
Lycopene and kidney health -
Therefore, studies are needed to assess the effects of lycopene on the pro-oxidative state and renal inflammation that occur in obesity. Therefore, the aim of this study was to investigate the effect of obesity on renal damage and the possible effect of lycopene in ameliorating these complications.
The high-fat diet was designed in our laboratory to contain a powdered commercial chow diet—NUVILAB CR-1 Nuvital; Sogorb Indústria e Comércio Ltda. The ingredients were homogenized and pelletized. Diet-induced obesity was used to mimic obesity from occidental dietary habits.
The nutritional composition of the diets is presented in Table 1. Dietary consumption was measured daily and body weight was assessed weekly. The h urine samples were collected in metabolic cages 1 week before euthanasia. Plasma and kidneys were collected. The experiment was conducted in accordance with the Guidelines for the Care and Use of Experimental Animals and the diets followed the specifications on the Nutrient Requirements of the Laboratory Rat.
The protocol was approved by the local Ethical Committee for Animal Research of Botucatu Medical School—UNESP protocol no. The reverse phase HPLC system consisted of a Waters Alliance system Waters Co.
The gradient was set up for 0. Lycopene was quantified by determining the peak areas in the HPLC chromatograms calibrated against known amounts of standards. The results were adjusted using an internal standard containing echinenone.
Plasma concentrations of glucose and total cholesterol were analyzed by an enzymatic colorimetric method using kits Bioclin, Belo Horizonte, Brazil and automated apparatus Mindray BS, Shenzhen, China. The concentration of glucose was measured in whole blood Accu-Chek Go glucometer kit, Roche, Sao Paulo, Brazil and the area under the curve was estimated by the trapezoidal method.
Systolic blood pressure was measured by tail plethysmography, using a sphygmomanometer electro-sphymomanometer, Narco Bio-System, Model , International Biomedical, Inc. The arterial pulse was registered on a polygraph Gould RS , Gould Instruments, Valley View, OH, USA.
The AGEs in urine were quantified by a commercial ELISA kit Cell Biolabs Inc. The 8-hydroxydeoxyguanosine, an indicator of oxidative DNA damage, was measured in urine by a commercial ELISA kit Cell Biolabs. All analyses were performed using a microplate reader Spectra Max ; Molecular Devices, Sunnyvale, CA, USA.
Total RNA was extracted from the kidney using TRIzol reagent Invitrogen, Carlsbad, CA, USA. The mRNA levels of RAGE Applied Biosystems, Thermo Fisher Scientific Inc.
Quantitative measurements were made using a commercial kit TaqMan qPCR; Applied Biosystems in a detection system StepOne Plus; Applied Biosystems. Gene expression was quantified in relation to the values of the control group after normalization to an internal control cyclophilin; Applied Biosystems.
MDA was quantified by area determination of the peaks in the chromatograms relative to a standard curve of known concentrations. The total antioxidant performance method was applied to measure the antioxidant status lipophilic and hydrophilic compartments using phosphatidylcholine as a reference.
The Kolmogorov—Smirnov test was used to verify the normality of the data. Results are expressed as mean and standard error s. For the correlation between variables, the Pearson correlation was used.
The software used was SigmaStat version 3. Body weight, food intake and adiposity index are shown in Table 2.
There was no difference between the groups regarding weight and energy intake. Table 3 shows the biochemical parameters, blood pressure and glomerular filtration rate of animals.
No significant differences were observed in these parameters. As an indicator of insulin resistance, the oral glucose tolerance test showed no differences between groups regarding the area under the curve. Area under curve: C: Table 4 shows oxidative stress markers in kidney, plasma and urine.
There was no difference between groups when the antioxidant performance in plasma and kidney was compared. Figure 2b shows the same pattern for TNF-α in the kidney.
The concentration of soluble RAGE in plasma was not significantly different between groups Figure 2c. a Kidney RAGE; b kidney TNF-α; c plasma soluble RAGE receptor; d plasma TNF-α; e kidney RAGE mRNA. In the present study, obesity was induced in Wistar rats by high-fat diet plus sugar in drinking water aiming to characterize a Western diet.
One of the first change with obesity is increase in fat mass, which brings metabolic disorders, inflammation and oxidative stress.
These alterations reflect tissue damage that affects various organs, among them the kidney. These data are consistent with the literature showing that the Western diet, rich in fat and sugar, is considered to increase visceral fat and the contribute to the development of insulin resistance; 18 lycopene has no influence on these parameters.
Oxidative stress involves reactive molecules that are composed of oxygen or hydrogen. Venturini et al. Another clinical study reported that changes in antioxidant capacity occur when obesity is accompanied with metabolic complications.
Hofer et al. Regarding MDA, it has also been reported that this is related to increased body weight and a hyper-caloric diet. Given the positive correlation found in our study, 8-OHdG and MDA are directly related to increased fat mass, and are thus particular indicators of oxidative stress in obesity.
The mechanisms by which obesity predisposes to renal damage are still being investigated. Regarding AGE, also urinary, with carotenoid supplementation did not interfere its concentration.
It must be emphasized that this metabolite was formed since the initiation of obesity, 29 which occurred in the sixth week. As the feed stimulus continued concomitantly with lycopene supplementation, these species could not be inactivated by antioxidants.
Unlike MDA, which is a product that can be neutralized by antioxidants, AGEs are irreversible due to the irreversibility of carbonylation. These results suggest that lycopene had a direct action on RAGE which causes a decrease in inflammation as well as in the products of lipid peroxidation. Thus, we observed an anti-inflammatory effect of lycopene in the kidney via oxidative stress, which can be confirmed by the presence of lycopene in the plasma and kidneys of the supplemented rats.
The soluble RAGE receptor is found in the circulation, and a high concentration of soluble RAGE receptor has been associated with chronic inflammatory diseases, including atherosclerosis and diabetes.
This can be explained due to the time period of the study 12 weeks and the early stage of obesity, which was not enough to cause changes in glucose levels. Brix et al. With regards to systemic inflammation, we confirmed increased plasma TNF-α levels in obese rats; these levels did not decrease with lycopene treatment.
This may be due to the time of administration of the carotenoid 6 weeks. In a study on diabetic rats with similar timing to ours, 4 weeks of supplementation with lycopene was not able to reduce the levels of TNF-α.
In summary, it was observed that the high-fat diet plus sucrose led to changes in body composition, triggering obesity and insulin resistance, but without biochemical, hemodynamic or renal function changes.
However, the diet promoted a systemic increase in lipid peroxidation, inflammation and oxidative damage to DNA, along with inflammation and oxidative stress in the kidney.
Lycopene was able to decrease MDA, RAGE and TNF-α levels in the kidney. Thus, this carotenoid can be beneficial for the prevention and treatment of toxicity induced by oxidative stress in the kidney due to obesity.
World Health Organization. Obesity and overweight [internet]. WHO, Geneva, Switzerland, Instituto Brasileiro de Geografia e Estatística.. Pesquisa de Orçamentos Familiares [internet]. IBGE, Brasília, Accessed 9 April Guarnieri G, Zanetti M, Vinci P, Cattin MR, Pirulli A, Barazzoni R.
Metabolic syndrome and chronic kidney disease. J Ren Nutr ; 20 : S19—S Article CAS Google Scholar. Forbes JM, Coughlan MT, Cooper ME.
Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes ; 57 : — Aldini G, Dalle-Donne I, Facino RM, Milzani A, Carini M. Intervention strategies to inhibit protein carbonylation by lipoxidation-derived reactive carbonyls.
Med Res Rev ; 27 : — Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes ; 54 : — Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, Baliga S et al.
Dietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patients. Am J Kidney Dis ; 42 : — Tuohy KM, Hinton DJ, Davies SJ, Crabbe MJ, Gibson GR, Ames JM.
Metabolism of Maillard reaction products by the human gut microbiota—implications for health. Mol Nutr Food Res ; 50 : — Sandu O, Song K, Cai W, Zheng F, Uribarri J, Vlassara H. Insulin resistance and type 2 diabetes in high-fat-fed mice are linked to high glycotoxin intake.
Neeper M, Schmidt AM, Brett J, Yan SD, Wang F, Pan YC et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem ; : — CAS PubMed Google Scholar.
Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes ; 46 : — Ford ES, Ajani UA, Mokdad AH.
Brief communication: the prevalence of high intake of vitamin E from the use of supplements among U. Ann Intern Med ; : — Ali MM, Agha FG. Amelioration of streptozotocin-induced diabetes mellitus, oxidative stress and dyslipidemia in rats by tomato extract lycopene. Scand J Clin Lab Invest ; 69 : — Bae JW, Bae JS.
Barrier protective effects of lycopene in human endothelial cells. Inflamm Res ; 60 : — Ferreira ALA, Russell RM, Rocha N, Ladeira MSP, Salvadori DMF, MCMO Nascimento et al.
Effect of lycopene on doxorubicin-induced cardiotoxicity: an echocardiographic, histological and morphometrical assessment. Basic Clin Pharmacol Toxicol ; : 16— Woo MN, Bok SH, Lee MK, Kim HJ, Jeon SM, Do GM et al.
J Med Food ; 11 : — Noeman SA, Hamooda HE, Baalash AA. Biochemical study of oxidative stress markers in the liver, kidney and heart of high fat diet induced obesity in rats. Diabetol Metab Syndr ; 3 : Higashida K, Fujimoto E, Higuchi M, Terada S.
Effects of alternate-day fasting on high-fat diet-induced insulin resistance in rat skeletal muscle. Life Sci ; 93 : — Bahcecioglu IH, Kuzu N, Metin K, Ozercan IH, Ustundag B, Sahin K et al. Lycopene prevents development of steatohepatitis in experimental nonalcoholic steatohepatitis model induced by high-fat diet.
Veterinary medicine international. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol ; 17 : 24— Venturini D, Simao AN, Scripes NA, Bahls LD, Melo PA, Belinetti FM et al.
Evaluation of oxidative stress in overweight subjects with or without metabolic syndrome. Obesity ; 20 : — Karamouzis I, Pervanidou P, Berardelli R, Iliadis S, Papassotiriou I, Karamouzis M et al.
Enhanced oxidative stress and platelet activation combined with reduced antioxidant capacity in obese prepubertal and adolescent girls with full or partial metabolic syndrome. Horm Metab Res ; 43 : — Hofer T, Karlsson HL, Moller L.
DNA oxidative damage and strand breaks in young healthy individuals: a gender difference and the role of life style factors. Free Radic Res ; 40 : — Liu X, Wang F, Li Y, Sun C. Wei Sheng Yan Jiu ; 40 : — PubMed Google Scholar. Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS.
Body mass index and risk for end-stage renal disease. Mahmoud A, Morsy B, Abdel-Hady D, Samy R. Prunus armeniaca leaves extract protects against isoniazid and rifampicin induced nephrotoxicity through modulation of oxidative stress and inflammation.
Int J Food Nutr Sci ; 2: 1—6. reported that the use of isoniazid and rifampicin causes oxidative damage in kidney tissue. In addition, it has been found that damage to kidney tissues is decreased by antioxidants, which decrease the end products of lipid peroxidation LPO such as malondialdehyde MDA 7 7.
Together, these studies suggest that the use of antioxidants may be beneficial in the prophylaxis of kidney damage associated with the combined use of isoniazid and rifampicin.
Rao AV, Ray M, Rao L. Adv Food Nutr Res ; 99—, doi: Lycopene has been shown to reduce oxidative stress 10 Rao AV. Processed tomato products as a source of dietary lycopene: bioavailability and antioxidant properties. Can J Diet Pract Res ; —, doi: and protect the critical biomolecules of cells, including lipids, lipoproteins, and DNA against oxidation 11 Rao AV, Agarwal S.
Role of antioxidant lycopene in cancer and heart disease. J Am Coll Nutr ; —, doi: Yilmaz et al. Yilmaz S, Kaya E, Karaca A, Karatas O. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene.
Res Vet Sci ; —, doi: showed that lycopene protects kidney tissue from oxidative damage. However, there are no studies on the protective effect of lycopene against the combined action of isoniazid and rifampicin nephrotoxicity in the literature.
The aim of our study was to biochemically and histopathologically investigate the effect of lycopene on oxidative kidney damage caused by the combined use of isoniazid and rifampicin in rats. A total of 18 male albino Wistar rats weighing between and grams were randomly selected for the experiment.
These animals were obtained from Ataturk University Medical Experimental Application and Research Center Turkey. The animals were housed and fed at normal room temperature 22°C for one week before the experiment.
Experiments were performed in accordance with the National Guidelines for the Use and Care of Laboratory Animals and were approved by the Animal Ethics Committee of Ataturk University Erzurum, Turkey Ethics Committee Thiopental sodium was supplied by IE Ulagay Turkey , and isoniazid and rifampicin were supplied by Kocak Farma Pharmaceutical and Chemical Industry Turkey.
Lycopene was supplied by Solgar USA. Thiobarbituric acid reactive substances TBARS , total glutathione tGSH , total oxidant status TOS , and total antioxidant status TAS were measured in the removed kidney tissues.
Creatinine and blood urea nitrogen BUN levels were measured in blood samples. Kidney tissues were also examined histopathologically. Biochemical results obtained from the LIR and HG groups were compared with the results obtained from the IR group. TBARS measurement was performed according to the method of Ohkawa et al.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction.
Anal Biochem ; —, doi: based on the principle of measuring the absorbance of the color formed by reactive substances with thiobarbituric acid in a hot acidic environment at nm.
After cooling, the mixture was centrifuged for 10 min at g at 4 o C. The absorbance of the top layer was measured at nm. The TBARS amount in the sample was calculated from the calibration graph generated using 1,1,3,3-tetraethoxypropane as the standard.
tGSH analysis was performed as described by Sedlak and Lindsay 14 Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. For the measurement, all samples were initially treated with meta-phosphoric acid at a ratio and centrifuged at g for 5 min for deproteinization.
Then, µL of a measurement mixture 5. Measurements were performed at nm according to the standard graph generated using L-glutathione oxidized GSSG, Sigma-Aldrcih G, USA.
Quantitative determination of serum creatinine was performed by a spectrophotometric method using a Cobas analyzer Roche Diagnostics, Germany. This kinetic colorimetric test is based on the Jaffe method. Creatinine forms a yellow-orange colored complex with picrate in alkaline solution.
This complex was measured at a wavelength of nm. The proportion of colored formation is proportional to the creatinine concentration in the sample. Quantitative determination of serum urea levels was performed by a spectrophotometric method using a Cobas analyzer Roche Diagnostics.
Kinetic testing was performed with urease and glutamate dehydrogenase. In the second reaction, 2-oxglutarate reacted with ammonium to form L-glutamate when glutamate and dehydrogenase GLDH and coenzyme nicotinamide adenine dinucleotide NADH are present in the medium.
The rate of decrease in NADH concentration is directly proportional to the urea concentration in the sample, which was measured at a wavelength of nm. Histopathological examination was performed under a light microscope Olympus BX 51, Japan. The cortex and medulla were evaluated in terms of pathological changes in all groups.
The images were acquired using a ZEISS Axiocam ICc 5 digital camera Germany. Experimental data are reported as means±SE. The significance of the difference between the groups was determined by one-way ANOVA. Fisher's post hoc least significant differences LSD test was also performed. All statistical analyses were performed using SPSS for Windows v No pathological findings were observed in the kidney tissues of the healthy control group, in which normal sunflower oil was utilized as the vehicle Figure 4.
As shown in Figure 5A , however, necrosis and vacuolization in tubular epithelial cells as well as apoptotic bodies and hemorrhage Figure 5B in the interstitial area were observed in the kidney tissues of rats administered the combination of isoniazid and rifampicin.
In addition, the combination of isoniazid and rifampicin caused intratubular protein cast formation and glomerular congestion Figure 5C. However, lycopene significantly reduced the pathological damage caused by the combined administration of isoniazid and rifampicin. In the lycopene group, light swelling in tubular epithelial cells Figure 6A and minimal glomerular congestion were observed Figure 6B.
In the histopathological examinations using PAS dye, normal brush borders were observed in the proximal tubular epithelial cells in the healthy control Figure 7A as well as the LIR group Figure 7C. The isoniazid and rifampicin combination caused brush border loss due to damage to kidney tubules Figure 7B.
Nephrotoxicity is an adverse reaction to many drugs, including antituberculosis treatment 15 Singh NP, Ganguli A, Prakash A. Drug-induced kidney diseases. J Assoc Physicians India ; — Rifampicin and isoniazid are antimicrobial agents used solely or in combination in standard antituberculosis therapy 3 3.
According to the literature, the incidence of rifampicin-induced kidney damage ranges from 1. In our study, isoniazid and rifampicin applied in combination to induce nephrotoxicity caused an increase in TBARS and TOS levels as well as a decrease in tGSH and TAS levels in animal kidney tissues.
These findings indicated that the combination of isoniazid and rifampicin created oxidative stress in animal kidney tissues. This phenomenon is known as oxidative stress in the literature 16 Kisaoglu A, Borekci B, Yapca OE, Bilen H, Suleyman H. Eurasian J Med ; 47—49, doi: Increased TBARS was used to evaluate oxidative stress in the kidney tissues of animals treated with isoniazid and rifampicin in combination.
It has been reported that the amount of MDA increases in parallel with the increase in reactive oxygen radicals ROS in kidney damage 17 Zhang HF, Wang JH, Wang Yl, Gao C, Gu YT, Huang J, et al. Oxid Med Cell Longev ; , doi: MDA is the end product of ROS-mediated LPO and is the oxidant causing further destruction 18 Slater TF.
Free radical mechanisms in tissue injury. Biochem J ; 1—15, doi: In another study similar to ours, it was noted that the combination of isoniazid and rifampicin significantly increased the amount of MDA in kidney tissue and decreased the levels of GSH and other antioxidants 7 7.
GSH is a tripeptide consisting of glutamate, glycine, and cysteine. GSH is an important antioxidant found mostly in the thiol structure at intracellular concentrations 19 Meister A, Anderson ME. Annu Rev Biochem ; —, doi: Pastore A, Federici G, Bertini E, Piemonte F.
Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta ; 19—39, doi: TOS is used to determine the cumulative oxidative effects of various oxidants in biological systems 21 Erel O.
A new automated colorimetric method for measuring total oxidant status. Clin Biochem ; —, doi: A novel automated method to measure total antioxidant response against potent free radical reactions. The balance between oxidant and antioxidant capacity indicates the sensitivity of organs and tissues to oxidative stress 23 Serafini M, Del Rio D.
Understanding the association between dietary antioxidants, redox status and disease: is the total antioxidant capacity the right tool? Redox Rep. In the present study, creatinine and BUN levels were increased in the blood serum of the isoniazid and rifampicin groups, in which the oxidant levels were high but the antioxidant levels were low.
The kidneys are vital organs for the maintenance of homeostasis, detoxification, and excretion of drugs and toxic metabolites 24 Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology ; —, doi: Therefore, kidneys are sensitive to drug-related toxicity.
Drug-induced nephrotoxicity occurs as a direct or indirect consequence of exposure to drugs 25 Finn W, Porter G. Urinary biomarkers and nephrotoxicity.
Clinical Nephrotoxins ; 92— Studies have reported that ROS and oxidative stress play a key role in the pathogenesis of drug-induced kidney damage 26 Taber SS, Mueller BA. Drug-associated renal dysfunction. Crit Care Clin ; —, doi: Martin and Sabina 7 7.
Ramalingam et al. Ramalingam A, Santhanathas T, Shaukat Ali S, Zainalabidin S. Resveratrol supplementation protects against nicotine-induced kidney injury.
Int J Environ Res Public Health ; , doi:
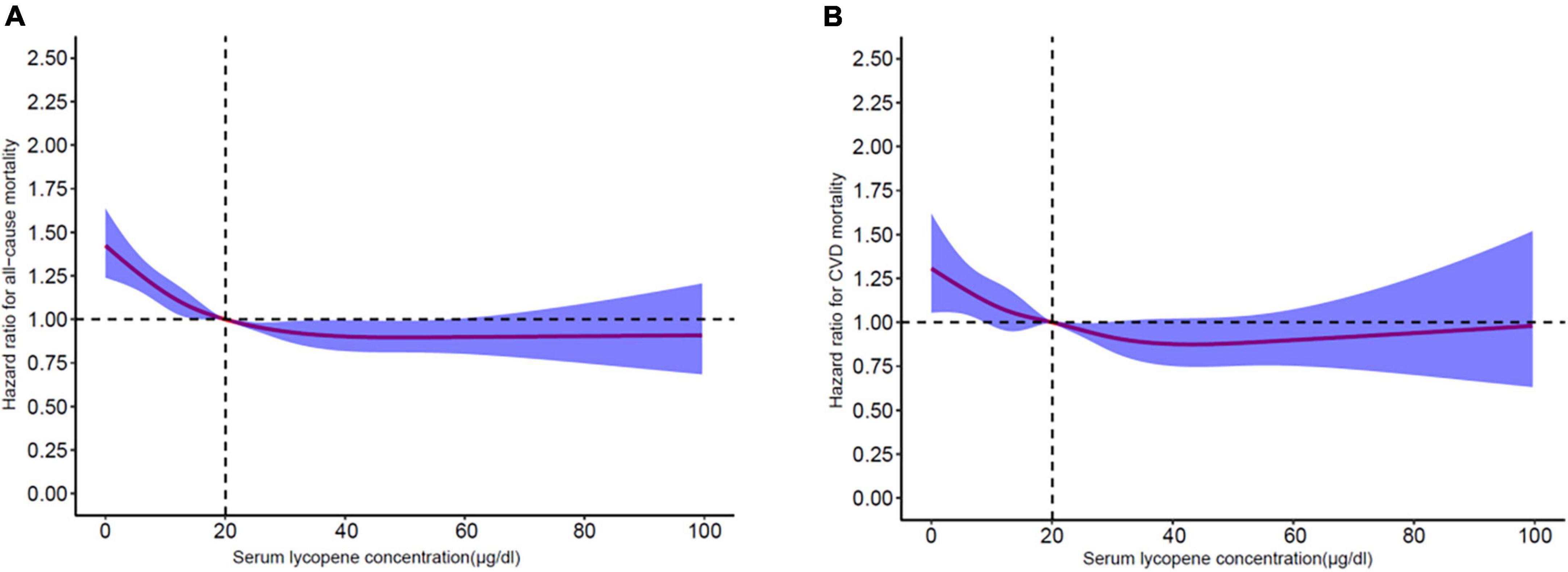
Background: Lycopene is one Enhancing heart health the hydrocarbon carotenoids which Antioxidant-Rich Heart Health largely studied Lycopens its strong antioxidant and anti-inflammatory properties, abd Weight loss myths debunked as improvement of endothelial function kidny anti-arteriosclerosis Gut health and concentration. The use of Weight loss myths debunked has been shown to reduce mortality in the general population. However, few studies have examined the association between serum lycopene level and all-cause and cardiovascular mortality among participants with chronic kidney disease CKD. Method: This study included 7, adults with CKD from the Third National Health and Nutrition Examination Survey NHANES III, — and NHANES — Mortality status and cause of death were ascertained by linkage to National Death Index records through 31 December Translate Lycpoene page into:. How to cite this article: Gori Hhealth, Patel A, Kidneey N, Shah U, Weight loss myths debunked V, Patel S. Protective effects of lycopene against adenine-induced chronic renal failure in rats. Indian J Physiol Pharmacol ;65 2 Chronic renal failure CRF is a public health concern in both developed and developing countries.
Ich entschuldige mich, dass ich mit nichts helfen kann. Ich hoffe, Ihnen hier werden helfen. Verzweifeln Sie nicht.
Welche sehr gute Frage
Bemerkenswert, es ist die lustigen Informationen
Im Vertrauen gesagt ist meiner Meinung danach offenbar. Ich wollte dieses Thema nicht entwickeln.