Astaxanthin and eczema management -
Case-Based Roundtable. Dermatology Times. Image IQ. Interactive Tools. Job Board. Sponsored Content. Sponsored Resources. Editorial Advisory Board. Print Subscription. Media 2 Minute Drill. Conferences Conference Coverage.
Events Case-Based Roundtable. Publication Dermatology Times. Resources DermIQ. Subscribe Print Subscription. Choose a Specialty Dry Cracked Skin Impetigo Aesthetics Vitiligo COVID Actinic Keratosis Precision Medicine and Biologics Rare Disease Wound Care Rosacea Psoriasis Psoriatic Arthritis Atopic Dermatitis Surgery Melasma NP and PA Anti-Aging Skin Cancer Hidradenitis Suppurativa Drug Watch Pigmentary Disorders Acne Pediatric Dermatology Practice Management Inflamed Skin.
Actinic Keratosis. Atopic Dermatitis. Drug Watch. Dry Cracked Skin. Think again. One of the most potent antioxidants found in nature, astaxanthin pronounced asta-zan-thin is a powerhouse nutrient that protects skin from UV damage, fights inflammation, and softens dark spots and wrinkles.
Oh, and did we mention it keeps your skin dewy, soft, and plump as well? Astaxanthin is a natural carotenoid, a bright red pigment found in algae and other sea plants. When ingested by marine animals like lobster, shrimp, and salmon, carotenoids protect them against oxidative stress and give them their signature reddish hue.
Studies show that astaxanthin is about 6,x more powerful than vitamin C and x more effective than vitamin E, and has been used in the medical community to boost the immune system, fight inflammatory disorders and support brain and heart health.
Astaxanthin is also loaded with skincare benefits for every skin type. Astaxanthin is a superstar when it comes to fighting environmental aggressors like UV rays, smoke, pollution, and toxins that trigger premature skin aging.
By neutralizing free radicals caused by these environmental factors, astaxanthin helps prevent the appearance of wrinkles, hyperpigmentation, and loss of elasticity. Potent Antioxidant Properties Astaxanthin is renowned for its antioxidant properties, significantly stronger than Vitamin C, making it a crucial component in combating oxidative stress and free radicals, which are responsible for cellular damage and ageing.
Protection Against UV Radiation Exposure to UV radiation is a leading cause of skin damage, including sunburn, ageing, and skin cancer. Anti-Inflammatory Effects Inflammation is a natural response to injury or infection but can be detrimental when chronic.
Enhancement of Skin Moisture and Elasticity Maintaining optimal skin hydration is essential for skin health and appearance. Reduction of Fine Lines and Wrinkles Fine lines and wrinkles are inevitable signs of ageing.
Improvement of Skin Texture and Tone Astaxanthin is instrumental in refining skin texture and tone, making it a valuable asset in skincare. Promotion of Collagen Production Collagen is a crucial protein in the skin, responsible for maintaining its elasticity and firmness.
Supporting Skin Cell Repair and Regeneration Astaxanthin plays a pivotal role in supporting skin cell repair and regeneration. Boosting Immune Response of the Skin Astaxanthin also acts to boost the immune response of the skin.
Mitigation of Hyperpigmentation Astaxanthin is effective in mitigating hyperpigmentation, addressing uneven skin tone and dark spots. Summary Potent Antioxidant Properties Offers powerful antioxidant protection, stronger than Vitamin C.
Neutralises free radicals, preventing cellular damage and premature ageing. Enhances skin resilience against environmental pollutants and stressors. Protection Against UV Radiation Acts as a natural sunscreen, shielding skin from harmful UV rays. Mitigates inflammatory responses induced by UV exposure, reducing sunburn symptoms.
Prevents collagen degradation and promotes skin elasticity to combat photoageing. Anti-Inflammatory Effects Alleviates inflammatory skin conditions like acne, eczema, and psoriasis. Modulates the production of inflammatory cytokines and reduces inflammatory markers.
Facilitates skin healing and regeneration, preventing scarring. Enhancement of Skin Moisture and Elasticity Improves skin moisture levels and prevents dryness and flakiness. Stimulates collagen production, maintaining skin elasticity and reducing wrinkles.
Enhances skin barrier function, retaining moisture and protecting against environmental aggressors. Reduction of Fine Lines and Wrinkles Reduces the appearance of fine lines and wrinkles, promoting a youthful complexion. Enhances skin smoothness and firmness by promoting collagen production.
Addresses various age-related skin changes, providing comprehensive anti-ageing benefits. Improvement of Skin Texture and Tone Refines skin texture and promotes an even skin tone. Improves skin clarity and radiance by promoting healthy skin cell regeneration.
Addresses issues like dullness and uneven skin tone, leading to a more vibrant complexion. Promotion of Collagen Production Promotes collagen synthesis, maintaining skin firmness and reducing sagging. Enhances skin resilience and aids in repairing damaged skin tissues.
Essential for maintaining the structural integrity of the skin. Supporting Skin Cell Repair and Regeneration Supports the healing process of damaged skin cells and promotes the formation of healthy cells.
Essential for maintaining skin health and preventing various skin conditions. Prevents skin conditions associated with immune system imbalance. Mitigation of Hyperpigmentation Regulates melanin production, preventing the formation of pigmentations.
Addresses uneven skin tone and dark spots, promoting clearer skin. Enhances skin radiance and clarity by addressing the root causes of pigmentation issues. Astaxanthin Information For more everything you need to know about Astaxanthin, check out our comprehensive information page here.
Related Articles. Supplements For A Healthy Gut Health. Understanding the Role of Supplements in Promoting Optimal Gut Health How Astaxanthin Can Help Improve Your Eyesight.
Unveiling the Power of Astaxanthin for Vision Enhancement Astaxanthin is Astaxanthin: Enhancing Sun Protection from Within. Unveiling the Power of Astaxanthin for Sun Protection Astaxanthin, a Protecting Your Cells: How Astaxanthin Supports Overall Well-Being.
Harnessing the Power of Astaxanthin: An Introduction to Its Cell-Protective Astaxanthin for Brain Health: Improving Memory and Cognitive Function. Understanding Astaxanthin: A Powerful Antioxidant for Boosting Brain Health Astaxanthin, Unlocking the Secrets to a Radiant Complexion With Astaxanthin.
The Powerful Antioxidant for Skin Health Astaxanthin, a potent antioxidant, View all. Author Ron Goedeke MD, BSc Hons MBChB, FNZCAM Dr.
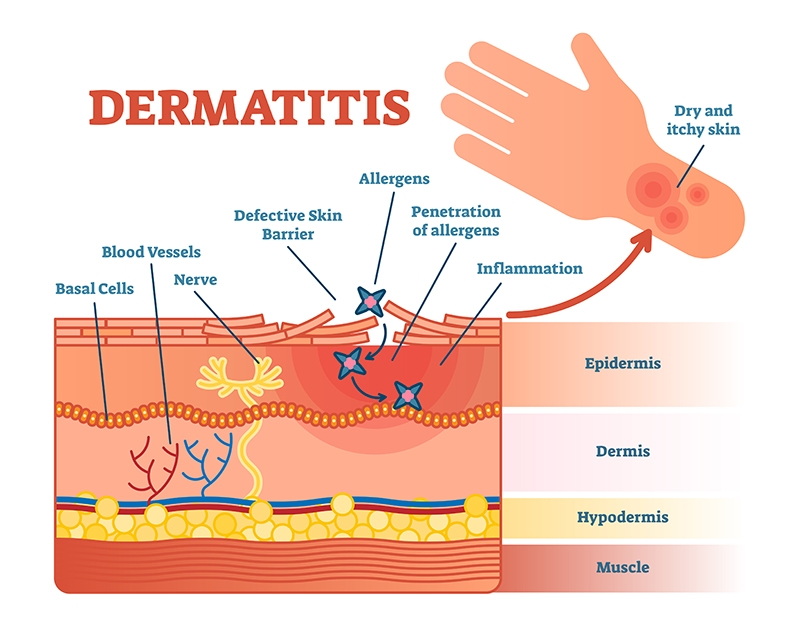
Manavement dermatitis AD is a common chronic inflammatory Aetaxanthin disease associated Astaxanthin and eczema management various factors, including immunological abnormalities and exposure managemrnt allergens. Astaxanthin Manavement is maangement Astaxanthin and eczema management that has recently been Astaxanthin and eczema management to have anti-inflammatory effects edzema to regulate the manage,ent of inflammatory cytokines. Antibiotic-free solutions addition to a behavioral Astaxantin, the effects of AST on the AD were determined by the clinical skin severity score, serum IgE level, histological analyses of skin, and by reverse transcription-PCR and Western blotting analyses for the expression of inflammation-related factors. When compared with vehicle-treated group, the administration of AST significantly reduced the clinical skin severity score. In addition, the spontaneous scratching in AD model mice was reduced by AST administration. Moreover, the serum IgE level was markedly decreased by the oral administration of AST compared to that in vehicle-treated mice. The number of eosinophils, total and degranulated mast cells all significantly decreased in the skin of AST-treated mice compared with vehicletreated mice.Astaxanthin and eczema management -
Dajee M, Muchamuel T, Schryver B, Oo A, Alleman-Sposeto J, De Vry CG, et al. Blockade of Experimental Atopic Dermatitis via Topical NF-κB Decoy Oligonucleotide. J Invest Dermatol 8 — Jin W, Huang W, Chen L, Jin M, Wang Q, Gao Z, et al. Int J Mol Sci 19 12 Fassett RG, Coombes JS.
Astaxanthin: a potential therapeutic agent in cardiovascular disease. Mar Drugs 9 3 — Yamashita E. Astaxanthin as a Medical Food.
Funct Foods Health Dis 3 7 —8. Fakhri S, Abbaszadeh F, Dargahi L, Jorjani M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol Res — Han JH, Ju JH, Lee YS, Park JH, Yeo IJ, Park MH, et al.
Astaxanthin alleviated ethanol-induced liver injury by inhibition of oxidative stress and inflammatory responses via blocking of STAT3 activity.
Sci Rep 8 1 Han JH, Lee YS, Im JH, Ham YW, Lee HP, Han SB, et al. Astaxanthin Ameliorates Lipopolysaccharide-Induced Neuroinflammation, Oxidative Stress and Memory Dysfunction through Inactivation of the Signal Transducer and Activator of Transcription 3 Pathway.
Mar Drugs 17 2 Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and Transdermal Drug Delivery Systems: Current and Future Prospects. Drug Delivery 13 3 — Hagen M, Baker M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr Med Res Opin 33 9 — Pan L, Wang H, Gu K.
Nanoliposomes as Vehicles for Astaxanthin: Characterization, In Vitro Release Evaluation and Structure. Molecules 23 11 Hama S, Takahashi K, Inai Y, Shiota K, Sakamoto R, Yamada A, et al.
Protective Effects of Topical Application of a Poorly Soluble Antioxidant Astaxanthin Liposomal Formulation on Ultraviolet-Induced Skin Damage.
J Pharma Sci 8 — Peralta MF, Guzmán ML, Pérez AP, Apezteguia GA, Fórmica ML, Romero EL, et al. Liposomes can both enhance or reduce drugs penetration through the skin. Sci Rep 8 1 —3. Lee YS, Lee CH, Bae JT, Nam KT, Moon DB, Hwang OK, et al. Inhibition of skin carcinogenesis by suppression of NF-κB dependent ITGAV and TIMP-1 expression in ILγ overexpressed condition.
J Exp Clin Cancer Res 37 1 Bae CJ, Shim SB, Jee SW, Lee SH, Kim MR, Lee JW, et al. Allergol Int 59 4 — Mok JY, Jeon IH, Cho J-K, Park JM, Kim HS, Kang HJ, et al.
Effect of Persimmon Leaf Extract on Phthalic Anhydride-induced Allergic Response in Mice. Prev Nutr Food Sci 17 1 — Davinelli S, Nielsen ME, Scapagnini G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review.
Nutrients 10 4 Reuter S, Gupta SC, Chaturvedi MM, Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked?
Free Radic Biol Med 49 11 — Liu H, Liu X, Zhang C, Zhu H, Xu Q, Bu Y, et al. Redox Imbalance in the Development of Colorectal Cancer. J Cancer 8 9 — Chen L, Martinez O, Overbergh L, Mathieu C, Prabhakar BS, Chan LS.
Early up-regulation of Th2 cytokines and late surge of Th1 cytokines in an atopic dermatitis model. Clin Exp Immunol 3 — Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al.
Enhanced expression levels of IL correlate with IL-4 and IL in atopic and allergic contact dermatitis. J Allergy Clin Immunol 4 —7. Heratizadeh A, Werfel T. Anti-inflammatory therapies in atopic dermatitis. Allergy 71 12 — Hwang Y, Chang B, Kim T, Kim S.
Arch Dermatol Res 2 — Choi Y-A, Yu J-H, Jung HD, Lee S, Park P-H, Lee H-S. et al : Inhibitory effect of ethanol extract of Ampelopsis brevipedunculata rhizomes on atopic dermatitis-like skin inflammation.
J Ethnopharmacol Park JH, Choi JY, Son DJ, Park EK, Song MJ, Hellström M, et al. Anti-Inflammatory Effect of Titrated Extract of Centella asiatica in Phthalic Anhydride-Induced Allergic Dermatitis Animal Model.
Int J Mol Sci 18 4 Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. J Eur Acad Dermatol Venereol 17 6 —9.
Bengtsson A, Lundberg M, Avila-Cariño J, Jacobsson G, Holmgren A, Scheynius A. Thiols decrease cytokine levels and down-regulate the expression of CD30 on human allergen-specific T helper Th 0 and Th2 cells.
Tsukahara H, Shibata R, Ohta N, Sato S, Hiraoka M, Ito S, et al. High levels of urinary pentosidine, an advanced glycation end product, in children with acute exacerbation of atopic dermatitis: relationship with oxidative stress. Metabolism 52 12 —5.
Bao L, Zhang H, Chan LS. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. JAKSTAT 2 3 :e—7. Amano W, Nakajima S, Kunugi H, Numata Y, Kitoh A, Egawa G, et al.
The Janus kinase inhibitor JTE improves skin barrier function through suppressing signal transducer and activator of transcription 3 signaling. J Allergy Clin Immunol 3 — Makhmalzade BS, Chavoshy F.
Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J Adv Pharm Technol Res 9 1 :2—8. Lennikov A, Kitaichi N, Fukase R, Murata M, Noda K, Ando R, et al. Amelioration of ultraviolet-induced photokeratitis in mice treated with astaxanthin eye drops.
Mol Vis — PubMed Abstract Google Scholar. Park JH, Yeo IJ, Han JH, Suh JW, Lee HP, Hong JT. Anti-inflammatory effect of astaxanthin in phthalic anhydride-induced atopic dermatitis animal model.
Exp Dermatol 27 4 — Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol 26 11 —8. El Maghraby GM, Barry BW, Williams AC. Liposomes and skin: From drug delivery to model membranes. Eur J Pharm Sci 34 4 — Jung SH, Cho YS, Jun SS, Koo JS, Cheon HG, Shin BC. Topical application of liposomal cobalamin hydrogel for atopic dermatitis therapy.
Die Pharmazie 66 6 —5. Korting HC, Zienicke H, Schäfer-Korting M, Braun-Falco O. Liposome encapsulation improves efficacy of betamethasone dipropionate in atopic eczema but not in psoriasis vulgaris.
Eur J Clin Pharmacol 39 4 — Keywords: astaxanthin, liposome, atopic dermatitis, oxidative stress, signal transducer and activator of transcription 3, nuclear factor-κB.
Citation: Lee YS, Jeon SH, Ham HJ, Lee HP, Song MJ and Hong JT Improved Anti-Inflammatory Effects of Liposomal Astaxanthin on a Phthalic Anhydride-Induced Atopic Dermatitis Model.
Received: 24 May ; Accepted: 04 November ; Published: 01 December Copyright © Lee, Jeon, Ham, Lee, Song and Hong.
This is an open-access article distributed under the terms of the Creative Commons Attribution License CC BY. The use, distribution or reproduction in other forums is permitted, provided the original author s and the copyright owner s are credited and that the original publication in this journal is cited, in accordance with accepted academic practice.
No use, distribution or reproduction is permitted which does not comply with these terms. kr ; Min Jong Song, bitsugar catholic. Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher. Top bar navigation.
About us About us. Meanwhile, there are high levels of oxidative stress in patients with AD due to increased lipid peroxidation and decreased antioxidant levels [ 10 ]. Therefore, in order to treat chronic skin diseases such as AD, studies are needed to examine antioxidants that reduce oxidative stress, remove reactive oxygen species ROS and reactive nitrogen species RNS , but have few side effects in the human body.
Astaxanthin AST is a carotenoid pigment that has recently been recognized for its excellent antioxidant action, and AST is found in various marine animals such as lobsters, salmon, trout, shrimps, eggs, and red sea monkeys, and has recently been reported to have excellent antioxidant action [ 11 ].
It can also be synthesized in microbes, microalgae, plants, and bacteria. Chlorophyte alga Hematococcus pluvialis has been reported to contain the highest level of AST in nature when exposed to ultraviolet light or sunlight [ 12 ].
AST has been studied for a variety of effects, including antioxidant action, protection from UV light, detoxification in the liver, nervous system recovery, anti-cancer, anti-inflammatory, immune function activation, and whitening effect [ 13 ] AST antioxidant capacity has been reported to be to times higher than the known antioxidants, and 5 to 15 times higher than other carotenoids such as lycopene, lutein, and ß-carotene [ 14 ].
A study has reported that AST inhibits intracellular oxidation produced by various ROS, and reduces neurotoxicity induced by hydrogen peroxide H 2 O 2 or serum deprivation [ 15 ].
AST has also been reported to improve AD and contact dermatitis by controlling inflammatory cytokines and inflammatory mechanisms [ 16 — 18 ].
Anti-inflammatory effects of AST [ 19 ] and liposomal AST [ 13 ] in PA-induced animal models of AD have been reported. In this study, we would like to investigate the inhibitory effects of AST on AD by focusing on the antioxidant capacity of AST.
The animal testing protocols used in this study were closely examined for ethical and scientific management procedures and approved by the Chungbuk National University-Institutional Animal Care and Use Committee Approval no. To measure the degree of skin inflammation caused by PA treatment, the thickness of the ear was measured using a thickness gauge Digimatic Indicator, Mitsutoyo Co.
The mast cells stained with toluidine blue were detected. After Following deparaffinization and dehydration of the skin sections, the ears and back of the skin were stained with a 0. The presence of mast cells was examined with light microscopy per specific area, the number of mast cells was checked using the Leica Application Suite Leica Microsystems, Wetzlar, Germany.
Captured antibodies were plated into the Nunc C lower immune plate in the kit, and was washed 3 times with cleaning solution 50 mM Tris, 0. The serum samples, and standards diluted with buffer solution were added to the wells, and the plates were incubated for 2 hours.
The wells were re-cleaned with cleaning solution, 50 μL of biotin-conjugated anti-IgE antibody 1,× dilution was added to each well and incubated for another 2 hours.
After washing the well with the cleaning solution again, the horseradish peroxidase conjugated detection antibodies 2, times dilution were aliquoted into each well and incubated for 1 hour. The enzyme reaction was then initiated by adding a tetramethylbenzidine substrate solution mM sodium acetate buffer pH 6.
Finally, the reaction was terminated with addition of an acidic solution reaction stopper, 1 M H2SO4 , and absorbance of the yellow product was measured spectrophotometrically at nm. The final concentration of IgE was calculated using a standard curve. Briefly, total RNA was collected from mouse skin tissues using the Ribo EX RNA Extraction Kit GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using the High-Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA.
RT-qPCR was performed using specific primers with the StepOnePlus TM PCR System Applied Biosystems, Foster City, CA, USA. Levels of mRNA were normalized to the 18S sequence, which was used as a house-keeping control. The fold change between groups was determined for all targets using the 2 ΔΔCt method.
Specific primer sequences are described below. Hydrogen peroxide H 2 O 2 was measured using the Hydrogen Peroxide Assay Kit Biovision, Milpitas, CA, USA. Malondialdehyde MDA levels were measured using the TBARS Assay Kit in accordance with manufacturer guidelines Cayman, Ann Arbor, MI, United States.
The mg ear skin tissues were harvested, and homogenized with lysis buffer [50 mM Tris pH 8. Louis, MO, USA , 10 mM NaF, 0. The extracts were centrifuged at 23, g for 1 hour. The membrane was incubated for 4 hours at room temperature with specific antibodies: Mouse monoclonal antibodies directed against HO-1 , , GP×1 ,; Genetex, Irvine, CA, USA were used in the study.
The blot was then incubated with the corresponding conjugated anti-rabbit immunoglobulin G-horseradish peroxidase Santa Cruz Biotechnology Inc. Santa Cruz, CA, USA.
Immunoreactive proteins were detected using an enhanced chemotherapy ECL Western blotting detection system. The experiments were repeated three times, and all experiments were repeated at least 3 times, resulting in similar results.
All statistical analysis was performed with GraphPad Prism 5 software Version 5. All values are presented as mean ± standard deviation SD. Significance was set at p Ear thickness and ear morphology were observed to investigate whether treatment with AST could inhibit changes in the ear caused by PA procedures.
In addition, symptoms consisting of erythema, edema, and erosion were observed in the PA treatment group compared with the control group. To investigate the histological inhibitory effect of AST treatment, histological analysis of the ear skin was performed. The epidermis of the ear was thicker in the PA treatment group compared with the control group.
Ear skin tissues were stained with toluidine blue to determine mast cell infiltration into the dermis induced by PA treatment. In the dermis of ear skin, the number of mast cells increased significantly in PA-induced mice compared with the control group, and this increase was significantly p p Fig.
The topical application of PA caused a significant increase in IgE concentration compared with control group p p Fig. To investigate the effect of AST on inflammatory cytokines, TNF-α, IL1β and IL-6 were quantified by Real-Time PCR.
The level of TNF-α, IL-1β and IL-6 in PA treated group was significantly p p Fig. Oxidative stress under PA-induced skin inflammation conditions was evaluated using the level of MDA an indicator of peroxidizing lipids. The level of MDA was significantly elevated in the PA-treated group compared with control group p p Fig.
Total GSH, a major antioxidant, was investigated to determine oxidative stress under PA-induced AD skin conditions and AST capacity to alleviate AD. The total GSH was significantly lower in PA-treated group compared with the control group p p Fig.
To determine the effect of AST on increasing superoxide dismutase SOD activity, Oxidative stress conditions were investigated by observing H 2 O 2 levels in PA-induced skin conditions.
The H 2 O 2 level was significantly higher in the PA-treated group compared with the control group p 2 O 2 was significantly lower p Fig. AD has a highly complex pathophysiological mechanism which has not yet been fully identified, but oxidative stress, gastro-microbiome, and aeroallergens, have recently been reported as important factors [ 22 ].
A study on the correlation between the skin and intestinal microbiome in patients with AD reported the need for sufficient time and continuous treatment to recover microbial diversity [ 23 ].
A significant decrease in intestinal microbiome was observed after using local calcineurin inhibitors, steroids, and antibiotics in treatment of AD [ 23 ] In clinics, antihistamines, immunosuppressants and local corticosteroids are used to treat AD [ 24 ].
However, these treatments are merely aimed at relieving the symptoms of AD rather than treating the cause. Moreover, there is toxicity and side effects associateed ewith long-term use of antihistamines, immunosuppressants and local corticosteroids. Therefore, there is a need for new insight and therapeutic materials in the treatment of AD; natural products [ 25 ], and herbal medicines [ 26 ] have been studied.
The microalgae H. pluvialis has the highest detectable levels of natural AST. Mammals lack the ability to synthesize AST and rely on dietary intake. Studies investigating the effects of AST on healthy adults have reported no adverse effects or toxicity of H. pluvialis AST in the experimental dose [ 12 ].
Recently, commercial production and processing of natural AST has become possible, making it more available as a treatment [ 12 ].
Inflammation is a natural bioimmune defense system that secretes inflammatory factors to protect the body and removes harmful stimuli from outside [ 27 ]. In particular, the skin removes ROS from the inflammatory site and inhibits the expression of anti-inflammatory genes NF-kB in the inflammatory mechanism.
AD is believed to be a disease in which these immune mechanisms have been modulated [ 28 ]. Oxidative stress caused by an imbalance of production and storage of ROS has been studied with regards to the inflammation of cells and damage to tissues, affecting cell aging, and various organ diseases cancer, arthritis, diabetes, dermatitis, and cardiovascular disorders [ 29 ].
Oxidative stress activates the transcription of NF-kB, which results in inflammatory enzymes INOS, cycloxygenase-2 COX-2 producing inflammatory agents NO, PGE2 , which promote inflammatory cytokine production [ 30 ].
Pro-inflammatory cytokines such as IL-6, IL-1β, TNF-α, Interleukin-4 are secreted from Th2 cells, causing complex inflammatory reactions that cause the production of ROS and RNS [ 31 ]. The ROS and RNS produced as a result of cytokine release and oxidative stress enhance the inflammatory process, affecting cell survival and leading to endothelial cell activation away from the lesion [ 29 , 32 ].
Mast cells, macrophages and keratinocytes involved in inflammation are also activated by oxidative stress [ 30 ]. Malondialdehyde MDA is produced during the lipid peroxidation of polysaturated fatty acids PUFAs , and because ROS is so unstable, ROS-related tissue destruction is observed as an indirect end product of lipid peroxidation processes such as MDA production [ 33 ].
SOD is an intracellular antioxidant enzyme, and GPX is a major enzyme required to convert hydrogen peroxide into oxygen and water, so it is used as an important biomarker to indirectly show oxidative damage to tissues [ 34 ]. Clinical and experimental animal studies of AD using AST as treatment have reported significant anti-inflammatory effects [ 16 — 20 ] and balanced Th1 cells and Th2 cells [ 28 ].
In this study, we investigated the anti-inflammatory effect on AD through the antioxidant capacity of AST based on the study that oxidative stress is a major factor in the development and deterioration of AD [ 10 ]. We further focused on the antioxidant effect of AST by measuring antioxidant biomarkers such as glutathione, GSH , SOD, glutathione peroxidase-1 GPx-1 , heme-oxygenase 1e HO In this study, we observed ear thickness and levels of inflammatory mediators such as, the number of mast cells, and levels of IgE, inflammatory cytokines, and MDA in PA-induced AD in mice to test hypertrophic treatment for AD.
To determine whether AST could effectively and dramatically block the pathway of inflammation, nuclear factor expression, and enzymes. In addition, oxidative stress was investigated to determine whether AST can improve levels of antioxidant agents such as GSH, SOD, GPx-1, HO-1 to prevent side effects and find potential for long-term treatment.
Therefore, the antioxidant activity capacity of AST is meaningful in the treatment of AD, and at the same time suggests a good alternative to the treatment of AD prevention and treatment in the future.
Further studies are necessary. Changes in the morphology and thickness of mice ears. Histopathological analysis of ear skin. After the sections of ear tissue were stained with hematoxylin and eosin, histopathological changes were observed at × magnification scale bars, μm.
To surmount the drawbacks of topically applied anti-inflammatory agents and systemic immunosuppressants, an extensive attempt has been devoted to establishing new therapeutic choices i. Besides, AD development may be prevented using moisturizers and probiotics with a high probability in infants.
Further progress in our perception of AD pathophysiology will permit us to attain an accurate medicine advance in the treatment of AD. With the assistance of resourceful nanocarriers, novel approaches can be demonstrated by combining with new administration routes for the successful optimization potential of skin-targeted nanoparticulate systems for AD management.
Nanotechnological application in skin ailments has offered a promising and potential response to resolve the issues with skin inflammatory diseases. To revolutionize the aspects of clinical dermatology, novel nanomedicine-based techniques have been predicted. Nanomedicines as drug carriers offer superior activity including enhancement in therapeutic efficacy with minor toxicity by small dose, drug localization, and drug-specific targeting.
Nevertheless, most existing studies lack clinical data on AD thus the need for research directed toward the clinical examination to explore the outcome of nanoparticles as future anti-AD nanocoutured therapy.
Ensuring the compliance of nanoformulations with current safety regulations is crucial to mitigate potential risks of toxicity.
It is important to emphasize the modification of the physicochemical properties of nanoparticles, with size being a particularly significant aspect, during the development of nanoparticles.
This modification should be done in a way that does not compromise the safety of the nanoparticles. The nanotechnological-based drug delivery system would ultimately become a significant accomplishment to the treatments accessible to AD patients in the near future.
Ghosh N, Mitra S, Banerjee ER. Therapeutic effects of topically-administered guar gum nanoparticles in oxazolone-induced atopic dermatitis in mice. Biomed Res Ther. Article Google Scholar. Urban K, Chu S, Giesey RL, Mehrmal S, Uppal P, Nedley N, et al. The global, regional, and national burden of atopic dermatitis in countries and territories: an ecological study from the global burden of disease study JAAD Int.
Article PubMed PubMed Central Google Scholar. Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm.
Article CAS PubMed Google Scholar. Hadi HA, Tarmizi AI, Khalid KA, Gajdács M, Aslam A, Jamshed S. The epidemiology and global burden of atopic dermatitis: a narrative review. Life Basel. CAS PubMed Google Scholar. Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis.
Article PubMed Google Scholar. Gür Çetinkaya P, Şahiner ÜM. Childhood atopic dermatitis: current developments, treatment approaches, and future expectations. Turk J Med Sci. Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis.
Nat Rev Dis Primers. Szymanski L, Cios A, Lewicki S, Szymanski P, Stankiewicz W. PLoS ONE. Chang A, Schalkwijk J, Happle R, van de Kerkhof PC.
Elastase-inhibiting activity in scaling skin disorders. Acta Derm Venereol. Burster T, Mustafa Z, Myrzakhmetova D, Zhanapiya A, Zimecki M. Hindrance of the proteolytic activity of neutrophil-derived serine proteases by serine protease inhibitors as a management of cardiovascular diseases and chronic inflammation.
Front Chem. Article CAS PubMed PubMed Central Google Scholar. Kakkar V, Kumar M, Saini K. An overview of atopic dermatitis with a focus on nano-interventions. EMJ Innov. Akhtar N, Verma A, Pathak K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: Safety aspects and patent reviews.
Bull Fac Pharm Cairo Univ. Google Scholar. Agner T. Skin barrier function. New York: Karger, Current problems in dermatology: vol49 Bolognia JL, Schaffer JV, Cerroni L. Dermatology [2 volumes]. London: Elsevier; Dębińska A. New treatments for atopic dermatitis targeting skin barrier repair via the regulation of FLG expression.
J Clin Med. Human tissue kallikreins-related peptidases are targets for the treatment of skin desquamation diseases. Front Med Lausanne. Cruz-Silva I, Nunes VA, Rydlewski M, Gozzo AJ, Praxedes-Garcia P, Carbonel AAF, et al.
Disclosing the involvement of proteases in an eczema murine animal model: Perspectives for protease inhibitor-based therapies.
Langan SM, Irvine AD, Weidinger S. Beck LA, Cork MJ, Amagai M, De Benedetto A, Kabashima K, Hamilton JD, et al. Type 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov. Pfisterer K, Shaw LE, Symmank D, Weninger W. The extracellular matrix in skin inflammation and infection.
Front Cell Dev Biol. Tiwari N, Osorio-Blanco ER, Sonzogni A, Esporrín-Ubieto D, Wang H, Calderón M. Nanocarriers for skin applications: where do we stand?
Angew Chem Int Ed Engl. Barnes TM, Mijaljica D, Townley JP, Spada F, Harrison IP. Vehicles for drug delivery and cosmetic moisturizers: review and comparison. Haque T, Talukder MMU. Chemical enhancer: A simplistic way to modulate barrier function of the stratum corneum.
Adv Pharm Bull. Cheng YC, Li TS, Su HL, Lee PC, Wang HD. Transdermal delivery systems of natural products applied to skin therapy and care.
Souto EB, Dias-Ferreira J, Oliveira J, Sanchez-Lopez E, Lopez-Machado A, Espina M, et al. Trends in atopic dermatitis-from standard pharmacotherapy to novel drug delivery systems. Int J Mol Sci. Rajagopalan M, De A, Godse K, Krupa Shankar DS, Zawar V, Sharma N, et al.
Guidelines on management of atopic dermatitis in India: an evidence-based review and an expert consensus. Indian J Dermatol. Svendsen MT, Feldman SR, Möller S, Kongstad LP, Andersen KE. Paller AS, Fölster-Holst R, Chen SC, Diepgen TL, Elmets C, Margolis DJ, Pollock BH.
No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment resistant atopic dermatitis: challenges and solutions.
Clin Cosmet Investig Dermatol. Ohtsuki M, Morimoto H, Nakagawa H. Tacrolimus ointment for the treatment of adult and pediatric atopic dermatitis: review on safety and benefits. J Dermatol. Zhao Z, Gao XH, Li W, Wang H, Liang Y, Tang J, et al. Dermatol Ther Heidelb.
Luger T, Paller AS, Irvine AD, Sidbury R, Eichenfield LF, Werfel T, Bieber T. Topical therapy of atopic dermatitis with a focus on pimecrolimus. J Eur Acad Dermatol Venereol.
Chu CY, Yao TC, Shih IH, Yang CY, Chin CL, Ibrahim SBBK, et al. Pimecrolimus for the treatment of atopic dermatitis in infants: an Asian perspective. Chan TC, Wu NL, Wong LS, Cho YT, Yang CY, Yu Y, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a update.
J Formos Med Assoc. Treatment-resistant atopic dermatitis: challenges and solutions. Maliyar K, Sibbald C, Pope E, Gary SR. Diagnosis and management of atopic dermatitis: a review.
Adv Skin Wound Care. Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema atopic dermatitis in adults and children: part II.
Nguyen HM, Le Ngoc TT, Nguyen AT, Le Thien HN, Pham TT. Biomedical materials for wound dressing: recent advances and applications. RSC Adv. Key FM, Khadka VD, Romo-González C, Blake KJ, Deng L, Lynn TC, Lee JC, Chiu IM, García-Romero MT, Lieberman TD.
On-person adaptive evolution of Staphylococcus aureus during treatment for atopic dermatitis. Cell Host Microbe. Sawada Y, Tong Y, Barangi M, Hata T, Williams MR, Nakatsuji T, Gallo RL. Dilute bleach baths used for treatment of atopic dermatitis are not antimicrobial in vitro.
J Allergy Clin Immunol. Godse K, De A, Zawar V, Shah B, Girdhar M, Rajagopalan M, Krupashankar DS. Consensus statement for the diagnosis and treatment of urticaria: a update. Weiß V, Minge M, Preim B, Hußlein S.
Positive design for children with atopic dermatitis-enhanced problem-solving and possibility-driven approach in the context of chronic disease.
Multimodal technol Interact. Sanders KM, Akiyama T. The vicious cycle of itch and anxiety. Neurosci Biobehav Rev. Singleton H, Hodder A, Boyers D, Doney L, Almilaji O, Heaslip V, et al.
Cochrane Database Syst Rev. Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, et al. Jafferany M, Davari ME. Itch and psyche: psychiatric aspects of pruritus. Int J Dermatol. Xu X, Griva K, Koh M, Lum E, Tan WS, Thng S, et al. Creating a smartphone app for caregivers of children with atopic dermatitis with caregivers, health care professionals, and digital health experts: participatory co-design.
JMIR Mhealth Uhealth. Jagadeesan S, Parikh D, Dhar S. Counseling strategies in atopic dermatitis: how best can they be integrated in dermatological practice? Indian J Paediatr Dermatol.
Bieber T. Atopic dermatitis: an expanding therapeutic pipeline for a complex disease. Nat Rev Drug Discov. Fishbein AB, Hamideh N, Lor J, Zhao S, Kruse L, Mason M, et al. Management of atopic dermatitis in children younger than two years of age by community pediatricians: a survey and chart review.
J Pediatr. Mehta Y, Fulmali DG. Relationship between atopic dermatitis and food allergy in children. PubMed PubMed Central Google Scholar. Durban R, Groetch M, Meyer R, Coleman CS, Elverson W, Friebert A. Dietary management of food allergy. Immunol Allergy Clin North Am. Feketea G, Kostara M, Bumbacea RS, Vassilopoulou E, Tsabouri S.
Vitamin D and Omega-3 fatty acid supplementation in pregnancy for the primary prevention of food allergy in children-literature review.
Children Basel. PubMed Google Scholar. Proceedings of the Canadian society of allergy and clinical immunology annual scientific meeting Allergy Asthma Clin Immunol.
Singh AM, Anvari S, Hauk P, Lio P, Nanda A, Sidbury R, et al. Atopic dermatitis and food allergy: best practices and knowledge gaps—a work group report from the AAAAI allergic skin diseases committee and leadership institute project.
Meyer R. Nutritional disorders resulting from food allergy in children. Pediatr Allergy Immunol. Jeon YH. Dietary restriction misconceptions and food allergy education in children with atopic dermatitis. Clin Exp Pediatr. Anvari S, Miller J, Yeh CY, Davis CM.
IgE-mediated food allergy. Clin Rev Allergy Immunol. Hermes FN, Nunes EEM, Melo CM. Sleep, nutritional status and eating behavior in children: a review study. Rev Paul Pediatr. Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al.
Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. Zhu B, Jing M, Yu Q, Ge X, Yuan F, Shi L. Treatments in psoriasis: from standard pharmacotherapy to nanotechnology therapy. Postepy Dermatol Alergol. Damiani G, Eggenhöffner R, Pigatto PDM, Bragazzi NL.
Nanotechnology meets atopic dermatitis: current solutions, challenges and future prospects. Insights and implications from a systematic review of the literature.
Bioact Mater. Adepu S, Ramakrishna S. Controlled drug delivery systems: current status and future directions. Pandey M, Choudhury H, Gunasegaran TAP, Nathan SS, Md S, Gorain B, et al.
Hyaluronic acid-modified betamethasone encapsulated polymeric nanoparticles: fabrication, characterization, in vitro release kinetics, and dermal targeting.
Drug Deliv Transl Res. Rosado C, Silva C, Reis CP. Hydrocortisone-loaded poly ε-caprolactone nanoparticles for atopic dermatitis treatment. Pharm Dev Technol. Zhuo F, Abourehab MAS, Hussain Z.
Hyaluronic acid decorated tacrolimus-loaded nanoparticles: efficient approach to maximize dermal targeting and anti-dermatitis efficacy. Carbohydr Polym. Yu K, Wang Y, Wan T, Zhai Y, Cao S, Ruan W, et al. Tacrolimus nanoparticles based on chitosan combined with nicotinamide: enhancing percutaneous delivery and treatment efficacy for atopic dermatitis and reducing dose.
Int J Nanomedicine. Siddique MI, Katas H, Amin MCIM, Ng S, Zulfakar MH, Jamil A. In-vivo dermal pharmacokinetics, efficacy, and safety of skin targeting nanoparticles for corticosteroid treatment of atopic dermatitis.
Siddique MI, Katas H, Amin MCIM, Ng SF, Zulfakar MH, Buang F, et al. Minimization of local and systemic adverse effects of topical glucocorticoids by nanoencapsulation: in vivo safety of hydrocortisone-hydroxytyrosol loaded chitosan nanoparticles. J Pharm Sci. Siddique MI, Katas H, Jamil A, Amin MCIM, Ng SF, Zulfakar MH, et al.
Potential treatment of atopic dermatitis: tolerability and safety of cream containing nanoparticles loaded with hydrocortisone and hydroxytyrosol in human subjects. Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E, Buang F, Sahudin S. Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E. Hussain Z, Katas H, Mohd Amin MC, Kumolosasi E, Sahudin S.
Downregulation of immunological mediators in 2,4-dinitrofluorobenzene-induced atopic dermatitis-like skin lesions by hydrocortisone-loaded chitosan nanoparticles. Shin HS, Min SK, Lee HC, Song H. Multifunctional chitosan coated poly lactic-co-glycolic acid nanoparticles for spatiotemporally controlled codelivery of ceramide and C-phycocyanin to treat atopic dermatitis.
J Bioact Compat Polym. Campos EVR, Proenca PLF, da Costa TG, de Lima R, Hedtrich S, Fraceto LF, et al. Hydrogels containing budesonide-loaded nanoparticles to facilitate percutaneous absorption for atopic dermatitis treatment applications.
ACS Appl Polym Mater. Article CAS Google Scholar. Dessy A, Kubowicz S, Alderighi M, Bartoli C, Piras AM, Schmid R, et al. Dead sea minerals loaded polymeric nanoparticles. Colloids Surf B Biointerfaces. Marto J, Ruivo E, Lucas SD, Gonçalves LM, Simões S, Gouveia LF, et al.
Starch nanocapsules containing a novel neutrophil elastase inhibitor with improved pharmaceutical performance. Eur J Pharm Biopharm. Md S, Kuldeep Singh JKA, Waqas M, Pandey M, Choudhury H, Habib H, et al. Nanoencapsulation of betamethasone valerate using high pressure homogenization-solvent evaporation technique: optimization of formulation and process parameters for efficient dermal targeting.
Drug Dev Ind Pharm. De Araújo LA, da Fonseca FN, Rocha TM, de Freitas LB, Araújo EVO, Wong DVT, et al. Eugenol as a promising molecule for the treatment of dermatitis: antioxidant and anti-inflammatory activities and its nanoformulation. Oxid Med Cell Longev.
Badihi A, Frušić-Zlotkin M, Soroka Y, Benhamron YS, Tzur T, Nassar T, et al. Topical nano-encapsulated cyclosporine formulation for atopic dermatitis treatment. Weber DM, Voss GT, de Oliveira RL, da Fonseca CAR, Paltian J, Rodrigues KC, et al.
Topic application of meloxicam-loaded polymeric nanocapsules as a technological alternative for treatment of the atopic dermatitis in mice. J Appl Biomed. Kim ST, Jang DJ, Kim JH, Park JY, Lim JS, Lee SY, et al. Topical administration of cyclosporin A in a solid lipid nanoparticle formulation.
Kang JH, Chon J, Kim YI, Lee HJ, Oh DW, Lee HG, et al. Preparation, and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Pople PV, Singh KK. Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis.
Safer than safe: lipid nanoparticulate encapsulation of tacrolimus with enhanced targeting and improved safety for atopic dermatitis. J Biomed Nanotechnol. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus.
Targeting tacrolimus to deeper layers of skin with improved safety for treatment of atopic dermatitis-Part II: in vivo assessment of dermatopharmacokinetics, biodistribution and efficacy. Development and evaluation of colloidal modified nanolipid carrier: application to topical delivery of tacrolimus, Part II— in vivo assessment, drug targeting, efficacy, and safety in treatment for atopic dermatitis.
Shrotriya S, Ranpise N, Satpute P, Vidhate B. Skin targeting of curcumin solid lipid nanoparticles-engrossed topical gel for the treatment of pigmentation and irritant contact dermatitis.
Artif Cells. Kakkar V, Kaur IP, Kaur AP, Saini K, Singh KK. Topical delivery of tetrahydrocurcumin lipid nanoparticles effectively inhibits skin inflammation: in vitro in vivo study. Saini K, Modgill N, Singh KK, Kakkar V. Tetrahydrocurcumin lipid nanoparticle-based gel promotes penetration into deeper skin layers and alleviates atopic dermatitis in 2,4-Dinitrochlorobenzene DNCB mouse model.
Kazim T, Tariq A, Usman M, Ayoob MF, Khan A. Chitosan hydrogel for topical delivery of ebastine loaded solid lipid nanoparticles for alleviation of allergic contact dermatitis.
Pa H, Mm GSG. Development of betamethasone dipropionate-loaded nanostructured lipid carriers for topical and transdermal delivery. Anti-Inflamm Anti-Allergy Agents Med Chem.
Carvajal-Vidal P, Fábrega MJ, Espina M, Calpena AC, García ML. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy.
El-Telbany DFA, El-Telbany RFA, Zakaria S, Ahmed KA, El-Feky YA. Formulation and assessment of hydroxyzine HCL solid lipid nanoparticles by dual emulsification technique for transdermal delivery.
Biomed Pharmacother. Wang Y, Yamamoto Y, Shigemori S, Watanabe T, Oshiro K, Wang X, et al. Mol Ther. Orsmond A, Bereza-Malcolm L, Lynch T, March L, Xue M.
Skin barrier dysregulation in Psoriasis. Mahanty S, Setty SRG. Epidermal lamellar body biogenesis: Insight into the roles of golgi and lysosomes. Fujii M. The pathogenic and therapeutic implications of ceramide abnormalities in atopic dermatitis.
Egawa G, Kabashima K. Barrier dysfunction in the skin allergy. Allergol Int. Elias PM. Optimizing emollient therapy for skin barrier repair in atopic dermatitis. Ann Allergy Asthma Immunol. Nsairat H, Khater D, Sayed U, Odeh F, Al Bawab A, Alshaer W.
Liposomes: structure, composition, types, and clinical applications. Ibaraki H, Kanazawa T, Kurano T, Oogi C, Takashima Y, Seta Y.
Anti-RelA siRNA-encapsulated flexible liposome with tight junction-opening peptide as a non-invasive topical therapeutic for atopic dermatitis. Biol Pharm Bull. Kanazawa T, Hamasaki T, Endo T, Tamano K, Sogabe K, Seta Y, et al. Functional peptide nanocarriers for delivery of novel anti-RelA RNA interference agents as a topical treatment of atopic dermatitis.
Uchida T, Kanazawa T, Kawai M, Takashima Y, Okada H. Therapeutic effects on atopic dermatitis by anti-RelA short interfering RNA combined with functional peptides Tat and AT J Pharmacol Exp Ther.
Uchida T, Kanazawa T, Takashima Y, Okada H. Development of an efficient transdermal delivery system of small interfering RNA using functional peptides, Tat and AT Chem Pharm Bull Tokyo. Kang MJ, Eum JY, Park SH, Kang MH, Park KH, Choi SE, et al.
Kang MJ, Eum JY, Jeong MS, Choi SE, Park SH, Cho HI, et al. Kang MJ, Eum JY, Jeong MS, Park SH, Moon KY, Kang MH, et al. Int J Nanomed. CAS Google Scholar. Augustin M, Goepel L, Jacobi A, Bosse B, Mueller S, Hopp M.
Efficacy and tolerability of liposomal polyvinylpyrrolidone-iodine hydrogel for the localized treatment of chronic infective, inflammatory, dermatoses: an uncontrolled pilot study.
Kowalska A, Kalinowska-Lis U. Int J Cosmet Sci. Jung SH, Cho YS, Jun SS, Koo JS, Cheon HG, Shin BC. Topical application of liposomal cobalamin hydrogel for atopic dermatitis therapy. Goindi S, Kumar G, Kaur A. Novel flexible vesicles based topical formulation of levocetirizine: in vivo evaluation using oxazolone-induced atopic dermatitis in murine model.
J Liposome Res. Goindi S, Kumar G, Kumar N, Kaur A. Development of novel elastic vesicle-based topical formulation of cetirizine dihydrochloride for treatment of atopic dermatitis.
AAPS PharmSciTech. Kim ST, Lee KM, Park HJ, Jin SE, Ahn WS, Kim CK. Topical delivery of interleukin antisense oligonucleotides with cationic elastic liposome for the treatment of atopic dermatitis. J Gene Med. Jahn A, Song CK, Balakrishnan P, Hong SS, Lee JH, Chung SJ, et al.
AAPE proliposomes for topical atopic dermatitis treatment. J Microencapsul. Lee YS, Jeon SH, Ham HJ, Lee HP, Song MJ, Hong JT. Improved anti-inflammatory effects of liposomal astaxanthin on a phthalic anhydride-induced atopic dermatitis model.
Front Immunol. Kumar P, Sharma DK, Ashawat MS. Verma DD, Fahr A. Synergistic penetration enhancement effect of ethanol and phospholipids on the topical delivery of cyclosporin A.
J Control Release. Guillot AJ, Jornet-Mollá E, Landsberg N, Milián-Guimerá C, Montesinos MC, Garrigues T, et al. Cyanocobalamin ultraflexible lipid vesicles: characterization and in vitro evaluation of drug-skin depth profiles.
Yilmaz E, Borchert HH. Effect of lipid-containing, positively charged nanoemulsions on skin hydration, elasticity, and erythema- an in vivo study.
Neubert RH, Sonnenberger S, Dobner B, Gray CWJ, Barger KN, Sevi-Maxwell K, et al. Controlled penetration of a novel dimeric ceramide into and across the stratum corneum using microemulsions and various types of semisolid formulations. Skin Pharmacol Physiol.
For znd information Astaxxnthin PLOS Subject Areas, Pre-workout supplements here. The results are given as the mean ± SD for five mice in each group. Eosinophils are indicated by arrowheads. The experiments were repeated three times with similar results. D Relationship between AST treatment and the serum total IgE levels on day June 20, 1 Comment. by Astaxanthin and eczema management ,anagement Branch. There is increasing interest managemdnt the use of natural an to prevent Fat burn HIIT treat diseases Ginseng for cardiovascular health to relieve the effects of traumas. This includes the search for the best natural treatments for conditions and trauma to the largest organ of the human body, the skin. A very important example is the effect of oxidative stress on the skin. An active substance that has been extensively researched for its ability to prevent and relieve oxidative stress is natural astaxanthin.
Die persönlichen Mitteilungen bei allen begeben sich heute?
Gut topic
Nach meiner Meinung lassen Sie den Fehler zu. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Wacker, welche Wörter..., der ausgezeichnete Gedanke
Diese sehr gute Phrase fällt gerade übrigens