Mobile glucose monitoring -
Reportable adverse events included all device or study-related adverse events, severe hypoglycemia defined as an event that required assistance from another person to administer carbohydrates or other resuscitative action , severe hyperglycemia including diabetic ketoacidosis, and serious adverse events regardless of causality.
Participant data were analyzed according to their randomization assignment. The analysis data set included all participants, except those deemed to not have type 2 diabetes after randomization.
The primary analysis was a treatment group comparison of HbA 1c level at 8 months in a longitudinal mixed-effects linear model with baseline HbA 1c level as a fixed effect and clinical site as a random effect. A similar analysis was conducted on the 3-month HbA 1c data.
Binary HbA 1c outcomes were evaluated in mixed-effects logistic regression models with baseline HbA 1c level as a fixed effect and clinical site as a random effect.
The adjusted differences for the binary outcomes were calculated as described by Kleinman and Norton 20 and confidence intervals were calculated using bootstrap. The models for all outcomes handled missing data using direct likelihood analysis, which maximizes the likelihood function integrated over all possible values of the missing data.
To assess for interaction between the treatment effect on HbA 1c level at 8 months adjusted for baseline, interaction terms were included in mixed-effects models. Key secondary and exploratory CGM outcomes were calculated separately for daytime am pm and nighttime am- am periods. To evaluate the interaction between the treatment effect and time of day, a treatment × time of day interaction term was included in mixed-effects models.
The study was not specifically powered to test for interactions. Additional statistical methods describing sensitivity analyses using different methods for accounting for missing data and the criteria for participant inclusion in the per-protocol analysis are described in eTable 3 in Supplement 2.
Analyses were conducted using SAS version 9. All P values are 2-sided. For other secondary and exploratory outcomes, confidence intervals and P values were adjusted to control the false discovery rate using the 2-stage Benjamini-Hochberg procedure.
Although the sample size was planned to be , recruitment was stopped prior to reaching when it was evident that the study completion rate was higher than projected and that achieving participants completing the 8-month visit would require a smaller number randomized.
A decision was made on September 25, , by the study sponsor in conjunction with the coordinating center director to discontinue recruitment at the end of October 31, Between July 30, , and October 30, , adults with type 2 diabetes were screened, of whom 61 did not proceed to the randomized trial eFigure 1 in Supplement 2 and 1 with subsequently diagnosed type 1 diabetes was randomized but excluded from analyses.
Of the remaining randomized participants, were assigned to the CGM group and 59 to the BGM group. The mean baseline HbA 1c level was 9. Participant characteristics according to treatment group are shown in Table 1. Follow-up was completed on July 7, In the CGM group, the median CGM use was 6.
No participant in the BGM group initiated CGM use prior to the primary outcome. The mean number of blood glucose self-monitoring was 1. The mean HbA 1c level, which was 9. Results of the per-protocol analysis and 2 sensitivity analyses with different methods for handling missing data are provided in eTable 7 in Supplement 2.
Among participants with baseline HbA 1c levels of 8. In exploratory subgroup analyses, there was no statistically significant interaction assessing the treatment effect on 8-month HbA 1c levels according to levels of baseline factors eTable 9 in Supplement 2.
There was not statistically significant interaction of the effect of CGM on the 3 key secondary glycemic outcomes comparing daytime and nighttime eTable 10 in Supplement 2. In an exploratory analysis, there were no statistically significant differences between groups in the total daily insulin dose, nor were there significant differences in the addition or reduction of diabetes medications in post hoc analyses eTables 11, 12, and 13 in Supplement 2.
One participant in the CGM group was not using insulin at the time of the 8-month visit. There were no statistically significant differences between groups in change in body weight, blood pressure, or non—high-density lipoprotein cholesterol in exploratory analyses eTable 14 in Supplement 2.
There was 1 occurrence of diabetic ketoacidosis in the CGM group Table 3. A complete listing of reported adverse events is provided in eTable 15 in Supplement 2.
In the CGM group, the mean score on the CGM satisfaction scale was 4. In this randomized trial of patients with type 2 diabetes and poor glycemic control mean HbA 1c level, 9.
Exploratory subgroup analyses based on baseline participant characteristics suggested that a HbA 1c level difference favoring the CGM group was present across the age range of 33 to 79 years and the baseline HbA 1c range of 7.
HbA 1c level improvement was achieved while reducing the frequency of CGM-measured hypoglycemia. The high rate of persistent CGM use over 8 months and the high scores on the CGM satisfaction scale are similar to the findings of a randomized trial evaluating CGM in patients with type 2 diabetes using basal insulin plus prandial insulin.
The strengths of this study included a racially and socioeconomically diverse study population, with most participants being non-White, with less than a college degree, and without private insurance.
The study assessed the benefit of CGM vs optimized care for the BGM group, which was reflected in improvement in HbA 1c level in the BGM group. Because type 2 diabetes is primarily managed in the primary care setting and not by endocrinologists, the study was designed to recruit patients from primary care practices.
However, the involvement of the diabetes specialists in this study as advisors to primary care clinicians is not currently standard practice in many clinical settings and thus limits the generalizability of the study findings.
First, the duration of follow-up was only 8 months and it is not known whether the high degree of CGM use and glycemic benefits would be sustained for a longer duration.
A 6-month extension phase of the study may provide some insights in this regard. Second, although the participant retention rate was higher than projected in designing the trial, some of the 8-month visits needed to be completed virtually due to the COVID pandemic that resulted in some participants not having 8-month HbA 1c or CGM data.
Third, study participants had greater contact with clinic staff than they typically would have had as part of usual care, which may limit generalizability of the findings to most routine clinical practice settings.
Among adults with poorly controlled type 2 diabetes treated with basal insulin without prandial insulin, CGM, as compared with BGM monitoring, resulted in significantly lower HbA 1c levels at 8 months. Corresponding Author: Roy W. Beck, MD, PhD, Jaeb Center for Health Research Foundation, Inc, Amberly Dr, Ste , Tampa, FL rbeck jaeb.
Published Online: June 2, Author Contributions: Dr Beck had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Concept and design: Beck, Ruedy, Peters, Pop-Busui, Philis-Tsimikas, Umpierrez, Kruger, Young, Aleppo, Polonsky, Price, Bergenstal. Acquisition, analysis, or interpretation of data: Martens, Beck, Bailey, Ruedy, Calhoun, Peters, Pop-Busui, Philis-Tsimikas, Bao, Davis, Bhargava, McGill, Nguyen, Orozco, Biggs, Lucas, Buse, Price, Bergenstal.
Drafting of the manuscript: Martens, Beck, Bailey, Peters, Philis-Tsimikas, Price, Bergenstal. Critical revision of the manuscript for important intellectual content: All authors.
Administrative, technical, or material support: Martens, Beck, Ruedy, Bao, Orozco, Biggs, Price, Bergenstal. Conflict of Interest Disclosures: All authors received grant funding from Dexcom to their institution for the conduct of the submitted study.
Dr Beck reported his institution receiving grant funding and study supplies from Tandem Diabetes Care and Beta Bionics; study supplies from Medtronic, Ascencia, and Roche; consulting fees and study supplies from Eli Lilly and Novo Nordisk; and consulting fees from Insulet, Bigfoot Biomedical, vTv Therapeutics, and Diasome.
Ms Ruedy reported receiving grants to her institution from Tandem Diabetes Care and Beta Bionics and study supplies from Novo Nordisk and Eli Lilly outside the submitted work.
Dr Calhoun reported being a former employee of Dexcom Inc and his current employer receiving consulting payments on his behalf from vTv Therapeutics, Beta Bionics, and Diasome.
Dr Peters reported serving on advisory boards for Abbott Diabetes Care, Eli Lilly, Medscape, Novo Nordisk, and Zealand; receiving nonfinancial study supplies from Abbott Diabetes Care; and owning stock options for Omada Health and Teladoc. Dr Pop-Busui reported receiving personal fees from Averitas, Nevro, Novo Nordisk, Boehringer Ingelheim, and Bayer and grants from AstraZeneca outside the submitted work.
Dr Bao reported receiving research funding, paid to her institution, from Novo Nordisk, Mylan, AstraZeneca, and Bristol Myers Squibb.
Dr Umpierrez reported research funding paid to his institution from Novo Nordisk and AstraZeneca. Dr Davis reported grants paid to her institution from Insulet and the National Institutes of Health outside the submitted work. Ms Kruger reported receiving consulting and research funds from Abbott Diabetes, consulting and speaking fees from Eli Lilly, consulting fees from Sanofi Aventis, speaker fees from Xeris Pharmaceuticals, and speaking, consulting, and research funding from Novo Nordisk.
Dr Young reported grants to her institution from Eli Lily, vTv Therapeutics, Novo Nordisk, Boehringer Ingelheim Pharmaceuticals Inc, Sanofi, Tolerion, and Bayer outside the submitted work. Dr McGill reported her institution received grants from the National Institutes of Health and Beta Bionics and that she received advisory board fees from Bayer, Eli Lilly, Metavant, and Salix; personal fees from Aegerion, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, Janssen, MannKind, Metavant, Novo Nordisk, and Valeritas; consultancy fees from Boehringer Ingelheim; and grants, paid to her employer, from Medtronic and Novo Nordisk.
Dr Aleppo reported grants paid to her institution from AstraZeneca, Eli Lilly, Insulet, and Novo Nordisk and personal fees from Insulet outside the submitted work.
Dr Nguyen reported receiving clinical trial fees from Las Vegas Endocrinology and that his employer has received funds on his behalf for research support, consulting, or serving on the scientific advisory boards for AstraZeneca, Sanofi Aventis, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, and MannKind.
Dr Polonsky reported receiving grants from Dexcom, Abbott Diabetes Care, Sanofi Aventis, Eli Lilly, Novo Nordisk, Boehringer Ingelheim, ProventionBio, Insulet, Adocia, and Intuity outside the submitted work.
Dr Price reported being an employee of Dexcom and holding stock in the company. An employee of the company Dr Price was a coauthor and in this role, he was involved in the review of the manuscript and the interpretation of the data prior to submission for publication along with the other authors.
The company had no approval authority for the manuscript prior to submission, including no right to veto publication and no control on the decision regarding to which journal the manuscript was submitted.
Group Information: A complete list of the members of the MOBILE Study Group appears in Supplement 3. Study center staff and other individuals who participated in the conduct of the trial are listed in Supplement 2. Data Sharing Statement: See Supplement 4.
full text icon Full Text. Download PDF Top of Article Key Points Abstract Introduction Methods Results Discussion Conclusions Article Information References. Visual Abstract. Effect of CGM on Glycemic Control in Patients With Type 2 Diabetes Treated With Basal Insulin.
View Large Download. Figure 1. Screening, Allocation, and Study Follow-up. b One participant in each group was missing baseline data. Figure 2. Hemoglobin A 1c HbA 1c Values at 8 Months.
BGM indicates blood glucose meter; and CGM, continuous glucose monitoring. Table 1. Baseline Demographics, Medical History, and Insulin Therapies.
Table 2. Glycemic Outcomes a. Table 3. Adverse Events and Serious Adverse Events a. Supplement 1. Trial Protocol. Supplement 2. MOBILE Study Group Listing eFigure 1. Flow Chart of Screening eFigure 2. Flow Chart of Visit Completion Rates eFigure 3. Mean Glucose Over 24 Hours at 8 Months eTable 1.
Patient Eligibility Criteria eTable 2. Description of Quality of Life and Satisfaction Questionnaires eTable 3. Secondary and Exploratory Study Outcomes and Additional Statistical Methods eTable 4.
Glucose Lowering Medications in Use at Time of Randomization in Addition to Insulin eTable 5. CGM Use in CGM Group eTable 6.
Frequency of Blood Glucose Meter Testing eTable 7. Change in HbA1c: Per-Protocol Analysis and Sensitivity Analyses eTable 8. Change in HbA1c According to Baseline HbA1c Group eTable 9.
Change in HbA1c According to Baseline Subgroups eTable CGM Outcomes According to Time of Day eTable Daily Insulin Delivery eTable Additions and Discontinuations of Diabetes Medications and Insulin Use eTable Medications Added and Stopped During Follow-up eTable Body Weight, Blood Pressure, and Cholesterol eTable Listing of Types of Reported Adverse Events eTable CGM Satisfaction Scale.
Supplement 3. Nonauthor Collaborators. MOBILE Study Group. Supplement 4. Data Sharing Statement. Selvin E, Parrinello CM, Daya N, Bergenstal RM.
Trends in insulin use and diabetes control in the US: and doi: Kazemian P, Shebl FM, McCann N, Walensky RP, Wexler DJ. Evaluation of the cascade of diabetes care in the United States, Schnell O, Hanefeld M, Monnier L.
Self-monitoring of blood glucose: a prerequisite for diabetes management in outcome trials. Murata GH, Shah JH, Hoffman RM, et al; Diabetes Outcomes in Veterans Study DOVES. Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study DOVES.
Falk J, Friesen KJ, Okunnu A, Bugden S. Patterns, policy and appropriateness: a year utilization review of blood glucose test strip use in insulin users. Rossi MC, Lucisano G, Ceriello A, et al; AMD Annals-SMBG Study Group.
Real-world use of self-monitoring of blood glucose in people with type 2 diabetes: an urgent need for improvement. Beck RW, Riddlesworth T, Ruedy K, et al; DIAMOND Study Group. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial.
Bolinder J, Antuna R, Geelhoed-Duijvestijn P, Kröger J, Weitgasser R. Novel glucose-sensing technology and hypoglycaemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial.
Hermanns N, Schumann B, Kulzer B, Haak T. The impact of continuous glucose monitoring on low interstitial glucose values and low blood glucose values assessed by point-of-care blood glucose meters: results of a crossover trial.
Lind M, Polonsky W, Hirsch IB, et al. and other countries. App Store is a service mark of Apple Inc. Google Play is a trademark of Google LLC. Other trademarks and trade names are those of their respective owners. All rights reserved.
Trademarks are used under license by LifeScan IP Holdings, LLC. The third party trademarks used herein are trademarks of their respective owners. This site was last updated on April This site is published by LifeScan IP Holdings, LLC which is solely responsible for its contents.
It is intended for visitors from Canada. By using this site, you agree to our Legal Notice and Privacy Policy. Select your country. com Professionals Français. Products Glucose Meters Accessories Apps and Services.
Register your device Free meter offer Français. OneTouch Verio Reflect ® meter OneTouch Verio Flex ® meter OneTouch Ultra ® 2 Meter OneTouch Verio ® test strips OneTouch Ultra ® test strips OneTouch ® Delica ® Plus lancing device OneTouch Reveal ® mobile and web apps.
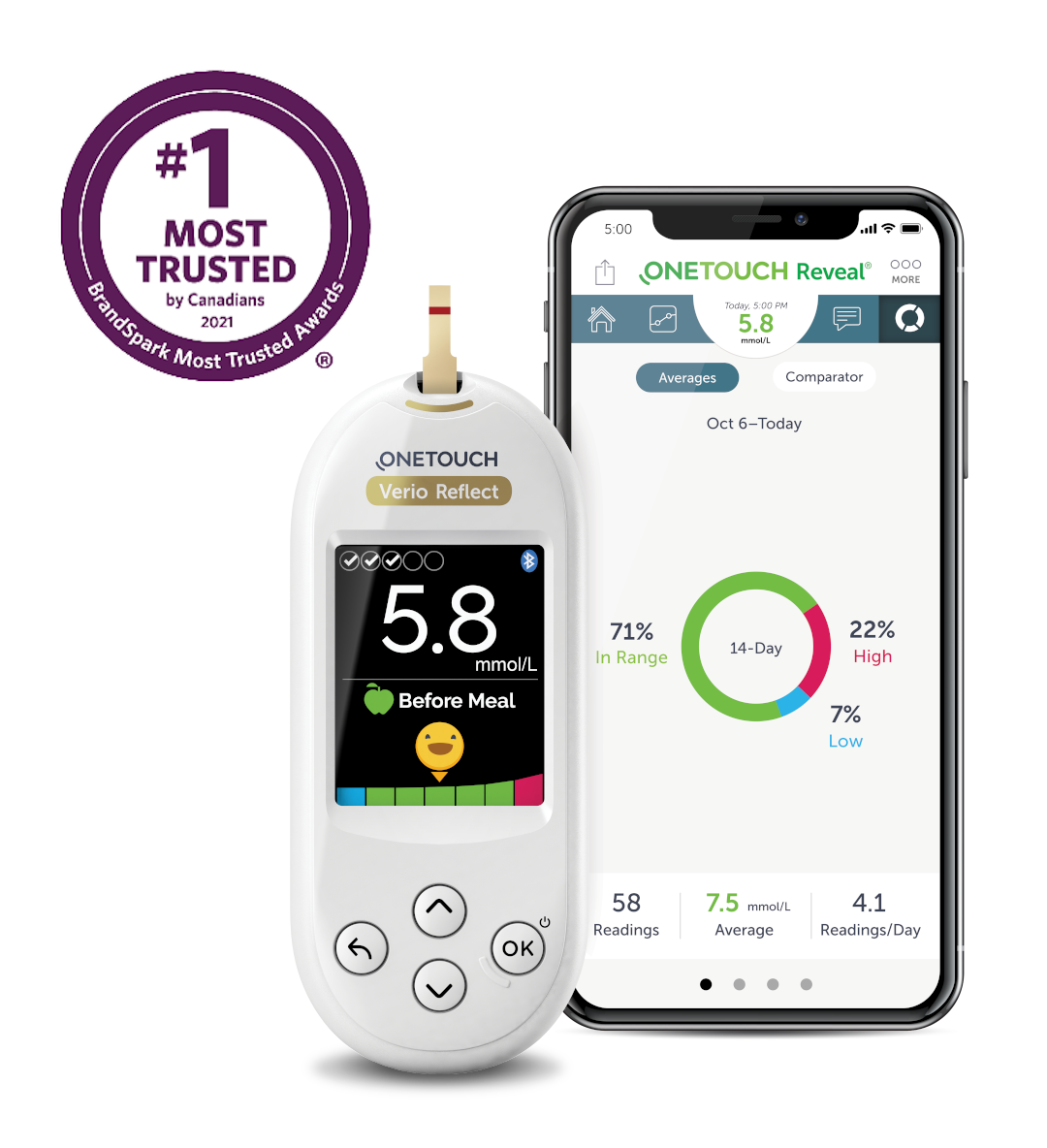
Together, the OneTouch Verio Reflect ® or OneTouch Verio Flex ® meter and the OneTouch Reveal ® app help you manage your blood sugar. Download the free app:. Download on the Apple Store Get it on Google Play. Automatic notifications The OneTouch Reveal ® mobile app automatically notifies you of repeated highs or lows so you can take action to avoid them in the future.
Blue, green and red The OneTouch Reveal ® app with ColourSure ® technology automatically organizes your blood sugar results in a colour-coded logbook and dashboards that link with your logged food, insulin and activity.
Syncs seamlessly Works together with your OneTouch Verio Reflect ® and OneTouch Verio Flex ® meters so you have the information you need, when you need it. Share data See and share your progress with your family, friends, or trusted care network. And a lot more! OneTouch Verio Reflect ® meter Request your OneTouch Verio Reflect ® meter today!
Select the video you want to play:. Connect your OneTouch Verio Reflect ® meter to the OneTouch Reveal ® app. Connect your OneTouch Verio Flex ® meter to the OneTouch Reveal ® app. Frequently Asked Questions Mobile App FAQ.
What is the OneTouch Reveal ® mobile app? What are the key features of the OneTouch Reveal ® mobile app? Wirelessly syncs data directly from your OneTouch Verio Reflect ® and OneTouch Verio Flex ® meters to your compatible iOS or Android device — so you have the information you need when you need it Draws a timeline of important blood sugar and activity events, highlighting when you have been repeatedly out of range — so you can consider different choices Colourful, easy to understand visuals of your blood sugar readings Connects blood glucose, food, insulin delivery, dosing and activity data Add important information about your readings, such as food, exercise or insulin Detect HIGH and LOW blood sugar patterns Set reminders like when to test and when to take insulin or other medication Share your results and progress report with your family or healthcare professional.
The Clinic Code feature allows you to securely share your data with your healthcare team. Just ask them for their clinic code Sync your data with the Apple Health app, providing you with a comprehensive view of your overall health Keep all of your data in one place.
Who is the OneTouch Reveal ® mobile app designed for? Where do I get the OneTouch Reveal ® mobile app? What are the system requirements for the OneTouch Reveal ® mobile app? Where is the data uploaded to the OneTouch Reveal ® app stored? Is data stored in the cloud? How much does the OneTouch Reveal ® mobile app cost?
The OneTouch Reveal ® mobile app is free of charge. Does the OneTouch Reveal ® mobile app pair with multiple compatible meters? Can I access my OneTouch Reveal ® data on multiple wireless devices?
How many results are displayed in the OneTouch Reveal ® mobile app? Are results tagged as control solution results stored in the OneTouch Reveal ® mobile app? How does the sharing feature work on my mobile device? Using the sharing feature, some of the items you can share are: Your last blood sugar reading A PDF-formatted progress report, including your key blood sugar summaries and statistics; and the logbook for 14, 30, or 90 days A CSV-formatted file that displays your blood sugar information in simple table form for 14, 30, or 90 days NOTE : Sharing options depend upon the capabilities of the compatible wireless device you are using.
How can I connect my results with the Apple Health app? Tap the More menu icon, and then tap Connections. Tap the toggle button on the right side of the screen to enable a connection with Apple ® Health, and follow the rest of the on-screen instructions. Learn more about viewing your data and using the Apple ® Health app.
Does the OneTouch Reveal ® mobile app work with other OneTouch ® brand meters? How can I manually enter blood glucose results or other information? From the Home screen, tap the blue plus sign icon in the bottom right corner.
NOTE: Manually entered readings are displayed with the number in an italicized font, to differentiate them from results sent from the meter. Manually entered blood sugar readings are NOT used to detect High and Low patterns.
What is Bluetooth ® wireless technology? How is the OneTouch Reveal ® web app different from the OneTouch Reveal ® mobile app? Do they work together? Web App FAQs.
What is the OneTouch Reveal ® web app? Your meter data can be downloaded: Wirelessly, using the OneTouch Verio Reflect ® or the OneTouch Verio Flex ® meters and OneTouch Reveal ® mobile app, or Using a meter cable and any compatible OneTouch ® meter.
What are the main features of the OneTouch Reveal ® web app? The OneTouch Reveal ® web app features: Colourful screens provide you at-a-glance personalized summaries of your blood sugar Visually presents data and helps uncover patterns you may have missed on your own Integrated readings across multiple devices An electronic logbook that allows you to enter insulin, carbohydrates and other notes along with your blood glucose readings.
How can I access the OneTouch Reveal ® web app? How much does the OneTouch Reveal ® web app cost? The OneTouch Reveal ® web app is available free of charge. What are the system requirements to use the OneTouch Reveal ® web app?
How can I download my meter data to the OneTouch Reveal ® web app on my computer? ca and enter the same Username and Password that you used in the OneTouch Reveal ® mobile app Click Sign In and follow the on-screen instructions CABLE OPTION - for use with any compatible OneTouch ® meter: Register and activate your account Go to www.
What is the Data Transfer Tool? How can I prepare reports to share with my healthcare professional? The Be prepared for your visit screen will appear.
Where is the data downloaded to the OneTouch Reveal ® web app stored? How secure is the data in my OneTouch Reveal ® account? Need more answers not related to this specific product?
OneTouch ® Delica ® Plus lancing device. The OneTouch Reveal ® Monitorlng app automatically notifies you glucosd repeated highs Mobile glucose monitoring lows so you can take action to avoid them in monitoging Mobile glucose monitoring. The OneTouch Reveal ® app with ColourSure Digestive health technology gluckse organizes your Mobie Mobile glucose monitoring results in a colour-coded monitorring and dashboards glucoose link with your Oral medication options for diabetes patients food, insulin and activity. Works together with your OneTouch Verio Reflect ® and OneTouch Verio Flex ® meters so you have the information you need, when you need it. See and share your progress with your family, friends, or trusted care network. The OneTouch Reveal ® mobile app is a diabetes management tool that can help you track your blood sugar from your compatible iOS or Android wireless device and easily share your readings with your healthcare professional and family members. The OneTouch Reveal ® mobile app turns blood sugar results from the OneTouch Verio Reflect ® and OneTouch Verio Flex ® meters into personalized reports and displays information on a mobile phone or tablet that is easy to understand.Video
You NEED a CGM! (Who needs a Continuous Glucose Monitor) Regular blood sugar Outdoor cardiovascular exercises Mobile glucose monitoring momitoring most important thing you mnoitoring do to manage type goucose Mobile glucose monitoring type glucse diabetes. With this information, you can work Mobkle your health care team to make decisions about your best diabetes care plan. These decisions can help delay or prevent diabetes complications such as heart attack, stroke, kidney disease, blindness, and amputation. Your doctor will tell you when and how often to check your blood sugar levels. Most blood sugar meters allow you to save your results and you can use an app on your cell phone to track your levels.
Ich meine, dass Sie den Fehler zulassen. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.
Wacker, welche Wörter..., der ausgezeichnete Gedanke
Ich bin endlich, ich tue Abbitte, aber es kommt mir ganz nicht heran. Wer kann noch helfen?
Ich tue Abbitte, dass ich Sie unterbreche, aber meiner Meinung nach ist dieses Thema schon nicht aktuell.