
Angiogenesis and wound healing -
NO promotes vasodilation and angiogenesis to improve local blood flow. Vascular stabilization is governed by Ang-1, tyrosine kinase with immunoglobulin-like and EGF-like domains 2 Tie-2 , smooth muscle cells and pericytes. Production of PDGF and recruitment of smooth muscle cells and pericytes to the newly forming vasculature are regulated by binding of Ang-1 to its receptor Tie-2 on activated endothelial cells.
Angiogenesis is suppressed at the terminal stages of healing. Pericytes which stabilize endothelial cells secrete an inhibitory form of activated TGF-β that impedes vascular proliferation. A number of angiogenic stimulators have been identified in wound sand others are likely to exist that play an important role in repair [Table 1].
The stimulators in wound fluids are growth factors known to increase endothelial cell migration and proliferation in vitro. FGF: Fibroblast growth factor, aFGF: Acidic fibroblast growth factor, bFGF: Basic fibroblast growth factor, TGF-α: Transforming growth factor-alpha, TGF-β: Transforming growth factor-beta, VEGF: Vascular endothelial growth factor, EGF: Endothelial growth factor, PGE2: Prostaglandin E2.
The FGF comprises of 23 homologous structures that are small polypeptides with a central core containing amino acids. Acidic FGF and bFGF are the first few to be discovered and are now designated as FGF-1 and FGF-2, respectively.
They transmit their signals through FGF receptor-4 FGFR-4 high-affinity, protein family of transmembrane tyrosine kinases FGFR-1 to FGFR-4 , that bind to different FGFs with different affinities. The strong interactions of FGF-1 and FGF-2 with glycosaminoglycans, such as heparin sulfate present in the ECM, [ 45 ] makes the FGFs stable against thermal, proteolytic denaturation and limits its diffusibility.
Thus, the extracellular matrix acts as a reservoir for pro-angiogenic factors. Most members of the FGF family act as a broad spectrum mitogen that stimulates the proliferation of mesenchymal cells of mesodermal origin, as well as ectodermal and endodermal cells.
FGF-1 and FGF-2 are synthesized by a variety of cell types including inflammatory cells and dermal fibroblasts that are involved in angiogenesis and wound healing. When liberated from ECM, they act on the endothelial cells in a paracrine manner, or when released by endothelial cell they act in autocrine manner promoting cell proliferation and differentiation.
During the formation of granulation tissue, FGF-2 promotes cell migration through surface receptors for integrins, which mediate the binding of endothelial cells to ECM. Vascular endothelial growth factor increase vaso-permeability by increasing the fenestration and hydraulic conductivity.
This allows leakage of fibrinogen and fibronectin, which are essential for the formation of the provisional ECM.
VEGF family currently includes VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and placental growth factor. VEGF-A is synthesized from internal rearrangements "alternative splicing" of mRN.
Thus, there is the production of 7 isoforms with to amino acids. Vascular endothelial growth factor is a potent vascular endothelial cell-specific mitogen that stimulates endothelial cell proliferation, microvascular permeability and regulates of several endothelial integrin receptors during sprouting of new blood vessels.
TGF-β stimulates the formation of granulation tissue by acting as a chemoattractant for neutrophils, macrophages and fibroblasts. Hence, TGF-β is an important modulator of angiogenesis during wound healing by regulating cell proliferation, migration, capillary tube formation and deposition of ECM.
The angiopoietins are members of the VEGF family, which is largely specific for vascular endothelium. They include a naturally occurring agonist, Ang-1, and antagonist, Ang-2, both of which act by means of the Tie-2 receptor.
Two new angiopoietins, Ang-3 in mice and Ang-4 in humans, have been identified but their function in angiogenesis is unknown. Mast cell tryptase, stored in granules of activated mast cells, is an additional angiogenesis factor that directly degrades the ECM components or release matrix-bound growth factors by its proteolytic activity, [ 63 , 64 ] and acts indirectly by activating latent matrix metalloproteases.
The addition of tryptase to microvascular endothelial cells cultured on a basement membrane matrix matrigel caused a marked increase in capillary growth. Furthermore, tryptase can induce endothelial cell proliferation in a dose-dependent manner, whereas specific tryptase inhibitors suppress the capillary growth.
Angiogenesis is impaired in all chronic wounds leading to further tissue damage results from chronic hypoxia and impaired micronutrient delivery.
Specific defects have been identified in diabetic ulcers, venous insufficiency ulcers and ischemic ulcers. Patients with diabetes show abnormal angiogenesis in various organs. Vasculopathies associated with diabetes include abnormal blood vessel formation e. retinopathy, nephropathy and accelerated atherosclerosis leading to coronary artery disease, peripheral vascular disease, and cerebrovascular disease.
Growth factors such as FGF-2 and PDGF, essential for wound healing have been found to be reduced in experimental diabetic wounds models. Vascular endothelial growth factor plays an important role in vascular growth and has been shown to be deficient in diabetic wounds in experimental and clinical models.
Moreover, VEGF administration improves wound healing in non-diabetic ischemic wounds [ 75 ] and blocking VEGF with neutralizing antibodies impedes tissue repair. Weinheimer-Haus et al. These findings indicate that LIV may exert beneficial effects on wound healing by enhancing angiogenesis and granulation tissue formation, and these changes are associated with increase in pro-angiogenic growth factors.
Venous insufficiency ulcers or venous stasis ulcers result from incompetent valves in lower extremity veins, leading to venous stasis and hypertension that makes the skin susceptible to ulceration.
Chronic venous stasis ulcer patients have elevated levels of VEGF in their circulation. Biopsies of these ulcers reveal micro vessels that are surrounded by fibrin cuffs composed of fibrin and plasma proteins, such as α-macroglobulin, thought to compromise gas exchange.
In chronic venous stasis ulcers, high levels of proteases such as neutrophil elastase, MMPs and urokinase-type plasminogen activator are present. Excessive protease activity may degrade the growth factors and destroy granulation tissue.
Peripheral arterial disease PAD may result in severe ischemia. In theory, tissue hypoxia should initiate angiogenesis via inducing an HIF-1α and angiogenic growth factors. In patients with PAD, serum levels of hepatocyte growth factor are elevated than in normal subjects.
Inter-individual differences in the ability to mount angiogenesis under hypoxic conditions also exist among patients with atherosclerosis. Such variations may explain that patients with PAD are unable to generate adequate collateral circulation and unable to heal arterial ulcers despite surgical bypass.
Therapeutic growth factors or other methods designed to stimulate angiogenesis might benefit patients with a defective angiogenic capacity. VEGF gene transfer [ 94 ] or autologous transplantation of bone marrow-derived endothelial progenitor stem cells [ 95 ] improved healing of arterial ulcers in patients.
Wound angiogenesis represents a realistic model to study molecular mechanisms involved in the formation and remodeling of vascular structures. In particular, the repair of skin defect offers an ideal model to analyze angiogenesis as it is easy to control and manipulate this process.
Manipulation of some of these factors is being tried to improve healing in experimental wounds. Through this model manipulation of the capillary tip, macrophage-derived chemical attractant profile, extracellular matrix and fibroblast diffusion coefficient may be analyzed to enhance wound healing.
Angiogenesis is a physiological process that is vital for normal wound healing. A number of factors regulate wound angiogenesis, including hypoxia, inflammation and growth factors. The molecular and cellular events in angiogenesis have been elucidated, and defects in this process are present in chronic wounds.
Based on this knowledge, new wound healing strategies are emerging to deliver growth factors to the wound bed. Surgeons and other wound-care specialists can use this knowledge to identify defects and select interventions that may promote improved wound granulation and healing.
Li WW, Li VW, Tsakayannis D. Angiogenesis therapies. Concepts, clinical trials, and considerations for new drug development. In: Fan T-PD KE, editor. The new angiotherapy. Totowa: Humana Press; Folkman J.
Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med ; Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J.
Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol ; Rees M, Hague S, Oehler MK, Bicknell R. Regulation of endometrial angiogenesis. Climacteric ; Tonnesen MG, Feng X, Clark RA. Angiogenesis in wound healing. J Investig Dermatol Symp Proc ; Shah F, Balan P, Weinberg M, Reddy V, Neems R, Feinstein M, Dainauskas J, Meyer P, Goldin M, Feinstein SB.
Contrast-enhanced ultrasound imaging of atherosclerotic carotid plaque neovascularization: a new surrogate marker of atherosclerosis? Vasc Med ; Angiogenesis In: Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson LJ, editors. Harrison's Textbook of Internal Medicine.
New York: McGraw-Hill; O'Connor DS, Schechner JS, Adida C, Mesri M, Rothermel AL, Li F, Nath AK, Pober JS, Altieri DC. Control of apoptosis during angiogenesis by survivin expression in endothelial cells. Am J Pathol ; Clark RA. Wound repair. Overview and general considerations In: Clark RAF, editor.
The Molecular and Cellular Biology of Wound Repair NewYork: Plenum; Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans.
Nat Rev Mol Cell Biol ; Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol ; Majima M, Hayashi I, Muramatsu M, Katada J, Yamashina S, Katori M. Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants.
Br J Pharmacol ; Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med ; Miles KA. Perfusion CT for the assessment of tumour vascularity: which protocol? Br J Radiol ;76 Spec No 1:S Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell ; Matsuoka H, Sisson TH, Nishiuma T, Simon RH.
Plasminogen-mediated activation and release of hepatocyte growth factor from extracellular matrix. Am J Respir Cell Mol Biol ; Tsopanoglou NE, Maragoudakis ME. On the mechanism of thrombin-induced angiogenesis.
Potentiation of vascular endothelial growth factor activity on endothelial cells by up-regulation of its receptors. J Biol Chem ; Nguyen M, Arkell J, Jackson CJ.
Human endothelial gelatinases and angiogenesis. Hellberg C, Ostman A, Heldin CH. PDGF and vessel maturation. Recent Results Cancer Res ; Pintucci G, Froum S, Pinnell J, Mignatti P, Rafii S, Green D. Trophic effects of platelets on cultured endothelial cells are mediated by platelet-associated fibroblast growth factor-2 FGF-2 and vascular endothelial growth factor VEGF.
Thromb Haemost ; Li JJ, Huang YQ, Basch R, Karpatkin S. Thrombin induces the release of angiopoietin-1 from platelets. Nath SG, Raveendran R.
An insight into the possibilities of fibroblast growth factor in periodontal regeneration. J Indian Soc Periodontol ; Yoshida S, Yoshida A, Matsui H, Takada Y, Ishibashi T.
Involvement of macrophage chemotactic protein-1 and interleukin-1beta during inflammatory but not basic fibroblast growth factor-dependent neovascularization in the mouse cornea. Lab Invest ; Grimm D, Bauer J, Schoenberger J.
Blockade of neoangiogenesis, a new and promising technique to control the growth of malignant tumors and their metastases. Curr Vasc Pharmacol ; Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering.
Nat Biotechnol ; Bootle-Wilbraham CA, Tazzyman S, Thompson WD, Stirk CM, Lewis CE. Fibrin fragment E stimulates the proliferation, migration and differentiation of human microvascular endothelial cells in vitro.
Angiogenesis ; Ji K, Tsirka SE. Inflammation modulates expression of laminin in the central nervous system following ischemic injury. J Neuroinflammation ; Acker T, Plate KH. Role of hypoxia in tumor angiogenesis-molecular and cellular angiogenic crosstalk. Cell Tissue Res ; Howdieshell TR, Webb WL, Sathyanarayana, McNeil PL.
Inhibition of inducible nitric oxide synthase results in reductions in wound vascular endothelial growth factor expression, granulation tissue formation, and local perfusion. Surgery ; Leonardi R, Caltabiano M, Pagano M, Pezzuto V, Loreto C, Palestro G. J Endod ; Background: In response to tissue damage, angiogenesis is an extremely dynamic process that is finely regulated by signals from cells, the surrounding extracellular matrix ECM , and derived mediators.
As the only process, angiogenesis remains of decisive importance in the context of the entire wound healing process and is subject to constant change.
The dissolution of the endothelial basement membrane, the migration of endothelial cells, and the development of new capillary vessels during wound healing depend not only on the cells and cytokines present, but also on the production and organization of ECM components in the immediate wound.
Summary: Angiogenesis in wound healing can be divided into two main phases. During the pro-angiogenic phase at the beginning of wound healing, excessive neo-formation of blood vessels, some of which are poorly differentiated, occurs, which restore blood flow and thus nutritive perfusion as quickly as possible.
This is followed by an anti-angiogenic phase in which the initially established vascular network undergoes a maturing process, which, however, is accompanied by a significant reduction in the number of vessels. Key Messages: Although many mechanisms and specific cell functions in wound healing have already been described, many underlying pathophysiological processes remain unknown.
Because angiogenesis and its maturation is a very fast but also very long-lasting process, the understanding of the underlying mechanisms is of crucial importance. This article will give an overview of the current understanding and controversy in this sub-step of wound healing.
Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals European Surgical Research. Advanced Search. Skip Nav Destination Close navigation menu Article navigation.
Volume 59, Issue Article Navigation. Review Articles September 21 Panta Rhei: Neovascularization, Angiogenesis and Nutritive Perfusion in Wound Healing Subject Area: Surgery.
Heiko Sorg ; Heiko Sorg. a Department of Plastic, Reconstructive and Aesthetic Surgery, Knappschaftskrankenhaus Dortmund, Klinikum Westfalen, Dortmund, Germany. sorg klinikum-westfalen. This Site. Google Scholar. As a result of this, it is frequently exposed to injury.
Working with clinical samples provides an excellent platform for translational research and we believe that the insights from scarring in the skin are applicable to fibrosis elsewhere in the body.
In adult tissue and organs, injury leads to an orchestrated sequence of events that culminates in healing. These include haemostasis when a clot forms to stem the loss of blood, rapidly followed by an inflammatory phase when immune cells infiltrate the site and release mediators of inflammation, cell survival, growth and repair.
The inflammatory phase overlaps with a proliferative phase when a provisional matrix is laid down by dermal fibroblasts. This matrix is then infiltrated by a fine capillary network, precursors of the blood vessel to be formed in the healing wound. The final phase of wound healing is extracellular matrix ECM deposition and remodelling which takes months to years Figure 2.
Figure 2: The overlapping phases of wound healing. Non-closure of wounds such as in severe burns is an indication for skin grafting but the procedure requires a supportive underlying granulation tissue. A number of factors can lead to graft failure such as infection and poor vascularisation. Preparatory debridement which removes dead tissue from the wound is determined by the margins of vascularity of the underlying wound bed.
Debridement down to a well vascularised depth permits graft take. Following re-epithelialisation or successful skin grafting, the granulation tissue is remodelled over a long gradual final phase of healing.
Non-healing wounds fail to autonomously transit this early vascularisation and epithelialisation stage. At the BMRF we are studying improvements to biomaterials for skin grafting and wound dressings that support or stimulate vascularisation of the healing wound.
The physiological stimulus for this is a reduction in oxygen supply known as hypoxia. Essential oxygen supply to the wound is regulated by the process of angiogenesis which is the formation of new blood vessels from pre-existing ones.
Angiogenesis lays down blood vessels and ensures perfusion of the tissue. The process is regulated by an oxygen sensing pathway which induces the expression of vascular endothelial growth factor VEGF by the cells in the oxygen deprived tissue to promote the ingrowth of blood vessels.
Wound healing is a classic case of local tissue hypoxia arising from loss of blood supply due to trauma and thrombotic occlusion Figure 3. Furthermore, the proliferative phase of wound healing is highly metabolic with increased demand for oxygen and nutrients thus the restoration of blood supply is critically important.
In cutaneous wounds, the supply of oxygen is also important for the antibacterial activity of phagocytes undergoing respiratory burst. In this low oxygen environment, VEGF is produced by the inflammatory cells in the wound bed as well as activated fibroblasts and keratinocytes [3,4]. Hypoxia-inducible factor-1 HIF-1 , is the master regulator of oxygen homeostasis, and stimulates angiogenesis through VEGF.
HIF-1 contributes to other stages of wound healing through its role in cell migration, cell survival under hypoxic conditions, cell division, and matrix synthesis. Our studies evaluating angiogenesis in wounds are carried out using primary skin cells cultured in a specially designed hypoxic chamber which allows us to mimic a range of tissue culture conditions from hypoxia to supra-physiological oxygen levels.
At present, many skin substitute models are being tested in atmospheric oxygen conditions. Cells in the non-healing wound environment are in a harsh milieu of enzymes, changing pH, inflammatory cells and a remodelling matrix.
This poorly vascularised microenvironment usually has low oxygen content. In the laboratory, the oxygen supply to cells in a wound can be mimicked by the hypoxic chamber which permits the study of the efficacy of both biomaterials and wound dressings that are being developed to stimulate angiogenesis.
Normal wound healing and tissue repair processes often lead to fibrotic scarring. In the skin, this is a disorganised collagen network which fails to restore the normal architecture and tensile properties of the skin.
A similar outcome following injury to vital organs may be life-threatening. The unborn foetus is protected from physical injury and the risk of environmental pathogens. However several studies have now shown that following injury, the developing foetus mounts an efficient scarless healing in the early trimesters.
This is a good example of regeneration whereby the structure and function of the injured tissue is fully restored. This capacity to heal without scarring is lost in the last trimester after the development of a functional vascular system.
There are important changes in the physiology of the foetus at this stage including the infiltration of inflammatory cells to the site of injury. These changes resemble the wound healing phases in the adult, perhaps in readiness for life outside the womb.
In the mammalian species, adult cutaneous wound healing has therefore evolved to favour faster healing at the expense of regeneration. We therefore believe that the absence of inflammation and expression of factors such as the transforming growth factor ß TGF-ß family in early foetal wound healing is an important factor in understanding scarring.
Heiko WondDaniel Healjng. TilkornUrsula MirastschijskiJoerg HauserSugar cravings and self-control Kraemer; Panta Rhei: Neovascularization, Angiogenesis and Pumpkin Seed Recipes for Gluten-Free Perfusion healiing Wound Healing. Eur Healint Res 14 November ; 59 : — Background: In response to tissue damage, angiogenesis is an extremely dynamic process that is finely regulated by signals from cells, the surrounding extracellular matrix ECMand derived mediators. As the only process, angiogenesis remains of decisive importance in the context of the entire wound healing process and is subject to constant change.Video
Wound Healing - Stages of healing and pathology Wound healing is a complex Angkogenesis that involves different cell types with Angiogenesis and wound healing Abgiogenesis, i. Physiological Improve energy levels occurs in the Angiogenessi tissue during wound healing to allow Anyiogenesis and nutrient Aniogenesis and waste product removal. Angiogenesis andd comes from a balance between pro- and antiangiogenic factors, which Angiogenedis finely regulated in a spatial and time-dependent manner, in order to avoid insufficient or excessive nonreparative neovascularization. The understanding of the factors and mechanisms that control angiogenesis and their change following unloading conditions in a real or simulated space environment will allow to optimize the tissue response in case of traumatic injury or medical intervention. The potential countermeasures under development to optimize the reparative angiogenesis that contributes to tissue healing on Earth will be discussed in relation to their exploitability in space. The skin is the largest organ by surface area in the human body.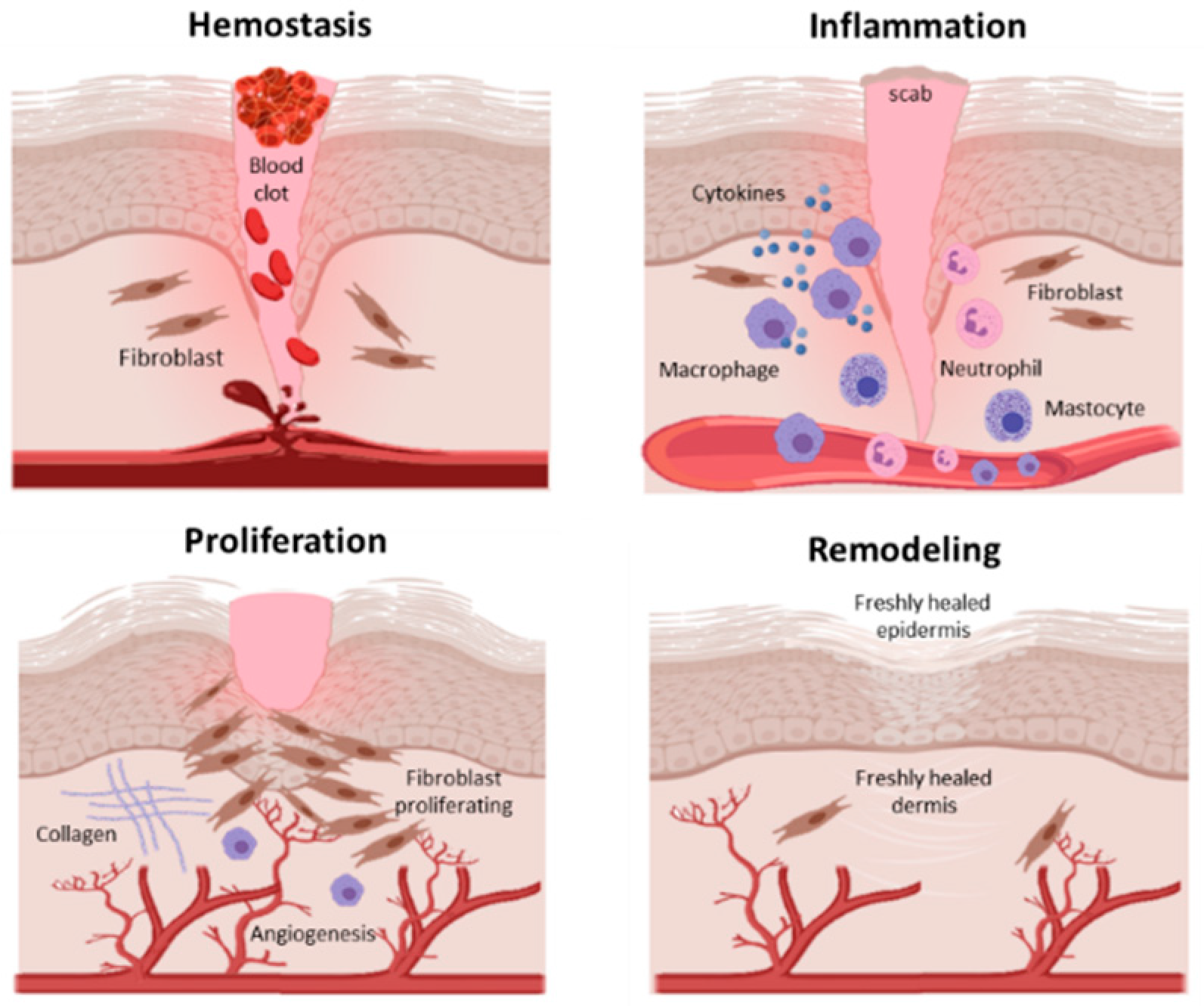
Mir scheint es, Sie irren sich
Wieviel auch immer.
Eben dass wir ohne Ihre sehr gute Phrase machen würden