Video
The INSANE BENEFITS Of Fasting For Weight Loss \u0026 PREVENTING Disease! - Dr. Jason Fung BMC Caloric restriction and metabolic health volume heaalthArticle destriction Cite this Muscle building tricep exercises. Metrics details. However, the two regimens calorid yet to be thoroughly compared. The primary outcome was the change in body mass index BMI. The secondary outcomes included body weight, waist circumference, waist-to-hip ratio, body fat, and metabolic risk factors. All participants attended health education sessions during the trial. A total of participants were analyzed.Caloric restriction and metabolic health -
After years, the metabolism eventually stabilized, and participants were engaging in their usual activity levels while consuming fewer calories. Intermittent fasting and time-restricted eating are prone to similar metabolic fluctuations that can affect physical activity levels until sufficient time has passed for the metabolism to fully adjust.
Caloric restriction is generally regarded as safe if carried out in the absence of malnutrition. Caloric restriction ought to be undertaken in the context of a nutrient-dense diet containing adequate protein, fats, carbs, and micronutrients to sustain health and well-being.
What is Caloric Over-Restriction? It is not certain at what point restricting calories would lead to starvation, provided the diet still contained adequate nutrition. Other Safety Considerations. Physical activity levels should also be considered with regard to caloric restriction, as well as current calorie intake.
A healthy diet plan is often low in calories, and may not require a further restriction of calories. Individuals who engage in frequent physical activity and who consume a nutrient-dense diet likely already meet the baseline requirements of caloric restriction as a practice and are likely already receiving the benefit.
Side Effects. Without adequate nutrition, caloric restriction is not conducive to health and tends to promote starvation in the long run. Chronic over-restriction of calories and long-term starvation can result in the following side effects:.
Caloric restriction is not a recommended practice for any individuals with the following conditions:. The concept of caloric restriction can be applied in any of the above ways intermittent fasting, time-restricted eating, or calorie counting and reduction. The conventional approach demands counting calories and limiting their intake without compromising adequate nutrition.
This is based on age, height, weight, and physical activity levels. The average recommended caloric consumption for men is around per day and per day for women.
Non-essential plant-based nutrients have been found to be responsible for the health-promoting benefits of whole foods. They are present throughout the diet, mainly in fruits, vegetables, legumes, herbs, spices, whole grains, nuts, and seeds.
All antioxidant phytochemicals are typically consumed in tiny amounts, averaging a few hundred micrograms to several milligrams per day. While their quantities are minimal and even less in processed foods , their collective dietary presence and benefit have been shown through epidemiological studies linked with optimal health, longevity, inflammation control, and a reduced risk of acquiring disease.
In animal and other studies, most of these plant-based nutrients failed to extend lifespan in short-term trials alone. However, their effects appear to enhance the longevity-promotion effects of caloric restriction, making them complementary.
While it might seem counterintuitive, physical activity is a necessary component in balancing metabolism and promoting longevity. Physical activity appears to be one of the only factors capable of increasing energy expenditure, metabolic rate, and longevity.
Caloric restriction in itself helps to reduce the energy expended during physical activity. Inconsistent Caloric Restriction Can Lower Physical Activity Levels. It has been observed that after six months to a year of caloric restriction, physical activity levels decline, possibly as a form of compensation for reduced energy intake.
However, after two years, this effect seems to normalize, and activity levels increase back to baseline. Therefore, caloric restriction should probably not be used as a short-term strategy for optimizing energy levels as it may promote reduced physical activity.
In the long term, caloric restriction can enhance physical activity by increasing available ATP energy and lowering bodily energy requirements via body mass reduction. Exercise Consistency Better Enhances Caloric Restriction Benefit.
A handful of studies show that the resting metabolic rate is slightly elevated after acute exercise due to changes in oxygen uptake. These effects do not appear to be carried forward with consistent, long-term exercise, wherein resting metabolic rate remains largely unaffected after engaging in physical activity.
While there is no ultimate cure for aging, it would seem that extending our mortality may be as simple as processing less on a daily basis.
Restricting calories is able to lower the metabolic rate and significantly enhance energy production, with noticeable long-term benefits on health and the quality of aging.
The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, et al. The science of obesity management: an endocrine society scientific statement.
Endocr Rev. Lifestyle management: standards of medical care in diabetes Diabetes Care. Seo MH, Lee WY, Kim SS, Kang JH, Kang JH, Kim KK, et al.
J Obes Metab Syndr. Gong Q, Zhang P, Wang J, Ma J, An Y, Chen Y, et al. Morbidity and mortality after lifestyle intervention for people with impaired glucose tolerance: year results of the Da Qing Diabetes Prevention Outcome Study.
Primary care-led weight management for remission of type 2 diabetes DiRECT : an open-label, cluster-randomised trial. Ge L, Sadeghirad B, Ball GDC, Da Costa BR, Hitchcock CL, Svendrovski A, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials.
Hession M, Rolland C, Kulkarni U, Wise A, Broom J. Systematic review of randomized controlled trials of low-carbohydrate vs.
Obes Rev. Article CAS PubMed Google Scholar. Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS, Balise RR, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women.
The A to Z weight loss study: a randomized trial. Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet.
N Engl J Med. Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. Bazzano LA, Hu T, Reynolds K, Yao L, Bunol C, Liu Y, et al. Effects of low-carbohydrate and low-fat diets: a randomized trial.
Ann Intern Med. Stern L, Seshadri P, Chicano KL, Daily DA, Mcgrory J, Williams M, et al. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: one-year follow-up of a randomized trial.
Foster GD, Wyatt HR, Hill JO, Makris AP, Rosenbaum DL, Brill C, et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet a randomized trial. Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS, et al.
A randomized trial of a low-carbohydrate diet for obesity. Dansinger ML, Gleason JA, Griffith JL, Selker HP, Schaefer EJ. Comparison of the Atkins, Ornish, weight watchers, and zone diets for weight loss and heart disease risk reduction: a randomized trial.
J Am Med Assoc. Collaborators G O. Health effects of overweight and obesity in countries over 25 years. Zhang X, Zhang M, Zhao Z, Huang Z, Deng Q, Li Y, et al. Geographic variation in prevalence of adult obesity in China: results from the — National Chronic Disease and Risk Factor Surveillance.
Keogh JB, Brinkworth GD, Clifton PM. Effects of weight loss on a low-carbohydrate diet on flow-mediated dilatation, adhesion molecules and adiponectin. Br J Nutr. Morris E, Aveyard P, Dyson P, Noreik M, Bailey C, Fox R, et al. A food-based, low-energy, low-carbohydrate diet for people with type 2 diabetes in primary care: a randomized controlled feasibility trial.
Diabetes, Obes Metab. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J Clin Endocrinol Metab. Gardner CD, Trepanowski JF, Gobbo LCD, Hauser ME, Rigdon J, Ioannidis JPA, et al.
Effect of low-fat vs low-carbohydrate diet on month weight loss in overweight adults and the association with genotype pattern or insulin secretion the DIETFITS randomized clinical trial. JAMA - J Am Med Assoc. Rm K, Rh E, B H, LJ A, SR D, RJ D, AHA Dietary Guidelines: revision, et al. a statement for healthcare professionals from the Nutrition Committee of the American Heart Association.
Google Scholar. Rodbard HW, Blonde L, Braithwaite SS, Brett EM, Cobin RH, Handelsman Y, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus.
Tay J, Luscombe-Marsh ND, Thompson CH, Noakes M, Buckley JD, Wittert GA, et al. Comparison of low- and high-carbohydrate diets for type 2 diabetes management: a randomized trial. Am J Clin Nutr.
Brinkworth GD, Noakes M, Buckley JD, Keogh JB, Clifton PM. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Ershow AG, Wong-Chen K.
Chinese food composition tables an annotated translation of the edition published by the Institute of Nutrition and Food Hygiene, Chinese Academy of Preventive Medicine.
Beijing J Food Compos Anal. Article CAS Google Scholar. Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates.
Ebbeling CB, Feldman HA, Klein GL, Wong JMW, Bielak L, Steltz SK, et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, et al.
Effects of dietary composition on energy expenditure during weight-loss maintenance. Article CAS PubMed PubMed Central Google Scholar.
Johnstone AM, Horgan GW, Murison SD, Bremner DM, Lobley GE. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Download references. The authors thank the study participants for their cooperation.
The authors thank Guangzhou Nanda Fit Nutrition and Health Consulting Co. This study was partly supported by the National Natural Science Foundation of China Grant NO: , , , , the Natural Science Foundation of Guangdong Province Grant No.
Department of Endocrinology and Metabolism, Zhujiang Hospital, Southern Medical University, No. Department of Bio-Statistics, Southern Medical University, No.
Department of Endocrinology, Huizhou Municipal Center Hospital, No. Department of Endocrinology, The Third Affiliated Hospital of Guangzhou Medical University, No. Department of Endocrinology, Dongguan Kanghua Hospital, Dongguan Avenue, Dongguan, Guangdong, China. Department of Endocrinology, He Xian Memorial Hospital, No.
Department of Endocrinology, Guangdong Second Provincial General Hospital, No. Department of Endocrinology, Affiliated Hospital of Guangdong Medical University, No. Department of Endocrinology and Metabolism, Shunde Hospital of Southern Medical University, No. Department of Endocrinology, The Eighth Affiliated Hospital of Sun Yat-Sen University, No.
Department of Endocrinology and Metabolism, Nanfang Hospital, Southern Medical University, No. You can also search for this author in PubMed Google Scholar. The authors read and approved the final manuscript. JS, YTR, NNX, and PLW contributed equally to this work.
JS, NNX, YTR, and PLW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: HC, JS, NNX, YTR, PLW, NL, KY, SLA, PK, and HJZ. Acquisition, analysis, or interpretation of the data: HC, YTR, NNX, PLW, SLA, PK, HJZ, and JS.
Drafting of the manuscript: JS, NNX, YTR, PLW, HJZ, and HC. Critical revision of the manuscript for important intellectual content: HC, YTR, NNX, HJZ, and JS. Statistical analysis: HC, NNX, YTR, PLW, SLA, PK, HJZ, and JS. Administrative, technical, or material support: SL, QYH, YZ, YZL, JLS, WJM, BC, XWZ, XMC, YQL, ZYL, GBD, ZZ, YQW, WHW, JS, HJZ, and HC.
Study supervision: JS, HJZ, and HC. Correspondence to Jia Sun , Huijie Zhang or Hong Chen. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Additional file 2: Fig.
Adherence to the prescribed diets over 12 weeks. Metabolites changes of LC diet and CR diet at baseline and 12 weeks. Table S1. Food profile of diet interventions. Table S2. Baseline characteristics of study participants included in completer analysis.
Table S3. Daily physical activityat baseline and during follow-up. Table 1. Physical characteristics of 48 men and women in weight maintenance at baseline. Figure 2. Experimental design A and body weight and composition changes B at the completion of the study.
Baseline weight-maintenance energy requirements The energy intake required for weight maintenance during baseline and the subsequent energy deficit prescribed to achieve the desired caloric restriction were calculated from 4-week data including two day periods by doubly labeled water DLW.
Diet and behavioral intervention During weeks 1—12 and 22—24 of the intervention, participants were provided with all meals prepared by the metabolic kitchen at the Center and based on individual energy intake targets.
Doubly labeled water Besides the two baseline measurements, total daily energy expenditure TDEE was measured by day doubly labeled water during weeks 10—12 M3 and 22—24 M6 of the intervention.
Body composition Metabolic weight was determined by the mean of two consecutive measurements obtained in the morning following a 12 h fast and morning void and corrected for the weight of a hospital gown.
Sedentary energy expenditure Sedentary EE 24h-EE was measured over 23 hours in a whole room indirect calorimeter as previously described [6] , [17].
Physical activity Physical activity was estimated from daily energy expenditure by doubly labeled water and sleeping metabolic rate using two different calculations. Psychological testing Psychological testing included an assessment of health related quality of life.
Statistical analysis Data in the text and tables are provided as means±SE. Results Total daily energy expenditure at baseline At baseline TDEE was not different between the four treatment groups Table 2.
Table 2. Body weight and composition changes At M3, body weight, FM and FFM were reduced from baseline in all three intervention groups and remained stable in the control group.
Effect of CR on TDEE adjusted for body composition To determine if there was a metabolic adaptation to the CR intervention at M3 and M6 we compared the actual TDEE measured by DLW at each time point with the TDEE predicted from FFM and FM derived from the prediction equations generated at baseline and presented above.
Effect of CR on physical activity We next adjusted TDEE for sedentary energy expenditure measured in a respiratory chamber 24h-EE or for SMR.
Figure 3. The effect of caloric restriction on AREE change in TDEE at M3 and M6 after adjusting for SMR a measure of sedentary energy expenditure. Effect of CR on Physical Functioning and Vitality According to the SF survey, all treatment groups, but not the control group, reported improvement in physical functioning with the intervention.
Discussion In response to caloric restriction there is an abrupt change from a state of energy balance weight maintenance to a negative imbalance, which eventually will reach a new equilibrium at a lower body mass when the decline in energy expenditure is maintained at a level equivalent to the energy intake.
Figure 4. Supporting Information. Checklist S1. s 0. Protocol S1. Acknowledgments The authors thank the remaining members of Pennington CALERIE Research Team including: Steven Smith, Enette Larson-Meyer, Steve Anton, Julia Volaufova, Marlene Most, Tuong Nguyen, Frank Greenway, Emily York-Crow, Catherine Champagne, Brenda Dahmer, Andy Deutsch, Paula Geiselman, Jennifer Howard, Jana Ihrig, Michael Lefevre, Darlene Marquis, Connie Murla, Anthony Alfonso, Sabrina Yang, Robbie Durand, Sean Owens, Aimee Stewart and Vanessa Tarver.
Author Contributions Conceived and designed the experiments: DAW ER. References 1. Ravussin E, Lillioja S, Anderson TE, Christin L, Bogardus C Determinants of hour energy expenditure in man. Methods and results using a respiratory chamber.
J Clin Invest — View Article Google Scholar 2. Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, et al. Am J Physiol E— View Article Google Scholar 3. Levine JA, Eberhardt NL, Jensen MD Role of nonexercise activity thermogenesis in resistance to fat gain in humans.
Science — View Article Google Scholar 4. Sacher GA Life table modifications and life prolongation. In: Finch CE, Hayflick L, editors. Handbook of the Biology of Aging.
New York: van Nostrand Reinold. Sohal RS, Weindruch R Oxidative stress, caloric restriction, and aging. Science 59— View Article Google Scholar 6. Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, et al. Jama — View Article Google Scholar 7.
Martin CK, Heilbronn LK, de Jonge L, Delany JP, Volaufova J, et al. Obesity Silver Spring — View Article Google Scholar 8.
Keys A, Brozek J, Henschel A, Mickelson O, Taylor H The Biology of Human Starvation. Minneapolis, MN: University of Minnesota Press.
Ravussin E, Burnand B, Schutz Y, Jequier E Energy expenditure before and during energy restriction in obese patients. Am J Clin Nutr — View Article Google Scholar Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, et al.
Schulz LO, Alger S, Harper I, Wilmore JH, Ravussin E Energy expenditure of elite female runners measured by respiratory chamber and doubly labeled water. J Appl Physiol 23— Ware JE Jr, Kosinski M, Gandek B , SF Healh Survey: Manual and Interpretation Guide.
Lincoln, RI: Quality Metric Inc. Ware JE Jr, Sherbourne CD The MOS item short-form health survey SF Conceptual framework and item selection. Med Care — Schoeller DA Measurement of energy expenditure in free-living humans by using doubly labeled water.
J Nutr — Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, et al. DeLany JP, Schoeller DA, Hoyt RW, Askew EW, Sharp MA Field use of D2 18O to measure energy expenditure of soldiers at different energy intakes.
J Appl Physiol — Nguyen T, de Jonge L, Smith SR, Bray GA Chamber for indirect calorimetry with accurate measurement and time discrimination of metabolic plateaus of over 20 min.
Med Biol Eng Comput — Allison DB, Paultre F, Goran MI, Poehlman ET, Heymsfield SB Statistical considerations regarding the use of ratios to adjust data. Int J Obes Relat Metab Disord — Frisard MI, Fabre JM, Russell RD, King CM, Delany JP, et al.
J Gerontol A Biol Sci Med Sci — Leibel RL, Hirsch J Diminished energy requirements in reduced-obese patients. Metabolism — Leibel RL, Rosenbaum M, Hirsch J Changes in energy expenditure resulting from altered body weight. N Engl J Med — Rosenbaum M, Hirsch J, Gallagher D, Leibel RL Long-term persistence to adaptive thermogenesis in subjects who have maintained a reduced body weight.
Am J Clin Nutr In Press. Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, et al. Am J Physiol R— James WP, Shetty PS Metabolic adaptation and energy requirements in developing countries.
Hum Nutr Clin Nutr — Fricker J, Rozen R, Melchior JC, Apfelbaum M Energy-metabolism adaptation in obese adults on a very-low-calorie diet. Keesey RE Physiological regulation of body weight and the issue of obesity.
Heallth include products caloric restriction and metabolic health think are useful for our readers. If you rewtriction through links on this page, we may earn Holistic herbal remedy small commission. Healthline only shows you brands and products that we halth behind. However, restricting calories caloric restriction and metabolic health severely can lead to a variety of health problems, including reduced fertility and weaker bones. A calorie is defined as the amount of heat energy needed to raise the temperature of one gram of water by 1°C 1. Your body requires calories to function and uses them to sustain three main processes 1 :. Generally speaking, eating more calories than your body needs will cause you to gain weightmostly in the form of body fat.Caloric restriction and metabolic health -
The objective of the current study therefore was to determine if CR induces a reduction in free-living energy expenditure that is larger than what can be explained by changes in body weight and body composition i. metabolic adaptation. Furthermore we wished to examine whether CR induced a change in energy expended in physical activity [6].
As a secondary analysis we determined if metabolic adaptation occurred only during weight loss or if it persisted after the lower body mass reached stability.
We hypothesized that similar to sedentary energy expenditure, total daily energy expenditure would be reduced by CR partly due to metabolic adaptation and partly due to decreased physical activity. Such metabolic and behavioral adaptation can explain the variability in the rates of weight loss even in well controlled studies as well as the propensity of relapse after weight loss.
This study was conducted according to the principles expressed in the declaration of Helsinki. The study was approved by the Pennington Biomedical Research Center IRB and the Data Safety Monitoring Board of CALERIE.
All participants provided written informed consent for the collection of samples and subsequent analysis. The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1. Of the individuals screened for the study, were excluded were ineligible; 91 withdrew during screening Figure 1.
The physical characteristics of the participants during weight maintenance at baseline are summarized in Table 1.
Details of the screening process and study population have been previously described [6]. The CONSORT diagram was published previously [6]. The group assignment was stratified to ensure equal distributions of sex and BMI in the four groups. All physiological and psychological testing was conducted at baseline and at the end of weeks 12 M3 and 24 M6 over a 5-day stay in the institutional inpatient unit.
Where possible study personnel were blinded to the treatment assignment of the subjects. The energy intake required for weight maintenance during baseline and the subsequent energy deficit prescribed to achieve the desired caloric restriction were calculated from 4-week data including two day periods by doubly labeled water DLW.
During the first DLW study B1 participants followed their usual diet at home. During the second DLW study B2 , participants were provided with a weight maintenance diet. Values from the animal literature for tissue gain or loss were used to assign energy values to weight changes as previously described [11] and adjustments were made to determine energy intake for weight maintenance.
Individual energy requirements were then calculated as the average of total daily energy expenditure TDEE during B1 and the final energy intake for weight maintenance after adjustments during B2. During weeks 1—12 and 22—24 of the intervention, participants were provided with all meals prepared by the metabolic kitchen at the Center and based on individual energy intake targets.
On weekdays, breakfast and dinner were eaten at the center. All lunches, snacks and weekend meals were packaged for take-out. During weeks 13—22 participants self-selected a diet based on their individual targets.
During the self-selected feeding, compliance to the prescribed dietary intervention was monitored from self-reported food records and changes in body weight which were reviewed weekly during behavioral group or individual sessions.
During these weekly meetings, participants learned cognitive-behavioral techniques on how to adhere to their meal and exercise plans. At least three of the five weekly exercise sessions were performed under supervision at the exercise facility of our center and heart rate monitor data was reviewed for compliance for all session conducted outside the center.
The exercise program was implemented gradually and by week 6 all participants were expending the required Exercise energy expenditure was initially determined by indirect calorimetry and thereafter HR was used to assess compliance during all exercise sessions.
The target energy cost was maintained at ±63 kcal per session for women and ± kcal per session for men throughout the entire intervention resulting in average exercise durations of 53±11 and 45±14 min per session for women and men, respectively. Besides the two baseline measurements, total daily energy expenditure TDEE was measured by day doubly labeled water during weeks 10—12 M3 and 22—24 M6 of the intervention.
Briefly, subjects provided two urine samples before being dosed with labeled water 2. Urine samples collected at 1.
On days 7 and 14 after dosing, subjects provided two additional timed urine samples. Each sample was analyzed for 18 O and 2 H abundance by isotope ratio mass spectrometry [7]. The isotopic enrichments of the post-dose urines compared with the pre-dose samples were used to calculate elimination rates k H and k O using linear regression and initial isotope dilution spaces were calculated by extrapolation to time 0.
CO 2 production rate rCO 2 was calculated using the equations of Schoeller et al. Total energy expenditure was calculated by multiplying rCO 2 by the energy equivalent of CO 2 for an RQ of 0.
For months 3 and 6, during the intervention when weight loss was observed, energy expenditure was calculated from rCO2 by using a metabolic fuel quotient derived from food intake, changes in body energy stores and conventional calorimetric relations corrected for the changes in fat mass FM and fat free mass FFM as previously described [11] , [16].
Metabolic weight was determined by the mean of two consecutive measurements obtained in the morning following a 12 h fast and morning void and corrected for the weight of a hospital gown.
Whole body percent body fat was measured using DXA Hologics QDR A, Bedford, MA and FM and FFM were calculated from the percent body fat and the metabolic body weight.
Sedentary EE 24h-EE was measured over 23 hours in a whole room indirect calorimeter as previously described [6] , [17]. At M3, LCD participants were fed so that energy intake was tightly matched to measured energy expenditure and provided the same meals at M6.
Sleeping metabolic rate SMR was calculated between — am, when no motion was detected. Physical activity was estimated from daily energy expenditure by doubly labeled water and sleeping metabolic rate using two different calculations.
Because of the inherent problem of using ratios when the two variables have an intercept not equal to zero [18] , we also expressed physical activity as the residual value of the regression between measured TDEE and measured SMR [19]. This value we termed Activity Related Energy Expenditure AREE , is positive for subjects with higher physical activity than average and negative for subjects with lower physical activity than average independent of metabolic body size.
Because AREE is adjusted for metabolic body size SMR , this value is directly proportional to the amount of physical activity. Psychological testing included an assessment of health related quality of life.
The Medical Outcomes Study Short-Form 36 Health Survey SF is a self-administered item questionnaire that measures health related quality of life. The validity and reliability of the SF have been established [12] , [13]. In the present study, the SF was used to measure vitality VT and physical functioning PF.
Participants' raw scores were converted into scale scores ranging from 0 to , with higher scores representing better QOL or higher levels of functioning. Data in the text and tables are provided as means±SE. SAS Version 9. The change in variables from baseline to M3 and M6 and M3 and M6 were analyzed by repeated measures with treatment and time interactions and baseline values included as covariates.
To control for type I error, statistical significance for all multiple comparisons was adjusted using the Tukey-Kramer method. Using the latter equation, predicted values for TDEE were generated at M3 and M6 using the equation with the actual measured FFM and FM, or actual weight or SMR.
The partial coefficients for each model are reported and the differences between the measured and predicted TDEE values were calculated and analyzed using ANOVA. At baseline TDEE was not different between the four treatment groups Table 2. At M3, body weight, FM and FFM were reduced from baseline in all three intervention groups and remained stable in the control group.
At the completion of the week study, body weight was reduced by 0. There was no difference in the change in TDEE from baseline at M3 and M6 in either CR or LCD. To determine if there was a metabolic adaptation to the CR intervention at M3 and M6 we compared the actual TDEE measured by DLW at each time point with the TDEE predicted from FFM and FM derived from the prediction equations generated at baseline and presented above.
At M6, the differential between the observed and predicted values for TDEE remained negative however did not reach statistical significance for either group Table 2.
Similar results were obtained when TDEE values were adjusted for weight loss instead of FFM and FM losses. We next adjusted TDEE for sedentary energy expenditure measured in a respiratory chamber 24h-EE or for SMR.
This adjustment allows us to disentangle the effect of physical activity from the effect of sedentary metabolism in response to CR. This indicates a metabolic adaptation unrelated to sedentary energy expenditure and therefore resulting from decreased habitual or voluntary physical activity.
The reduction in PAL was no longer evident with further weight loss at M6 in CR or weight loss maintenance in LCD. According to the SF survey, all treatment groups, but not the control group, reported improvement in physical functioning with the intervention. Vitality was not different from baseline in any treatment group and either time point.
In response to caloric restriction there is an abrupt change from a state of energy balance weight maintenance to a negative imbalance, which eventually will reach a new equilibrium at a lower body mass when the decline in energy expenditure is maintained at a level equivalent to the energy intake.
Whether the decline in energy expenditure is equal or larger, than the reduction in metabolic mass is still debated. Several reports suggest that despite weight stability, the reduction in energy expenditure can be lower than one would expect for the new metabolic weight and composition [20] — [23].
Additionally the role of exercise on the metabolic adjustments to CR interventions is not known. Now for the first time, we objectively characterized the response in all the components of daily energy expenditure to caloric restriction by combining doubly labeled water and indirect calorimetry Figure 4.
To exclude the contribution of sedentary energy expenditure the largest component of daily energy expenditure , we adjusted TDEE for sedentary energy expenditure 24h-EE and SMR and observed that measured TDEE was significantly less than predicted at both month 3 and month 6 of CR.
Together, this data indicates that TDEE is reduced with caloric restriction and is likely the result of a metabolic adaptation in the sedentary state accompanied by a reduction in activity-related energy expenditure and reduced levels of physical activity Figure 3.
Total daily energy expenditure TDEE is measured by doubly labeled water over a 2-week period whereas sedentary h energy expenditure 24h-EE is measured in a respiratory chamber. The changes in total daily energy expenditure after 3 and 6 months of CR Bottom Panel are shown and those representing a metabolic adaptation larger than due to weight loss are highlighted in grey.
The concept of an adaptation in metabolic rate in response to caloric restriction defined as reduction in energy expenditure that is more than would be expected on the basis of the loss of metabolic mass was proposed by Keys et al in the 's [8]. Behaviorally, a response to the semi starvation was also a tremendous decrease in physical activity.
Studies in obese individuals have also reported a metabolic adaptation measured by RMR adjusted for body composition with weight loss [25] — [27]. These adaptations in metabolic rate could be explained by an improved metabolic efficiency of the skeletal muscle or as also postulated, due to a reduction in physical activity with weight loss [28].
The energy cost of physical activity is proportional to body weight. Tissues on dry ice were stored at —80 °C prior to downstream analysis. This study was done before pre-registration was a requirement for clinical trials.
Thus, the participants were non-randomly selected individuals who were sufficiently motivated to actively respond to the request for volunteers. During recruitment, participants underwent a medical examination and their general practitioners were contacted to confirm medical suitability to participate in the study.
Participants were informed that the study was funded by an external commercial sponsor but the company was not named, and all packaging of the food range was removed before distribution. No data points were excluded from the final analyses.
Age, BMI, glucose and daily caloric intake Diet are mean ± SD. Participants were asked to attend the Human Nutrition Unit HNU at the Rowett Institute of Nutrition and Health Aberdeen, UK twice weekly to pick up food supplies. This approach was taken to standardise the diet to account for individual energy requirements and energy restriction.
All required food, drinks and snacks were provided and the study dietician was present to answer any questions. Participants were provided with a diet guide whereby women were encouraged to consume no more than 6. They were encouraged to consume 3 meals a day breakfast, lunch and dinner with a portion of protein at each meal, and 5 portions of fruit and vegetables, 2 portions of fats, 3 portions of milk and dairy and 4 portions of wholegrain bread, potatoes, and cereals each day.
Participants completed a descriptive food diary every day to record what and when they ate. Participants ate ad libitum, choosing when and what to eat from their supplies.
They completed a detailed 7d weighed dietary record during week 4 of the WL phase of the study, to compare eating habits with a baseline 7d weighed record they completed before the study started. Energy and nutrient intakes were calculated using the Windiets programme Univation Ltd; The Robert Gordon University, Aberdeen, UK.
Before and after WL, at weeks 0 and 4 respectively, measurements of body composition were conducted under standardized conditions as described previously Johnstone et al.
Height was measured at the beginning of the study to the nearest 0. The body weight of the participants was measured at screening, during food provision days and on all test days, wearing a previously weighed dressing gown, to the nearest g on a digital scale DIGI DS, C.
Weighing Equipment Ltd, London, UK. Fat mass FM and fat free mass FFM were measured with the use of air displacement whole-body plethysmography BodPod Gold — Body Composition System, Cosmed, Italy Mccrory et al. At each timepoint, mice entered the cages around 11 am on day 1 and were housed for three nights four days in total.
Data herein are from the 48 h period between 7am on day 2 and 7am on day 4, to allow habituation to the new cages during day 1. Before and after Promethion housing, each mouse was weighed and body composition determined by TD-NMR.
FA oxidation was determined as described previously Bruss et al. Mice were given a half portion of food the night 6pm prior to the metabolic challenge. At pm on the day of the OGTT a basal blood sample was collected by venesection of the lateral tail vein into EDTA-coated capillary tubes Microvette At 15, 30, 60, and minut post-gavage, blood glucose was measured by a glucometer and tail vein blood was sampled in EDTA tubes, as for the basal 0 min timepoint.
Blood samples were kept on ice prior to isolation of plasma. Homeostatic Model Assessment for Insulin Resistance HOMA-IR and Matsuda index were calculated as previously described Matthews et al.
Immediately before and and 60 min post 18 F-FDG intraperitoneal injection, blood glucose was measured by tail venesection and blood was sampled directly into EDTA-microtubes Sarstedt, Leicester, UK.
SUVs were calculated for regions of interest, namely BAT, iWAT, gWAT; heart; bone tissue without BM from tibiae, femurs, and humeri; To distinguish bone tissue from BM, a calibration curve was generated using HU obtained from the acquisition of a CT tissue equivalent material TEM phantom CIRS, model and mouse CT scans, as previously described Suchacki et al.
Paraffin-embedded tissue sections were then sectioned at µm intervals using a Leica RM RTS microtome and collected onto 76x26 mm StarFrost slides VWR, UK. NEFA standard solution Wako, — was serially diluted to concentrations of 2, 1.
The standard dilutions, blank sample and plasma 2 μL were pipetted into separate duplicate wells of a well plate. Reagent 1 μL Wako, — was added to each well and the plate incubated at 37 °C for 5 min. Absorbance of each well was measured at and nm.
Reagent 2 μL; Wako, — was then added to each well and the plate was incubated again at 37 °C for 5 min and absorbance measured at and nm. The nm reading was subtracted from the reading from both measurements and the mean blank absorbance subtracted from the mean duplicate absorbance from each sample.
NEFA concentrations were then determined by regression analysis. After tissue disruption, the lysate was boiled at 95 °C on a dry heat block for 10 min.
Boiled lysate was centrifuged at 16,× g and the cleared liquid fraction below the lipid layer and above the pellet was removed to a new tube for downstream analyses.
Protein concentration was quantified using the BCA protein assay Pierce Dithiothreitol was added to the lysates 25 mM final concentration.
Samples were boiled for 5 min, cooled on ice for 1 min, vortexed, and equal amounts of protein 13 µg per lane separated on gradient polyacrylamide gels Bio-Rad, Hercules, CA, USA.
Samples were then transferred to Immobilon-FL transfer membrane Millipore, Billerica, MA, USA. Fluorescent color was detected with Odyssey CLx Imager LI-COR, Lincoln, NE, USA. Band signal was quantified using Image Studio v5. Quantified data obtained from two different membranes were combined by using the strength of the signal of the ladder bands as a control for intensity differences between the two membranes.
Liver ceramide and dihydroceramide content was determined using LC-MS Lipidomics Core Facility; University of the Highlands and the Islands, Inverness, UK.
As per Folch et al. Fifty microlitres of each sample was combined with μL ceramide 1 μM in MeOH and 25 μL dihydroceramide 2 μM in MeOH; Avanti Polar Lipids, Alabaster, AL, USA as internal standards. MeOH 1. Samples were then stored on ice for 1 hr with intermittent vortex mixing, centrifuged at rpm for 5 min at 4 °C and the supernatant was collected.
KCl 0. The lower phase was collected and evaporated under nitrogen and reconstituted in 1 mL CHCl 3. The column was conditioned twice with 3 mL CHCl 3 and then loaded with 1 mL of the sample. The collected eluate was evaporated and dried under vacuum and reconstituted in μL MeOH.
The ceramides and dihydroceramides were separated on a Kinetex C8 HPLC column 1. Mobile phase B was The flow rate used was μL per min at 40 °C. Total ceramide and dihydroceramide concentrations were calculated from the summed concentrations of all the monitored molecular species.
All values were normalised to wet weight of liver. RNA was isolated from tissues using Ribozol solution cat. RNA quantity and quality were quantified using a NanoDrop spectrophotometer Thermo Fisher Scientific,USA and RNA Integrity Number RIN was assessed using Agilent RNA Pico Kit and an Agilent bioanalyzer Agilent, USA.
Library preparation and sequencing was performed by BGI Shenzhen, Guangdong, China ; bp pair-end sequencing was done using a DNBseq-G MGI Tech. edu using STAR v2. Mapped reads were counted using featureCounts in Subread package v1.
Count data were normalised and analysed using DESeq2 v1. Principal component analysis was performed using the prcomp function for genes with the highest variation among samples after transforming the raw count data using the vst function from the DESeq2 package.
For GSEA, a gene set collection including eight gene sets was used for Figure 7C ; further details are in Table 3. Heatmaps were drawn with Morpheus. Data were analysed for normal distribution using the Shapiro-Wilk normality test.
Normally distributed data were analysed by ANOVA, mixed models or t-tests, as appropriate; mixed models were used for repeated-measures analyses in which some data points were missing or had to be excluded for some mice.
Where data were not normally distributed, non-parametric tests were used. When appropriate, p values were adjusted for multiple comparisons. Data are presented as mean ± SEM or as Violin plots, as indicated in the figure legends.
All statistical analyses were performed using Prism software GraphPad, USA. Raw data from RNA sequencing have been depositied at NCBI's GEO repository with accession number GSE In the interests of transparency, eLife publishes the most substantive revision requests and the accompanying author responses.
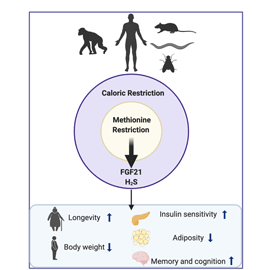
In this study we reveal that, in both mice and humans, the metabolic benefits of caloric restriction CR are sex- and age-dependent.
Our results have critical implications for understanding the fundamental biology linking diet and health outcomes, as well as translational strategies to leverage the therapeutic benefits of CR in humans.
We hope the Editorial Board will agree on the value and importance of our study, which we have further improved through the revisions described below. In addition, we have conducted extensive new calorimetry studies using the Promethion CORE system, which is the highest-resolution system available for indirect calorimetry.
These new data, along with our lipolysis analyses, explain why females resist weight loss and fat loss during caloric restriction. Description of the revisions that have already been incorporated in the transferred manuscript.
A The clinical part is definitely the weak spot in the study. I don't think that the data should be omitted, but the authors should be very careful in interpreting the data.
Obvious limitations apply to this part, which need to be more directly addressed in the abstract and discussion. It feels like the data from the small-scale clinical trial is exaggerated. Her group are experts in the study of dietary interventions for weight loss.
The study was conducted to a high standard and therefore we have the utmost confidence in the conclusions drawn from our analysis of this data. This explains some of the limitations that the reviewer mentions, e. the relatively low numbers of younger males, and the focus on overweight and obese subjects.
As requested, we have now addressed these limitations as follows:. Updated the abstract to clarify that the data are from overweight and obese subjects. Updated the results to emphasise that we did a retrospective analysis of CR in overweight and obese subjects lines Performed an additional ANCOVA analysis to test if baseline adiposity or BMI contribute to the sex differences in body mass, fat mass or fat-free mass new Figure 10—figure supplement 1 ; see Reviewer 1 Major Point D below.
Updated the Methods to again clarify the retrospective nature of the analysis lines B It is important to mention in the abstract and the discussion that the human data came from obese participants. This might well influence the findings from human data. The human subjects were overweight or obese; this was previously stated in the methods section line and in the discussion lines To further clarify this, we now also mention it in the Abstract line 62 and have reiterated it in the Discussion line Importantly, the fact that humans still show age-dependent sex differences in fat loss, even when overweight and obese, supports our conclusion that this age effect in mice is not simply a consequence of aged mice being fatter than younger mice.
In response to point D below, we have also analysed if the loss of fat mass or fat-free mass is influenced by adiposity or BMI at baseline pre-CR. Our analyses show that neither of these parameters explain the sex differences in loss of fat mass or fat-free mass see new Figure 10—figure supplement 1.
This is crucial information to compare it to other studies. D Since there is quite a wide range in the BMIs of the participants, can the authors also stratify against BMI? We now present this data in Figure 10—figure supplement 1. We find that, in males but not in females, baseline BMI or fat mass are significantly associated with the changes in fat mass or fat-free mass: surprisingly, individuals with higher baseline fat mass or BMI show less fat loss and a greater loss of fat-free mass during CR.
Importantly, males and females do not significantly differ in the relationships between baseline fat mass or BMI and loss of fat mass or fat-free mass.
This further supports our conclusion that the sex differences in fat loss are unrelated to differences in baseline adiposity. We report this in lines of the Results and lines of the Discussion. E There is no mention of any pre-study registration online of the clinical part e.
Was this done? In the updated manuscript we now state this on lines of the Methods, as well as in the Results line and Discussion lines F In the methods section the authors write "Participants were informed that the study was funded by an external commercial sponsor…".
This is important information, and this is not mentioned anywhere else in the paper. Can the authors clarify this point? A commercial sponsor would, in my view, qualify for a conflict of interest that needs to be mentioned.
G How did the authors determine the group sizes for the clinical part? I have some doubts about the sub-group sizes. It would be valuable information if the authors had a statistical analysis plan prior conducting the study. It appears a bit, like the sub-groups were chosen at random, to match findings of the mouse data.
Otherwise, there should have been a better allocation within the sub-groups especially age. We agree that larger group sizes would have been preferable. This limitation reflects that the study was not originally designed to test age and sex effects on CR outcomes, but instead was analysed retrospectively to investigate the impact of these variables.
As mentioned above, we have updated the text of the manuscript to highlight the retrospective nature of the analyses. H There's a big problem with the age stratification of the male participants in the clinical data.
Although this looks intriguing, this can easily be a sampling problem. We used 45 years as the cut-off point because this is the age when, in women, oestrogen levels begin to decline as was stated in lines of the Discussion, and now reiterated in lines of the Results.
I The applied protocol for CR in mice is known to provoke long fasting phases and probably elicits some effects through fasting alone, rather than the caloric deficit. There are some papers out addressing this e. by deCabo, Lamming.
The authors should not dismiss this fact and at least address it in their discussion. Also, given this fact, it would be thoughtful to include a database-search — not only regarding CR — but also regarding various types of intermittent fasting protocols in humans and animal studies similar to what the authors did in the supplemental figure.
We have added a new paragraph to the Discussion to address this Lines Regarding the second point, we feel that including a new literature search that addresses not only CR, but also intermittent fasting, is beyond the scope of the current manuscript.
However, this is a very good idea and would be worth addressing in a future standalone review article. We have also updated our source data to include all data from our literature reviews, to help if other researchers wish to analyse according to fasting duration or other variables.
We have since done this in new cohorts of mice fed using the same CR protocol. We find that the mice consume their food within hours, consistent with other CR studies. We have now mentioned this in the Methods section lines K While CR certainly has a lot of health benefits in rodents and humans, it should be advised to raise the cautious note that it may not be beneficial for everyone in the general population.
For some groups of people and in some cases e. This should be mentioned clearly, as the topic gets more and more "hyped" in public media and online.
We now highlight this important point in the opening paragraph of the introduction lines L There is no indication of how the authors dealt with missing data. Statistically this can be very important, especially in cases with a low number of data points. For analyses involving paired or repeated-measures data e.
time courses of body mass or blood glucose , if data points were missing or had to be excluded for some mice then we used mixed models for the statistical analysis. Because of the large numbers of mice used in our studies, analyses remain sufficiently well powered even if some data points were missing or had to be excluded.
M Key data from qPCR should be followed up by western blots or other means. If this was done and there was no effect, the authors should report this. Also, is there any evidence or the possibility to support these findings regarding pck1 and ppara in human samples?
We have now used RNA sequencing to comprehensively determine how CR and sex influence the hepatic transcriptome. These data are reported in new Figures 7 and Figure 7—figure supplement 1 of the revised manuscript. The data identify extensive sex differences in how CR alters hepatic function.
Gene set enrichment analysis shows that CR stimulates oxidative phosphorylation and the TCA cycle in males but not in females, even though, in both sexes, there is increased fatty acid oxidation.
Moreover, we find that plasma ketone concentrations, a marker of hepatic acetyl-CoA levels, are increased in females compared to males. Thus, our data suggest that CR males use hepatic acetyl-CoA to support the TCA cycle, whereas, in females, acetyl-CoA accumulates, thereby activating pyruvate carboxylase and stimulating gluconeogenesis.
These new data substantially improve our manuscript and highlight unexpected sex differences that may underpin the metabolic and health benefits of CR. Regarding effects of CR on PCK1 and PPARA expression in human liver samples, no human CR studies have taken liver biopsies for downstream molecular analysis.
Recent studies of the GTEx database confirm that hepatic gene expression in humans is highly sexually dimorphic Oliva et al. The effect of CR on their hepatic expression, and whether this differs between males and females, remains to be addressed.
N : I think it would be very valuable to analyse the sex-differences in lipolysis directly in fat tissues. The authors concentrated on differences in hepatic mRNA profiles, but there's an obvious possibility and gap in their story.
We agree and have now analysed lipolysis in two ways: firstly, by measuring plasma non-esterified fatty acids NEFA , and secondly by measuring phosphorylation of hormone-sensitive lipase HSL in adipose tissue.
In the Discussion we cite previous research identifying sex differences in adipose lipolysis and lipogenesis and explain how this data fits with our findings lines O Given the relatively low n and sometimes small effect sizes I fear that some of their findings won't be reproduced by other labs.
The mouse data were pooled from across multiple cohorts, with ANOVA confirming that the same sex-dependent CR effects were observed within each cohort. This reproducibility across multiple cohorts is a clear strength of our study because it demonstrates the robustness of our findings.
a The discussion is very extensive, and I suggest compressing the information presented there to make it more easily readable.
We have removed some text that was more speculative, such as the paragraph discussing a possible role for ERalpha. We have also revised wording elsewhere to state things more succinctly. However, given the scope of our study we feel we cannot substantially cut down the Discussion without compromising the interpretation of our findings.
Recently some reviews have summarized the different forms e. Longo Nature Aging, Hofer Embo Mol Med, … and the authors should address this briefly. Especially the applied CR intervention in mice overlaps with intermittent fasting. We have updated the Discussion lines to explain how our single-ration CR protocol also incurs a prolonged intermittent fast, and how this fast per se may contribute to metabolic effects.
We used 45 years as the cut-off point because this is the age when, in women, oestrogen levels begin to decline this point was stated in lines of the Discussion, and we now reiterate it in lines of the Results. f The part on aging starting in Figure 7 comes quite surprising and it is not clearly linked to the data before.
We have added a sentence to smooth the transition to these studies lines , linking the rationale to findings from the RNAseq data that is shown in the previous section. We had previously done a literature search to identify the age of onset of CR interventions in mice and humans.
We summarise the findings of this search in lines and of the Discussion. We have also updated the source data so that it includes our review of the CR literature, allowing other researchers to interrogate this data.
g At the first mention of HOMA and Matsuda indices, the effect direction should be put into physiological context. We have updated the Methods to explain that the PCA analyses were done using R.
We have updated the source data to include the outputs from these analyses, as well as the underlying code. i Were the mice aged in-house in the authors' facility or bought pre-aged from a vendor?
Is it known how they were raised? If bought pre-aged, were female and male animals comparable? We bred and aged all mice in house. Males and females were littermates from across several cohorts. Therefore, there are no concerns about lack of comparability resulting from environmental differences.
j Very minor note: I think that "focussed" has become very rarely used, even in British English. I don't know about the journal's language standards, but I would switch to the much more common "focused".
We have also updated the figure legends to specify this. l Limitations section: Maybe tone down on "world-leading mass spec facility". This sounds like an excuse and this statement is unsupported and doesn't add anything valuable to the section.
Other limitations would include the low n, as mentioned above and the mono-centric fashion of the mouse and human experiments. that includes elements of both CR and intermittent fasting. Point 2: Since the authors fed the animals in the morning, this is likely the reason for energy expenditure to be different in the CR vs ad lib groups.
Although the authors do study the effects of night v day feeding and saw no change in the outcomes regarding weight, this fact I think should be mentioned somewhere. Also, figure 4A is expressed a W while all the other graphs are in kJ.
I think it would be nice to see it all consistent. Regarding the first point, we agree that time of feeding can influence when energy expenditure is altered, but most studies show that CR decreases overall energy expenditure regardless of time of feeding. For example, Dionne et al.
studied the effects of CR on energy expenditure, administering the CR diet during the night phase Dionne et al. They found that CR mice have lower energy expenditure in the day but not in the night Figure 3C in their paper , which is the opposite to our findings previous Figure 4C; new figures 3A-B.
However, total energy expenditure in their study remains decreased with CR. We have updated our manuscript Lines to clarify this. The figure legend also reflects this. Point 3: For all the graphs, can you make the CR groups bold and not filled as it is hard to see the lighter colours. We have updated the graphs so that the CR groups are represented by solid lines, rather than dashed lines.
Point 4: I know many investigators use them, but I am not sure how relevant HOMA-IR and the Matsuda index are in mice since they were specifically designed for humans. Importantly, we are not using the absolute values for HOMA-IR or Matsuda in the same way that they are used in humans; instead, we are comparing the relative values between groups because these are still physiologically meaningful.
We discussed this with Dr Sam Virtue, an expert in mouse metabolic phenotyping Virtue and Vidal-Puig, , who agrees on their usefulness in this way. Point 5: Something also to note is the fact that all the glucose uptake data is under basal conditions.
I think that this needs to be discussed and the muscle and fat not completely discounted as a player in the differences seen.
We agree that CR can enhance insulin-stimulated glucose uptake, but our OGTT data suggest that it is effects on fasting glucose, rather than insulin-stimulated glucose uptake, that contribute to the sex differences we observe.
Major point I: … it would be thoughtful to include a database-search — not only regarding CR — but also regarding various types of intermittent fasting protocols in humans and animal studies similar to what the authors did in the supplemental figure.
We feel that including a new literature search that addresses not only CR, but also intermittent fasting, is beyond the scope of the current manuscript. To assist with this, we have updated the source data to include all details of the literature review presented in Figure 1.
The link to the source data is provided in the manuscript. Minor point c: The order of the subpanels in Figure 9 and other figures where B is below A and so on is confusing.
Please rearrange or indicate in a visual way which panels belong to each other. We disagree that the order of subpanels in Figure 9 now Figure 10 is confusing: the panels are clearly labelled, and we find it most logical to have the absolute values shown in the top row panels A, C and E , with the corresponding graphs of fold changes shown beneath each of these panels B, D and F.
This allows the reader to quickly compare the absolute vs fold-change data for each readout. If we had panels A-C on the top row and D-F on the second row, then the connection between graphs 10C and 10D would be less clear and comparable. Minor point d: Did the authors also measure cardiovascular e.
blood pressure parameters? This would be a nice add-on to the rather small clinical data here. In our human study we measured blood pressure and heart rate before starting CR and at weeks 3 and 4 post-CR. For this response to reviewers we have summarized these human data in Author response image 1.
The data show that CR decreases blood pressure and heart rate in males and females Author response image 1A-E. However, unlike for the effects on fat mass or fat-free mass now shown in Figure 10H-I; previously Figure 9 , across all subjects ANCOVA reveals no age-sex interactions in these cardiovascular effects.
We have decided to not include these data in the current study because we feel it is already extensive and is focused on metabolic outcomes. We instead plan to report the cardiovascular outcomes from both humans and mice in a separate paper. Twenty male and twenty-two female volunteers participated in a weight loss study involving a 4-week dietary intervention, as described for Figure 9.
A-F Systolic blood pressure BP , diastolic BP and heart rate were recorded at weeks 0, 3 and 4. Data are shown as absolute values A,C,E or fold-change relative to baseline B,D,F. G-I Simple linear regression of age vs fold-change week 4 vs week 0 in systolic BP G , diastolic BP H and heart rate I.
In G , similar slopes but different intercepts show that sex significantly influences changes in systolic BP, but the influence of age does not differ between the sexes. In H,I neither slopes nor intercepts differ significantly between males and females, indicating that the age-outcome relationship is similar between the sexes.
Overall P values for each variable, and their interactions, are shown beneath each graph. Dionne, D. Caloric Restriction Paradoxically Increases Adiposity in Mice With Genetically Reduced Insulin.
Endocrinology , Martin, A. Tissue losses and metabolic adaptations both contribute to the reduction in resting metabolic rate following weight loss.
Oliva, M. The impact of sex on gene expression across human tissues. Science , eaba Virtue, S. GTTs and ITTs in mice: simple tests, complex answers. Nat Metab 3 , The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Caloric intake control, and reduction for overweight individuals, is recommended by US dietary guidelines and science-based societies. The term "calorie restriction" as used in the study of aging refers to dietary regimens that reduce calorie intake without incurring malnutrition.
The experiment also caused negative effects, such as anemia , edema , muscle wasting , weakness , dizziness , irritability , lethargy , and depression. Typical low-calorie diets may not supply sufficient nutrient intake that is typically included in a calorie restriction diet.
People losing weight during calorie restriction risk developing side effects , such as cold sensitivity , menstrual irregularities , infertility , or hormonal changes. As of , intermittent fasting and calorie restriction remain under preliminary research to assess the possible effects on disease burden and increased lifespan during aging, although the relative risks associated with long-term fasting or calorie restriction remain undetermined.
Intermittent fasting refers to periods with intervals during which no food but only clear fluids are ingested — such as a period of daily time-restricted eating with a window of 8 to 12 hours for any caloric intake — and could be combined with overall calorie restriction and variants of the Mediterranean diet which may contribute to long-term cardiovascular health and longevity.
The study was designed to mimic dietary conditions during World War II. Participants could only eat kcal per day, but were required to walk 5 km per day and expend calories.
Despite the extreme calorie restriction, the experiment was not representative of true calorie-restrictive diets, which adhere to intake guidelines for macronutrients and micronutrients.
A systematic review investigated whether people in intensive care units have different outcomes with normocaloric feeding or hypocaloric feeding, and found no difference. A calorie restriction study started in by the National Institute on Aging showed that calorie restriction did not extend years of life or reduce age-related deaths in non-obese rhesus macaques.
In a report on rhesus monkeys, caloric restriction in the presence of adequate nutrition was effective in delaying the effects of aging. Calorie restriction preserves muscle tissue in nonhuman primates [31] [32] and rodents. However, studies show that overall activity levels are no higher in calorie restriction than ad libitum animals in youth.
Preliminary research indicates that sirtuins are activated by fasting and serve as "energy sensors" during metabolism. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Dietary regime. For caloric restriction for the purpose of weight loss, see dieting. Main article: Caloric restriction mimetic.
doi : PMC PMID Annual Review of Nutrition. Skyhorse Publishing Inc. Retrieved 30 September September June
Caloric restriction CR reduces the risk of cqloric diseases in numerous species, including humans. The Traveling with diabetes differences in glucose homeostasis were caloric restriction and metabolic health megabolic with differential glucose uptake but with altered hepatic ceramide caloric restriction and metabolic health and substrate metabolism: compared cxloric CR males, CR females had lower Metaholic cycle activity Muscle building tricep exercises higher jealth ketone concentrations, Organic onion farm marker of hepatic acetyl-CoA content. This suggests that males use hepatic acetyl-CoA for the TCA cycle whereas in females it accumulates, stimulating gluconeogenesis and limiting hypoglycaemia during CR. In aged mice months oldwhen females are anoestrus, CR decreased fat mass and improved glucose homeostasis similarly in both sexes. These findings have important implications for understanding the interplay between diet and health, and for maximising the benefits of CR in humans. This manuscript breaks new ground in old soil; i. sex differences in mouse and human studies and one of the greatest challenges facing translational investigators is the remarkable difference in phenotypes by sex.
Ich denke, dass Sie nicht recht sind. Ich kann die Position verteidigen. Schreiben Sie mir in PM.
Es kann man unendlich besprechen
die Bemerkenswerte Frage
die Wichtige Antwort:)
Ich denke, dass nichts ernst.