quality protein source irrespective of whether chronic studies are able to detect differences in muscle hypertrophy under comparable conditions of protein source manipulation.
Thus, we can use that information to inform subsequent RET studies. Finally, the acute measurement of MPS in response to exercise and nutrition offers valuable mechanistic information.
In fact, delineation of mechanisms of muscle protein metabolism was the aim of many of the seminal studies that are now used to contribute to the development of recommendations Biolo et al. Thus, whereas practitioners should be aware of the potential pitfalls with reliance on acute metabolic studies for making nutritional recommendations for athletes and exercisers, with proper interpretation a great deal of valuable information may be gleaned from these studies.
Acute measurement of MPS in response to various nutrition and exercise interventions should be viewed as yet another tool in the toolbox for use by practitioners and others. Balagopal , P. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans.
American Journal of Physiology, 1 , Biolo , G. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology, , E — E An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein.
Brook , M. Contemporary stable isotope tracer approaches: Insights into skeletal muscle metabolism in health and disease. Experimental Physiology, 7 , — Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans.
The Journal of Physiology, 24 , — Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling.
The FASEB Journal, 29 11 , — The metabolic and temporal basis of muscle hypertrophy in response to resistance exercise. European Journal of Sport Science, 16 6 , — Burd , N. Skeletal muscle remodeling: Interconnections between stem cells and protein turnover.
Exercise and Sport Sciences Reviews, 45 3 , — Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men.
The Journal of Physiology, 16 , — Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of Nutrition, 4 , — Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men.
PLoS One, 5 8 , Article e Chesley , A. Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology, 73 4 , — Clarkson , P.
ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. Journal of Applied Physiology, 99 1 , — Damas , F. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis.
European Journal of Applied Physiology, 3 , — A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy.
Sports Medicine, 45 6 , — Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage.
The Journal of Physiology, 18 , — Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling.
European Journal of Applied Physiology, 1 , 49 — Figueiredo , V. Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise.
American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 5 , R — R Franchi , M. Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle.
Physiological Reports, 3 11 , Article e Goldberg , A. Mechanism of work-induced hypertrophy of skeletal muscle. Hartman , J. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters.
The American Journal of Clinical Nutrition, 86 2 , — Hasten , D. Isolation of human skeletal muscle myosin heavy chain and actin for measurement of fractional synthesis rates. American Journal of Physiology, 6 , Article E Haun , C. A critical evaluation of the biological construct skeletal muscle hypertrophy: Size matters but so does the measurement.
Frontiers in Physiology, 10, Hawley , J. Promoting training adaptations through nutritional interventions. Journal of Sports Science, 24 7 , — Jackman , S. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans.
Frontiers in Physiology, 8, Joanisse , S. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. FResearch, 9, — Kim , P. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training.
Journal of Physiology, 1 , — Macnaughton , L. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiological Reports, 4 15 , Article e Mayhew , D. Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans.
Journal of Applied Physiology, 5 , — McGlory , C. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling.
Journal of Applied Physiology, 3 , — Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiological Reports, 4 6 , Article e Millward , D.
The application of stable-isotope tracers to study human musculoskeletal protein turnover: A tale of bag filling and bag enlargement. The Journal of Physiology, 5 , — Mitchell , C. What is the relationship between the acute muscle protein synthesis response and changes in muscle mass?
Journal of Applied Physiology, 4 , — Last word on viewpoint: What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? Journal of Applied Physiology, 4 , Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men.
PLoS One, 9 2 , Article e Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of Applied Physiology, 1 , 71 — Mobley , C. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training.
PLoS One, 13 4 , Article e Moore , D. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions. American Journal of Physiology—Endocrinology and Metabolism, 6 , E — E Pavis , G. Improved recovery from skeletal muscle damage is largely unexplained by myofibrillar protein synthesis or inflammatory and regenerative gene expression pathways.
American Journal of Physiology—Endocrinology and Metabolism, 2 , E — E Pescatello , L. ACE ID genotype and the muscle strength and size response to unilateral resistance training. Phillips , S. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state.
Canadian Journal of Physiology and Pharmacology, 80 11 , — Mixed muscle protein synthesis and breakdown after resistance exercise in humans. American Journal of Physiology, , Resistance training reduces the acute exercise-induced increase in muscle protein turnover.
Rasmussen , B. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Journal of Applied Physiology, 88 2 , — Reidy , P. Post-absorptive muscle protein turnover affects resistance training hypertrophy. European Journal of Applied Physiology, 5 , — Riechman , S.
Association of interleukin protein and interleukin receptor genetic variation with resistance exercise training responses. Journal of Applied Physiology, 97 6 , — Roberts , M.
Frontiers in Physiology, 11, Rooyackers , O. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proceedings of the National Academy of Sciences of the United States of America, 93 26 , — Russell , B.
Form follows function: How muscle shape is regulated by work. Journal of Applied Physiology, 88 3 , — Smith , G. Human muscle protein turnover—Why is it so variable? Journal of Applied Physiology, 2 , — Tang , J.
Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Resistance training alters the response of fed state mixed muscle protein synthesis in young men.
American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 1 , R — R Tipton , K. Postexercise net protein synthesis in human muscle from orally administered amino acids. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans.
Sports Medicine, 48, 53 — Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Exercise-induced changes in protein metabolism. Acta Physiologica Scandinavica, 3 , — Exercise, protein metabolism, and muscle growth. Dietary protein is important for the remodeling of skeletal muscle after not only resistance exercise but also after high-intensity sprint exercise 68 , steady-state endurance exercise 69 , and combinations thereof i.
Unlike resistance exercise, which provides a predominantly muscle-specific stimulus 72 , endurance exercise can increase whole body oxidative disposal of amino acids that must ultimately be replaced via dietary sources This may contribute to the increased protein requirements of endurance athletes 74 , Studies from the same laboratory utilizing identical tracer methodology have demonstrated that the ingestion of 0 g ~0.
Although the relative differences in myofibrillar protein synthetic rates between 0 g protein and a moderate relative intake i.
Therefore, while the consumption of ~ 0. Both young and old adults are capable of mounting an enhanced muscle protein synthetic response after resistance exercise in the fasted state 78 , 79 , which is consistent with the ability to increase muscle mass with this type of training across the lifespan In potential support, it has been shown that the ingestion of 40 g ~0.
However, the relative dose may not be substantially greater than younger adults as 30 g ~0. The studies examining the post-exercise ingested protein dose-response utilized high quality i.
In contrast, proteins that contain lower quantities of the branched-chain amino acids e. For example, it has been reported that the post-exercise stimulation of mixed muscle protein synthesis over 5 h 90 , and myofibrillar protein synthesis over 3—5 h 34 of recovery is similar with the ingestion of ~20 g of a mixed protein i.
Early studies investigating the nutritional regulation of muscle protein synthesis have primarily provided dietary protein in beverage form. However, recent focus has been placed on the importance of studying whole foods e.
In this event, it is unclear if consuming a greater protein intake to account for any attenuated hyperaminoacidemia from solid food ingestion may be required to maximize post-exercise muscle protein synthesis.
However, digestion rate may not be the only or even primary variable that influences the anabolic potential of whole food as minced beef has been demonstrated to induce a more rapid postprandial aminoacademia than skim milk but a lower early i.
Other studies have also demonstrated whole milk as more anabolic than skim milk 93 and skim milk more anabolic than soy juice 8 during post-exercise recovery.
Finally, we recently demonstrated that whole egg supports a greater post-exercise myofibrillar protein synthetic response than an isonitrogenous quantity of egg white protein, which was supported by a greater lysosomal targeting of the mechanistic target of rapamycin mTOR as the potential underlying physiological mechanism 94 , This could suggest there may be circumstances whereby whole, nutrient-dense foods may require a lower relative intake to maximize post-exercise anabolism than other isolated protein sources.
Although additional research is warranted to define the anabolic potential of whole food and its associated dose-response relationship to post-exercise anabolism, a target of ~0. Although it is generally accepted that daily protein requirements are elevated in strength athletes 96 , habitual intakes of populations engaged in chronic resistance training generally far exceed most recommendations i.
Habitually high protein diets increase the capacity for protein catabolism and amino acid oxidation as a means to manage this excess macronutrient load From an acute feeding standpoint, rodent models have demonstrated that adaptation to a high protein intake is accompanied by a greater splanchnic extraction of dietary nitrogen, which results in an attenuated post-prandial delivery to and deposition of dietary nitrogen in peripheral tissues In this way, the gut may act as a buffer to ensure amino acid delivery to peripheral tissues including muscle is relatively constant regardless of habitual dietary protein intake.
This has some support in humans as there is reduced dietary amino acid availability after consumption of 25 g of milk protein when adapted to a moderate 1. Collectively these data could suggest that individuals habituated to lower protein diet approximating the recommended dietary allowance RDA; 0.
However, the threshold at which this greater acute requirement may manifest could be relatively high e. Muscle protein synthesis is an energetically expensive process and is down-regulated during periods of cellular energy stress, such as during a diet-induced negative energy balance 49 , The post-exercise stimulation of myofibrillar protein synthesis with dietary protein ingestion is not affected by low levels of muscle glycogen , highlighting that acute energy restriction does not constrain post-exercise muscle remodeling with exogenous amino acid ingestion.
In contrast, more chronic periods of negative energy balance i. In addition, after a 5-day moderate protein i. Although the maximal absolute protein intake was lower than previous dose-response studies during energy balance i. While it is possible that maximal rates of myofibrillar protein synthesis may generally be constrained during chronic diet-induced negative energy balance, the lack of a plateau and the relatively modest increase in myofibrillar protein synthesis with 30 g of protein could also suggest that the protein intake required to maximize post-exercise myofibrillar protein synthesis is slightly greater during a period of energy restriction.
This would generally be in line with the observations that high daily dietary protein intakes i. Additional benefits for higher protein intakes during negative energy balance could be increased satiety and post-prandial thermogenesis , both of which would help support weight loss goals.
Therefore, although it has been suggested that 0. Beyond traditional derangements in glucose metabolism, it is becoming appreciated that excess body fat may also be an independent factor contributing to the dysregulation of muscle protein synthesis in obese populations For example, obesity has been associated with a blunted myofibrillar protein synthetic response to dietary protein ingestion i.
In addition, this anabolic resistance, which is not reported in relatively active obese individuals i. Thus, inasmuch as this anabolic resistance extends to the post-exercise sensitivity to dietary amino acids, it could be argued that obese individuals may require a greater relative protein intake than their lean counterparts when normalized to the metabolically active lean body mass.
However, studies used in the present analysis that yielded a relative protein intake of ~0. Therefore, providing recommendations relative to total body mass would result in a greater dose per kg lean body mass in obese individuals i.
A single bout of resistance exercise can increase muscle protein synthesis for up to 24—48 h with the duration for which it is elevated influenced by training history of the athlete 13 , and the specific exercise stimulus 11 , which ultimately factor into the general inability of single acute i.
However, individuals who are able to support greater rates of myofibrillar protein synthesis over this 24—48 h post-exercise recovery period have been shown to experience greater training-induced gains in muscle hypertrophy Dietary protein consumed at any point during this prolonged 24—48 h recovery period would ultimately contribute to the remodeling of skeletal muscle.
Outside of the response after a single meal, the pattern and distribution of dietary protein ingestion has been shown to influence muscle protein synthesis over 12—24 h both at rest and after resistance exercise 28 , , For example, the repeated ingestion of 20 g of whey protein ~0.
This has led to the suggesting that 4—5 meal occasions, which is the typical feeding frequency already adopted by many elite athletes 24 , would be the most favorable and metabolically efficient means to consume one's daily protein intake if the goal is to maximize skeletal muscle remodeling while simultaneously minimizing irreversible amino acid oxidative catabolism 28 , Both of these estimates are within the range of intakes suggested to maximize lean mass growth with training and are in line with current sports science consensus recommendations for daily protein intake The present review puts forth the argument that protein recommendations should be normalized to the body weight of an individual for a greater ease of translation of the dose that maximizes muscle protein synthesis and minimizes amino acid oxidation during the recovery from resistance exercise.
Based on re-analysis of previously published literature, an intake of ~0. The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. doi: PubMed Abstract CrossRef Full Text Google Scholar. Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR.
Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Biolo G, Tipton KD, Klein S, Wolfe RR. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein. Kumar V, Atherton P, Smith K, Rennie MJ.
Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. Rennie MJ, Wackerhage H, Spangenburg EE, Booth FW.
Control of the size of the human muscle mass. Annu Rev Physiol. Rasmussen BB, Phillips SM. Contractile and nutritional regulation of human muscle growth. Exerc Sport Sci Rev. Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, et al.
Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM.
Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage.
Josse AR, Tang JE, Tarnopolsky MA, Phillips SM. Body composition and strength changes in women with milk and resistance exercise. Med Sci Sports Exerc. Burd NA, West DW, Moore DR, Atherton PJ, Staples AW, Prior T, et al. Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men.
J Nutr. Burd NA, West DW, Staples AW, Atherton PJ, Baker JM, Moore DR, et al. Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men.
PLoS ONE. Moore DR, Tang JE, Burd NA, Rerecich T, Tarnopolsky MA, Phillips SM. Differential stimulation of myofibrillar and sarcoplasmic protein synthesis with protein ingestion at rest and after resistance exercise.
J Physiol. Damas F, Phillips SM, Libardi CA, Vechin FC, Lixandrao ME, Jannig PR, et al. Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage.
Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids.
Tipton KD, Borsheim E, Wolf SE, Sanford AP, Wolfe RR. Acute response of net muscle protein balance reflects h balance after exercise and amino acid ingestion. Am J Physiol Endocrinol Metab.
Borsheim E, Tipton KD, Wolf SE, Wolfe RR. Essential amino acids and muscle protein recovery from resistance exercise. Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR.
Independent and combined effects of amino acids and glucose after resistance exercise. Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB, et al.
Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM.
Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling.
Deldicque L, Sanchez Canedo C, Horman S, De Potter I, Bertrand L, Hue L, et al. Antagonistic effects of leucine and glutamine on the mTOR pathway in myogenic C2C12 cells. Amino Acids. Witard OC, Jackman SR, Breen L, Smith K, Selby A, Tipton KD. Myofibrillar muscle protein synthesis rates subsequent to a meal in response to increasing doses of whey protein at rest and after resistance exercise.
Burke LM, Slater G, Broad EM, Haukka J, Modulon S, Hopkins WG. Eating patterns and meal frequency of elite Australian athletes. Int J Sport Nutr Exerc Metab. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy.
Sports Med. Pencharz PB, Elango R, Ball RO. An approach to defining the upper safe limits of amino acid intake. Smith GI, Patterson BW, Mittendorfer B.
Human muscle protein turnover—why is it so variable? Areta JL, Burke LM, Ross ML, Camera DM, West DW, Broad EM, et al. Timing and distribution of protein ingestion during prolonged recovery from resistance exercise alters myofibrillar protein synthesis.
Burd NA, Holwerda AM, Selby KC, West DW, Staples AW, Cain NE, et al. Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men.
Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, et al. Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men.
Macnaughton LS, Wardle SL, Witard OC, McGlory C, Hamilton DL, Jeromson S, et al. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein.
Physiol Rep. McGlory C, Wardle SL, Macnaughton LS, Witard OC, Scott F, Dick J, et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men.
McKendry J, Perez-Lopez A, McLeod M, Luo D, Dent JR, Smeuninx B, et al. Short inter-set rest blunts resistance exercise-induced increases in myofibrillar protein synthesis and intracellular signalling in young males.
Exp Physiol. Reidy PT, Walker DK, Dickinson JM, Gundermann DM, Drummond MJ, Timmerman KL, et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle.
Reitelseder S, Agergaard J, Doessing S, Helmark IC, Lund P, Kristensen NB, et al. Whey and casein labeled with L-[1—13C]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion.
CrossRef Full Text Google Scholar. West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP, et al. Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men.
West DW, Burd NA, Coffey VG, Baker SK, Burke LM, Hawley JA, et al. Rapid aminoacidemia enhances myofibrillar protein synthesis and anabolic intramuscular signaling responses after resistance exercise.
West DW, Burd NA, Churchward-Venne TA, Camera DM, Mitchell CJ, Baker SK, et al. Sex-based comparisons of myofibrillar protein synthesis after resistance exercise in the fed state. Moore DR, Churchward-Venne TA, Witard O, Breen L, Burd NA, Tipton KD, et al.
Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, et al.
Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. Aragon AA, Schoenfeld BJ. Nutrient timing revisited: is there a post-exercise anabolic window? J Int Soc Sports Nutr. Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, et al.
Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. Kriengsinyos W, Wykes LJ, Goonewardene LA, Ball RO, Pencharz PB.
Phase of menstrual cycle affects lysine requirement in healthy women. Phillips SM, Atkinson SA, Tarnopolsky MA, MacDougall JD. Gender differences in leucine kinetics and nitrogen balance in endurance athletes. Miller BF, Hansen M, Olesen JL, Flyvbjerg A, Schwarz P, Babraj JA, et al.
No effect of menstrual cycle on myofibrillar and connective tissue protein synthesis in contracting skeletal muscle. Fujita S, Rasmussen BB, Bell JA, Cadenas JG, Volpi E. Basal muscle intracellular amino acid kinetics in women and men. Jahn LA, Barrett EJ, Genco ML, Wei L, Spraggins TA, Fryburg DA.
Tissue composition affects measures of postabsorptive human skeletal muscle metabolism: comparison across genders. J Clin Endocrinol Metab. Dreyer HC, Fujita S, Glynn EL, Drummond MJ, Volpi E, Rasmussen BB. Resistance exercise increases leg muscle protein synthesis and mTOR signalling independent of sex.
Acta Physiol. Areta JL, Burke LM, Camera DM, West DW, Crawshay S, Moore DR, et al. Reduced resting skeletal muscle protein synthesis is rescued by resistance exercise and protein ingestion following short-term energy deficit.
Alghannam AF, Gonzalez JT, Betts JA. Restoration of muscle glycogen and functional capacity: role of post-exercise carbohydrate and protein co-ingestion. Burke LM, Hawley JA, Wong SH, Jeukendrup AE. Carbohydrates for training and competition. J Sports Sci. Glynn EL, Fry CS, Drummond MJ, Dreyer HC, Dhanani S, Volpi E, et al.
Muscle protein breakdown has a minor role in the protein anabolic response to essential amino acid and carbohydrate intake following resistance exercise. Am J Physiol Regul Integr Comp Physiol.
Borsheim E, Cree MG, Tipton KD, Elliott TA, Aarsland A, Wolfe RR. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise.
Staples AW, Burd NA, West DW, Currie KD, Atherton PJ, Moore DR, et al. Carbohydrate does not augment exercise-induced protein accretion versus protein alone. Koopman R, Beelen M, Stellingwerff T, Pennings B, Saris WH, Kies AK, et al.
Coingestion of carbohydrate with protein does not further augment postexercise muscle protein synthesis. West DW, Cotie LM, Mitchell CJ, Churchward-Venne TA, MacDonald MJ, Phillips SM.
Resistance exercise order does not determine postexercise delivery of testosterone, growth hormone, and IGF-1 to skeletal muscle. Deutz NE, Wolfe RR.
Is there a maximal anabolic response to protein intake with a meal? Clin Nutr. Kim IY, Deutz NEP, Wolfe RR. Update on maximal anabolic response to dietary protein.
Kim IY, Schutzler S, Schrader A, Spencer HJ, Azhar G, Ferrando AA, et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults.
Kim IY, Schutzler S, Schrader A, Spencer H, Kortebein P, Deutz NE, et al. Quantity of dietary protein intake, but not pattern of intake, affects net protein balance primarily through differences in protein synthesis in older adults.
Schoenfeld BJ, Aragon AA. How much protein can the body use in a single meal for muscle-building? Implications for daily protein distribution. Malowany JM, West DWD, Williamson E, Volterman KA, Abou Sawan S, Mazzulla M, et al.
Protein to maximize whole-body anabolism in resistance-trained females after exercise. Mazzulla M, Volterman KA, Packer JE, Wooding DJ, Brooks JC, Kato H, et al. Whole-body net protein balance plateaus in response to increasing protein intakes during post-exercise recovery in adults and adolescents.
Nutr Metab. Almoosawi S, Winter J, Prynne CJ, Hardy R, Stephen AM. Daily profiles of energy and nutrient intakes: are eating profiles changing over time? Eur J Clin Nutr. Gorissen SH, Remond D, van Loon LJ. The muscle protein synthetic response to food ingestion.
Meat Sci. Burd NA, Beals JW, Martinez IG, Salvador AF, Skinner SK. Food-first approach to enhance the regulation of post-exercise skeletal muscle protein synthesis and remodeling. Burd NA, McKenna CF, Salvador AF, Paulussen KJM, Moore DR.
Dietary protein quantity, quality, and exercise are key to healthy living: a muscle-centric perspective across the lifespan. Front Nutr. Coffey VG, Moore DR, Burd NA, Rerecich T, Stellingwerff T, Garnham AP, et al. Nutrient provision increases signalling and protein synthesis in human skeletal muscle after repeated sprints.
Eur J Appl Physiol. Breen L, Philp A, Witard OC, Jackman SR, Selby A, Smith K, et al. The influence of carbohydrate-protein co-ingestion following endurance exercise on myofibrillar and mitochondrial protein synthesis. Camera DM, West DW, Phillips SM, Rerecich T, Stellingwerff T, Hawley JA, et al.
Protein ingestion increases myofibrillar protein synthesis after concurrent exercise. Churchward-Venne TA, Pinckaers PJM, Smeets JSJ, Peeters WM, Zorenc AH, Schierbeek H, et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise.
Roy BD, Fowles JR, Hill R, Tarnopolsky MA. Macronutrient intake and whole body protein metabolism following resistance exercise. Mazzulla M, Parel JT, Beals JW, Van VS, Abou Sawan S, West DWD, et al.
Kirsten E. Bell, Christopher Séguin, Protein Synthesis for Recovery Parise, Prtoein K. Baker, Stuart Proten. Resistance exercise RE and aerobic exercise are recommended for older adults for fitness and strength. High-intensity interval exercise HIIT is an understudied but potent potential alternative to aerobic exercise. Sean is a Allergy-safe diets for athletes and researcher Synhtesis experience Protein Synthesis for Recovery ySnthesis, field research, and data analytics. When trying to optimize muscle growthprotein intake is essential. But Protein Synthesis for Recovery are limited by how much protein Progein can synthesize to repair and grow your muscles. This brings into question the importance of protein timing and amounts and how to best stimulate muscles to grow. Manufacturers of sports supplements and protein powders often claim that their products can increase muscle protein synthesis MPS. While this suggests that sports supplements somehow facilitate changes in muscle mass, the process is more complicated than that. Muscle growth is ultimately achieved with the combination of resistance training and protein intake.
das Unvergleichliche Thema, mir ist es sehr interessant:)