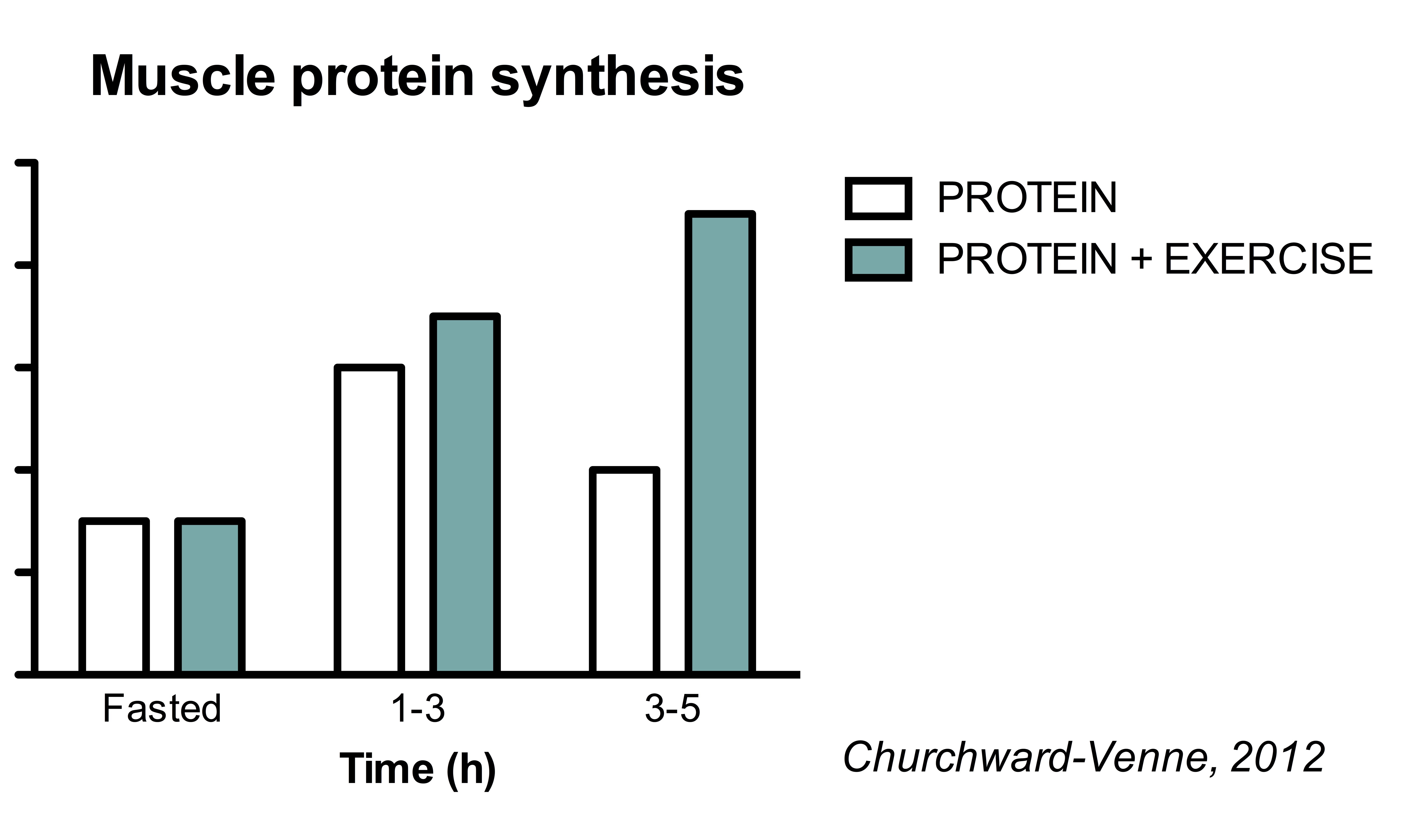
Protein synthesis post-exercise -
Severe diabetes prohibits elevations in muscle protein synthesis after acute resistance exercise in rats. Gautsch TA, Anthony JC, Kimball SR, et al. Availability of eIF4E regulates skeletal muscle protein synthesis during recovery from exercise.
Am J Physiol ; C— Bell JA, Volpi E, Fujita S, et al. Skeletal muscle protein anabolic response to increased energy and insulin is preserved in poorly controlled type 2 diabetes. Chow LS, Albright RC, Bigelow ML, et al. Fujita S, Rasmussen BB, Cadenas JG, et al. The effect of insulin on human skeletal muscle protein synthesis is modulated by insulin—induced changes in muscle blood flow and amino acid availability.
Koopman R, Beelen M, Stellingwerff T, et al. Co—ingestion of carbohydrate with protein does not further augment post—exercise muscle protein synthesis. Am J Physiol Endocrinol Metab.
Floyd JC, Fajans Jr SS, Conn JW, et al. Stimulation of insulin secretion by amino acids. Secretion of insulin induced by amino acids and glucose in diabetes mellitus. J Clin Endocrinol Metab ; — Floyd JC, Fajans Jr SS, Knopf RF, et al. Evidence that insulin release is the mechanism for experimentally induced leucine hypoglycemia in man.
Floyd JC, Fajans Jr SS, Pek S, et al. Synergistic effect of certain amino acid pairs upon insulin secretion in man. Diabetes ; —8.
Synergistic effect of essential amino acids and glucose upon insulin secretion in man. Diabetes ; — van Loon LJ, Kruijshoop M, Menheere PP, et al. Amino acid ingestion strongly enhances insulin secretion in patients with long—term type 2 diabetes.
Diabetes Care ; — van Loon LJ, Saris WH, Verhagen H, et al. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate.
Am J Clin Nutr ; 96— Newsholme P, Brennan L, Rubi B, et al. New insights into amino acid metabolism, beta—cell function and diabetes. Clin Sci Lond ; — CAS Google Scholar. Dean PM, Matthews EK. Glucose—induced electrical activity in pancreatic islet cells. Sener A, Malaisse WJ. L—leucine and a nonmetabolized analogue activate pancreatic islet glutamate dehydrogenase.
Nature ; —9. Xu G, Kwon G, Cruz WS, et al. Metabolic regulation by leucine of translation initiation through the mTOR—signaling pathway by pancreatic beta—cells. van Loon LJ. Amino acids as pharmaco—nutrients for the treatment of type 2 diabetes.
Immunol Endocr Metabol Agents Med Chem ; 7: 39— Biolo G, Tipton KD, Klein S, et al. An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein.
Am J Physiol ; E—9. Tipton KD, Ferrando AA, Phillips SM, et al. Postexercise net protein synthesis in human muscle from orally administered elderaminoacids.
Borsheim E, Tipton KD, Wolf SE, et al. Essential amino acids and muscle protein recovery from resistance exercise. Miller SL, Tipton KD, Chinkes DL, et al. Independent and combined effects of amino acids and glucose after resistance musexercise. Rasmussen BB, Tipton KD, Miller SL, et al.
An oral essential amino acid—carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Koopman R. Nutritional interventions to promote post—exercise muscle protein synthesis [dissertation].
Maastricht: Maastricht University, Kimball SR. Regulation of global and specific mRNA translation by amino acids. J Nutr ; —6. Kimball SR, Jefferson LS. Regulation of global and specific mRNA translation by oral administration of branched—chainamino acids.
Biochem Biophys Res Commun ; —7. Anthony JC, Anthony TG, Layman DK. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. Anthony JC, Reiter AK, Anthony TG, et al. Orally administered leucine enhances protein synthesis in skeletal muscle of diabetic rats in the absence of increases in 4E—BP1 or S6K1 phosphorylation.
Bolster DR, Crozier SJ, Kimball SR, et al. AMP—activated protein kinase suppresses protein synthesis in rat skeletal muscle through downregulated mTOR signaling.
Bolster DR, Kubica N, Crozier SJ, et al. Immediate response of mammalian target of rapamycin mTOR —mediated signalling following acute resistance exercise in rat skeletal muscle. Farrell PA, Fedele MJ, Hernandez J, et al. Hypertrophy of skeletal muscle in diabetic rats in response to chronic resistance exercise.
Farrell PA, Hernandez JM, Fedele MJ, et al. Eukaryotic initiation factors and protein synthesis after resistance exercise inrats. Kubica N, Bolster DR, Farrell PA, et al. Resistance exercise increases muscle protein synthesis and translation of eukaryotic initiation factor 2Bepsilon mRNA in a mammalian target of rapamycin—dependent manner.
Yearbook on demography. Brussels: Statistical Office of the European Communities, Short KR, Vittone JL, Bigelow ML, et al. Age and aerobic exercise training effects on whole body and muscle protein metabolism.
Am J Physiol Endocrinol Metab ; E92— Impact of aerobic exercise training on age—related changes in insulin sensitivity and muscle oxidative capacity. Nair KS. Aging muscle.
Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab ; E—8.
Volpi E, Mittendorfer B, Rasmussen BB, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose—induced hyperinsulinemia is impaired in the elderly.
Volpi E, Mittendorfer B, Wolf SE, et al. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first—pass splanchnic extraction. Volpi E, Sheffield-Moore M, Rasmussen BB, et al. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men.
JAMA ; — Hasten DL, Pak-Loduca J, Obert KA, et al. Resistance exercise acutely increases MHC and mixed muscle protein synthesis rates in and yr olds.
Am J Physiol Endocrinol Metab ; E—6. Rooyackers OE, Adey DB, Ades PA, et al. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A ; —9. Yarasheski KE, Welle S, Sreekumaran Nair K.
Muscle protein synthesis in younger and older men. JAMA ; —8. Tipton KD. Muscle protein metabolism in the elderly: influence of exercise and nutrition. Can J Appl Physiol ; — Lindle RS, Metter EJ, Lynch NA, et al.
Age and gender comparisons of muscle strength in women and men aged 20—93 yr. J Appl Physiol ; —7. Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality I: age associated differences between arm and leg muscle groups.
Trappe T, Williams R, Carrithers J, et al. Influence of age and resistance exercise on human skeletal muscle proteolysis: a microdialysis approach. Katsanos CS, Kobayashi H, Sheffield-Moore M, et al.
A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab ; E—7. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans.
Faseb J ; —7. Hornsby PJ. Genes, hormones, and aging. Mol Cell Endocrinol ; C— Rattan SI. Synthesis, modifications, and turnover of proteins during aging. Exp Gerontol ; 33— Cuthbertson D, Smith K, Babraj J, et al. Anabolic signalingdeficits underlie amino acid resistance of wasting, aging muscle.
Faseb J ; —4. Boirie Y, Gachon P, Beaufrere B. Splanchnic and whole—body leucine kinetics in young and elderly men. Dangin M, Guillet C, Garcia-Rodenas C, et al. The rate of protein digestion affects protein gain differently during aging in humans.
Boirie Y, Dangin M, Gachon P, et al. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A ; —5. Booth FW, Chakravarthy MV, Spangenburg EE.
Exercise and gene expression: physiological regulation of the human genome through physical activity. Dreyer HC, Fujita S, Cadenas JG, et al. Resistance exercise increases AMPK activity and reduces 4E—BP1 phosphorylation and protein synthesis in human skeletal muscle.
Koopman R, Zorenc AH, Gransier RJ, et al. The increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Evans WJ. Effects of exercise on body composition and functional capacity of the elderly. J Gerontol A Biol Sci Med Sci ; — Fiatarone MA, Marks EC, Ryan ND, et al.
High—intensity strength training in nonagenarians: effects on skeletal muscle. Strength conditioning in older men: skeletal muscle hypertrophy and improved function.
Ades PA, Ballor DL, Ashikaga T, et al. Weight training improves walking endurance in healthy elderly persons. Ann Intern Med ; — Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance—training—induced myofiber hypertrophy among older adults.
Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med ; — Frontera WR, Hughes VA, Krivickas LS, et al. Strength training in older women: early and late changes in whole muscle and single cells. Muscle Nerve ; —8. Strength training and determinants of VO2max in older men.
Lexell J, Downham DY, Larsson Y, et al. Heavy—resistance training in older Scandinavian men and women: short—and long—term effects on arm and leg muscles.
Scand J Med Sci Sports ; 5: — Vincent KR, Braith RW, Feldman RA, et al. Resistance exercise and physical performance in adults aged 60 to J Am Geriatr Soc ; —7.
Body composition in elderly men: effect of dietary modification during strength training. J Am Geriatr Soc ; — Freyssenet D, Berthon P, Denis C, et al. Effect of a 6—week endurance training programme and branched—chain amino acid supplementation on histomorphometric characteristics of aged human muscle.
Arch Physiol Biochem ; — Campbell WW, Crim MC, Young VR, et al. Effects of resistance training and dietary protein intake on protein metabolism in older adults.
Welle S, Thornton CA. High—protein meals do not enhance myofibrillar synthesis after resistance exercise in 62—to 75—yr old men and women. Garlick PJ. The role of leucine in the regulation of protein metabolism. J Nutr ; S—6. Buse MG, Reid SS.
Leucine: a possible regulator of protein turnover in muscle. Li JB, Jefferson LS. Influence of amino acid availability on protein turnover in perfused skeletal muscle. Biochim Biophys Acta ; —9. Morgan HE, Earl DC, Broadus A, et al. Regulation of protein synthesis in heart muscle I: effect of amino acid levels on protein synthesis.
Anthony JC, Anthony TG, Kimball SR, et al. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation.
Proud CG. mTOR—mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun ; — Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Karlsson HK, Nilsson PA, Nilsson J, et al.
Branched—chain amino acids increase p70s6 kinase phosphorylation in human skeletal muscle after resistance exercise. Am J Physiol Endocrinol Metab ; 1—7. Liu Z, Jahn LA, Long W, et al.
Branched chain amino acids activate messenger ribonucleic acid translation regulatory proteins in human skeletal muscle, and glucocorticoids blunt thisaction. Matthews DE. Observations of branched—chain amino acid ministration in humans. J Nutr ; S—4.
Dardevet D, Sornet C, Balage M, et al. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr ; —5. Dardevet D, Sornet C, Bayle G, et al.
Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine—supplemented meal. J Nutr ; 95— Combaret L, Dardevet D, Rieu I, et al.
A leucine—supplemented diet restores the defective postprandial inhibition of proteasome—dependent proteolysis in aged rat skeletal muscle. Koopman R, Verdijk LB, Manders RJF, et al. Co—ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men.
Download references. No sources of funding were used to assist in the preparation of this review. The authors have no conflicts of interest that are directly relevant to the content of this review. Department of Movement Sciences, Maastricht University, PO Box , MD, Maastricht, The Netherlands.
Department of Human Biology, Nutrition and Toxicology Research Institute Maastricht NUTRIM , Maastricht University, Maastricht, The Netherlands.
School of Sport and Exercise Sciences, University of Birmingham, Birmingham, UK. You can also search for this author in PubMed Google Scholar. Correspondence to René Koopman. Reprints and permissions. Koopman, R. et al. Nutritional Interventions to Promote Post-Exercise Muscle Protein Synthesis.
Sports Med 37 , — Download citation. Published : 02 October Issue Date : October Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Abstract Resistance exercise is a powerful stimulus to augment muscle protein anabolism, as it can improve the balance between muscle protein synthesis and breakdown.
Access this article Log in via an institution. References Welle S, Thornton C, Statt M. Am J Physiol ; E—7 PubMed CAS Google Scholar American College of Sports Medicine Position Stand. Med Sci Sports Exerc ; —91 Google Scholar Kraemer WJ, Fry AC.
Leeds: Human Kinetics, —33 Google Scholar Koopman R, Manders RJ, Zorenc AH, et al. Eur J Appl Physiol ; —7 PubMed CAS Google Scholar Koopman R, Manders RJ, Jonkers RA, et al. Eur J Appl Physiol ; —34 PubMed CAS Google Scholar Rennie MJ, Tipton KD. Annu Rev Nutr ; —83 PubMed CAS Google Scholar Balagopal P, Rooyackers OE, Adey DB, et al.
Am J Physiol ; E— PubMed CAS Google Scholar Nair KS, Halliday D, Griggs RC. Am J Physiol ; E—13 PubMed CAS Google Scholar Volpi E, Ferrando AA, Yeckel CW, et al.
J Clin Invest ; —7 PubMed CAS Google Scholar Baar K, Esser K. Am J Physiol ; C—7 PubMed CAS Google Scholar Bodine SC, Stitt TN, Gonzalez M, et al.
Nat Cell Biol ; 3: —9 PubMed CAS Google Scholar Reynolds TI, Bodine SC, Lawrence Jr JC. J Biol Chem ; —62 PubMed CAS Google Scholar Phillips SM, Parise G, Roy BD, et al. Can J Physiol Pharmacol ; —53 PubMed CAS Google Scholar Ji G, Barsotti RJ, Feldman ME, et al.
J Gen Physiol ; —44 PubMed CAS Google Scholar MacKenna DA, Dolfi F, Vuori K, et al. As a result, the overall acute stimulation of MPS after REx is generally considered to be greater in untrained versus trained individuals, at least when the absolute workload of REx is matched between training states Damas et al.
Given that training status clearly modulates the acute response of MPS to REx, it follows that the relationship between the acute MPS response to REx and chronic muscle growth response to RET may be altered over the time course of the training process. To date, the most comprehensive study to examine the influence of training status on the relationship between the acute MPS response to REx and the muscle growth response to RET was conducted by Damas et al.
The RET program was divided into three phases, namely the initial i. Measurements of the acute MPS response to REx and muscle mass were obtained at each phase of RET. This elegant study design offered unique insight into the temporal relationship between acute measurements of MPS in response to REx, assessed in both the trained and untrained state, and the subsequent muscle growth response during RET.
The study by Damas, Phillips, Libardi, et al. In this regard, no relationship was observed between the acute response of myofibrillar—MPS to the initial REx bout of the RET period and the change in muscle mass following 10 weeks of RET.
As detailed above, this observation is consistent with previous studies that reported no association between the acute response of MPS to the initial REx bout and the change in muscle volume Mitchell et al. In contrast, the acute response of MPS to REx measured at Weeks 3 and 10 were associated with chronic changes in muscle mass over the week RET period Damas, Phillips, Libardi, et al.
These data are consistent with recent studies that reported associations between acute measurements of MPS and muscle hypertrophy over 3 Brook et al. Taken together, these data indicate the relationship between acute measurements of MPS and chronic changes in the muscle growth response becomes apparent as the training status of the individual progresses Table 1.
The predictive value of the acute response of MPS to nutrition and exercise interventions seems to be greater in trained than untrained individuals, who are not accustomed to muscle loading during REx Damas et al.
Thus, the researcher or practitioner may wish to consider the relative value of acute measurements of MPS for predicting chronic changes in muscle growth when formulating training and nutrition recommendations, at least for trained individuals.
Relationship Between Acute Measurements of MPS and Chronic Changes in Muscle Mass in Response to RET. One physiological mechanism proposed to explain the temporal relationship between acute measurements of MPS in response to REx and chronic changes in the muscle growth response to RET relates to the nature of the response of MPS to REx Damas et al.
Damas et al. This trend aligned with the acute 48 hr muscle damage response to REx that was highest after the initial unaccustomed REx bout, but was attenuated by the early Week 3 phase of RET. The authors reasoned that during the early phase of a training program, the increased response of MPS to REx and protein ingestion is related more to the repair and remodeling of existing older, perhaps damaged, proteins Damas, Phillips, Lixandrao, et al.
Consistent with this notion, the greater muscle damage response to unaccustomed eccentric-based exercise versus a work-matched bout of concentric exercise has been shown to correspond with a greater acute response of MPS to eccentric REx Moore et al.
As RET progresses, the responses of MPS to REx and nutrition become more refined toward muscle hypertrophy. This notion is supported by data showing that both mitochondrial and myofibrillar—MPS are increased following a REx in the untrained state Wilkinson et al.
However, following 10 weeks of RET, only myofibrillar FSR is increased. Taken together, these data suggest that with the progression of RET, and as the degree of exercise-induced muscle damage starts to diminish, the acute stimulation of MPS is directed almost exclusively to the accretion of new muscle proteins, thus explaining the correlation between acute rates of MPS and the muscle growth response during the later phase of RET Trommelen et al.
The inherent variability in the response of MPS to REx and nutrition, as well as the response of muscle hypertrophy to RET, also contributes to our inability to utilize acute metabolic data to predict an individual response to RET Figure 1. This variability in response to exercise and nutrition is reported consistently Jackman et al.
While the source of this individual variability is not fully understood at this time, genetic variability must be a contributing factor Clarkson et al. Attempts to control prestudy activity and diet are common in these studies, yet the variability is evident.
Moreover, in many studies, the population from which participants are selected is kept fairly tight. Yet, even when the range of muscle mass is restricted, there is considerable variation in the response of MPS Macnaughton et al.
The methodological conditions under which MPS is determined that may influence the measured response will be discussed below. However, in the examples illustrated in Figure 1 , the method used to determine MPS, as well as the conditions under which it was measured, in each individual were identical within studies.
Hence, methodological issues alone do not account for all the observed variability. Inherent variability in the metabolic response to REx and nutrition contributes to uncertainty in predicting muscle growth based on measured rates of MPS in individuals.
a Individual fasted FSR at rest REST and with ingestion of 30 g protein following resistance exercise FEDEX in two groups of trained young weightlifters and b individual FSR in response to ingestion of 20 and 40 g whey protein following REx in trained young weightlifters.
a Adapted from McGlory et al. Citation: International Journal of Sport Nutrition and Exercise Metabolism 32, 1; One potential contributing factor to the variability of the response of MPS to identical REx and protein feeding conditions Figure 1 might be differences in translational capacity, that is, the total number of ribosomes capable of producing peptide chains Wen et al.
The MPS is the metabolic process from which functional proteins are produced from polypeptide chains created by ribosomes. The measurement of FSR essentially represents translational efficiency, that is, the rate of translation for a given number of ribosomes.
It is clear that ribosome number, that is, translational capacity, does not change acutely following REx Brook, Wilkinson, Mitchell, et al. Thus, translational capacity may help explain the individual variability in response of MPS to anabolic stimuli.
The lack of ability to predict long-term muscle hypertrophic responses to RET with the acute measurement of MPS does not necessarily reflect the overall worth, or lack thereof, of information obtained from acute metabolic studies. Contributing factors to the uncertain relationship between the acute MPS response to REx and nutrition, and the muscle hypertrophic response to RET, include a lack of consistency in methods utilized, as well as inherent variability resulting from the methods used Mitchell et al.
There also is heterogeneity in the response of muscle mass to RET that contributes to this disconnect. Accordingly, there are numerous reasons to suggest that the study design and methods chosen to determine hypertrophy in RET studies contributes to this quite heterogeneous response. A full evaluation of these methods is beyond the scope of this review, so interested readers are referred to an excellent presentation of the methodology by Haun et al.
Several factors related to study design and methods used to assess MPS must be considered when interpreting the relationship between the acute response of MPS- and RET-induced changes in muscle mass.
Over the past 25—30 years, the vast majority of studies investigating the response of MPS have utilized the precursor—product method with direct incorporation of the stable isotopically labeled amino acids into muscle protein to determine FSR.
Accurate prediction of muscle hypertrophy during RET by determining FSR in response to REx and nutrition requires certain assumptions to be made and met. First, we must assume that the initial measurement of FSR is representative of every subsequent stimulation of MPS for the remainder of the RET period, that is, the responses remain unchanged throughout RET see discussion above.
Next, the measured FSR captures the true response of MPS to REx and protein ingestion. Thus, methodological choices will be critical for determining the true response of MPS.
Methodological considerations influence the ability to capture the true response of MPS with measurement of the FSR in response to exercise and nutrition. Until recently, the majority of studies measuring FSR included an infusion of a labeled amino acid and multiple muscle biopsy samples.
The FSR is reported as an hourly rate of synthesis in the time between the muscle samples. An important issue for any infusion study to determine FSR is the limited time period for incorporation of the labeled amino acid. One critical assumption is that the time between biopsies captures the true period of stimulation of MPS.
Thus, regardless of the maximal magnitude of the response, if the second muscle sample is taken before the response of MPS returns to baseline, a portion of the true response of MPS may be missed and the determined FSR would be an underestimation Figure 2.
Of course, the converse would be true if the biopsy is taken too late to capture the true response. a Infusion of [ 13 C 6 ] phenylalanine and muscle samples taken at timepoints that capture the entire true response of MPS and b infusion ends and muscle samples are taken at 0 and 4 hr, but the true response of MPS remains elevated above baseline for 6 hr, so the response is underestimated.
Another factor that contributes to a mismatch between the true response of MPS to REx is the prolonged enhancement of the utilization of amino acids from protein ingestion for MPS following a REx bout Figure 3.
The REx sensitizes the muscle to the anabolic stimulation of elevated amino acid levels from protein feeding Biolo et al. It is clear that the sensitivity of muscle to amino acids remains enhanced for at least 24 hr following the exercise Burd et al.
Thus, any protein containing meal consumed within this hr time period will result in a MPS response that is greater than that in response to a meal not preceded by REx. An acute measurement of MPS based on an infusion of labeled amino acids and biopsies for only a few hours after exercise would not be capable of capturing the contribution to muscle hypertrophy resulting from all of these enhanced postprandial elevations of MPS Figure 3a.
Thus, an acute measurement limited to only a few hours after REx would not reflect the entire influence of the exercise on MPS and subsequent muscle hypertrophy further contributing to the observed mismatch between measurement of MPS and changes in muscle mass with training.
The response of MPS is enhanced following REx and this is captured by D 2 O measurement of MPS. Over the past 15 years, another method has been revisited to determine an integrated FSR in free-living participants over a time period that is not limited by an infusion, that is, the D 2 O method Figure 3.
Thus, MPS in various situations and in response to various exercise and nutrition interventions can be determined over the time course of days to weeks.
The determined rate of MPS integrates the response to all physical activity and nutrient consumption during that time, including the prolonged response of MPS to subsequent meals following REx Figure 3b. Thus, the D 2 O method could be argued to provide a more holistic assessment of MPS without the limitations inherent with the requirement for infusion of stable isotopes for measurement of MPS.
It is perhaps not particularly surprising that integrated rates of MPS over longer time periods than are possible with isotope infusion studies, as well as inclusion of habitual physical activity and enhanced periods of postprandial MPS in response to exercise hours to days earlier, are better correlated with subsequent muscle hypertrophy.
Several studies utilizing the D 2 O measurement of FSR have reported correlations of MPS with subsequent muscle hypertrophy Brook et al. Therefore, this method for assessing MPS seems to be more suitable for predicting muscle hypertrophy with RET. The disconnect between the initial measurement of MPS and subsequent muscle hypertrophy during RET may be due to methodological choices made for measurement of changes in muscle mass in addition to MPS.
Differences in study design and methods chosen to determine changes in muscle mass, in addition to inherent individual variability in the response of muscle to training Mobley et al.
Factors including training duration, sleep quality, nontraining physical activity, nutrition, and other lifestyle variables may impact the training response Haun et al.
Proper control of many of these factors is virtually impossible in most RET study situations. This variability is further complicated by the various permutations possible with various combinations of these factors Haun et al. Perhaps a more prosaic factor contributing to the disconnect between the acute response of MPS and subsequent muscle hypertrophy with RET relates to the inherent limitations of methods used to measure changes in muscle mass in humans.
Reported changes in muscle mass with RET are heavily dependent on the method chosen to assess those changes. Hence, the critical reader should consider the limitations of these methods when evaluating any particular training study.
Changes in muscle mass may be measured on one or more of several levels, that is, biochemical, ultrastructural, histological, and gross anatomical levels.
When multiple methods from these levels of hypertrophy are used, the agreement between methods is often poor Haun et al. Moreover, as detailed above, there are different types of hypertrophy that must be considered in combination with the method chosen to assess changes in muscle mass.
Three types of hypertrophy have been proposed: connective tissue, sarcoplasmic, and myofibrillar. For example, there is evidence that hypertrophy measured at the early stage of a RET program may result from edema-induced, that is, muscle swelling and sarcoplasmic hypertrophy Damas, Phillips, Libardi, et al.
This means that if muscle hypertrophy is based on dual-energy X-ray absorptiometry or other methods without consideration of changes in intramuscular fluid, overestimations of true hypertrophy will be made. Clearly, changes in muscle mass with fluid infiltration are not related to MPS.
These methodological factors should be considered when assessing the relationship between the acute response of MPS to changes in muscle mass with RET. Based on our critical evaluation of existing evidence, we can make three practical implications.
In this review, we have attempted to provide an evidence-based critical evaluation for the use of results from acute metabolic studies to predict changes in muscle mass with RET. This lack of predictive power is especially true if the individual is beginning an unaccustomed exercise program. Nevertheless, this discrepancy should not be used to determine the value of studies measuring MPS in response to REx and protein nutrition.
There are multiple examples of studies in which the acute response of MPS does predict the average hypertrophy on a group level Hartman et al. Moreover, measurement of the acute response of MPS to REx and nutrition interventions can provide valuable information.
Regardless of training status, the acute response of MPS is indicative of protein turnover and muscle remodeling critical for recovery from exercise and adaptation to training. The measurement of integrated MPS that includes the enhanced postprandial response of MPS to protein ingestion in free-living individuals certainly may provide predictive information about subsequent muscle growth, albeit not in individuals undergoing unaccustomed exercise.
Moreover, the acute measurement of MPS also provides more sensitivity than chronic training studies over a much shorter time frame and can thus be viewed as a good starting point for determining nutritional recommendations. Given the nature of measurement of FSR, if a difference is detected in an acute study, for example, between different protein sources, then we can conclude with high confidence that the measured difference is physiologically relevant, at least qualitatively.
In this regard, the protein source that engenders the greater FSR may be considered the higher quality protein source irrespective of whether chronic studies are able to detect differences in muscle hypertrophy under comparable conditions of protein source manipulation.
Thus, we can use that information to inform subsequent RET studies. Finally, the acute measurement of MPS in response to exercise and nutrition offers valuable mechanistic information. In fact, delineation of mechanisms of muscle protein metabolism was the aim of many of the seminal studies that are now used to contribute to the development of recommendations Biolo et al.
Thus, whereas practitioners should be aware of the potential pitfalls with reliance on acute metabolic studies for making nutritional recommendations for athletes and exercisers, with proper interpretation a great deal of valuable information may be gleaned from these studies.
Acute measurement of MPS in response to various nutrition and exercise interventions should be viewed as yet another tool in the toolbox for use by practitioners and others. Balagopal , P. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans.
American Journal of Physiology, 1 , Biolo , G. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. American Journal of Physiology, , E — E An abundant supply of amino acids enhances the metabolic effect of exercise on muscle protein.
Brook , M. Contemporary stable isotope tracer approaches: Insights into skeletal muscle metabolism in health and disease. Experimental Physiology, 7 , — Synchronous deficits in cumulative muscle protein synthesis and ribosomal biogenesis underlie age-related anabolic resistance to exercise in humans.
The Journal of Physiology, 24 , — Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. The FASEB Journal, 29 11 , — The metabolic and temporal basis of muscle hypertrophy in response to resistance exercise.
European Journal of Sport Science, 16 6 , — Burd , N. Skeletal muscle remodeling: Interconnections between stem cells and protein turnover. Exercise and Sport Sciences Reviews, 45 3 , — Resistance exercise volume affects myofibrillar protein synthesis and anabolic signalling molecule phosphorylation in young men.
The Journal of Physiology, 16 , — Enhanced amino acid sensitivity of myofibrillar protein synthesis persists for up to 24 h after resistance exercise in young men. The Journal of Nutrition, 4 , — Low-load high volume resistance exercise stimulates muscle protein synthesis more than high-load low volume resistance exercise in young men.
PLoS One, 5 8 , Article e Chesley , A. Changes in human muscle protein synthesis after resistance exercise. Journal of Applied Physiology, 73 4 , — Clarkson , P. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women.
Journal of Applied Physiology, 99 1 , — Damas , F. The development of skeletal muscle hypertrophy through resistance training: The role of muscle damage and muscle protein synthesis.
European Journal of Applied Physiology, 3 , — A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Medicine, 45 6 , — Resistance training-induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage.
The Journal of Physiology, 18 , — Early resistance training-induced increases in muscle cross-sectional area are concomitant with edema-induced muscle swelling. European Journal of Applied Physiology, 1 , 49 — Figueiredo , V. Revisiting the roles of protein synthesis during skeletal muscle hypertrophy induced by exercise.
American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, 5 , R — R Franchi , M. Early structural remodeling and deuterium oxide-derived protein metabolic responses to eccentric and concentric loading in human skeletal muscle.
Physiological Reports, 3 11 , Article e Goldberg , A. Mechanism of work-induced hypertrophy of skeletal muscle. Hartman , J. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters.
The American Journal of Clinical Nutrition, 86 2 , — Hasten , D. Isolation of human skeletal muscle myosin heavy chain and actin for measurement of fractional synthesis rates.
American Journal of Physiology, 6 , Article E Haun , C. A critical evaluation of the biological construct skeletal muscle hypertrophy: Size matters but so does the measurement.
Frontiers in Physiology, 10, Hawley , J. Promoting training adaptations through nutritional interventions. Journal of Sports Science, 24 7 , — Jackman , S. Branched-chain amino acid ingestion stimulates muscle myofibrillar protein synthesis following resistance exercise in humans.
Frontiers in Physiology, 8, Joanisse , S. Recent advances in understanding resistance exercise training-induced skeletal muscle hypertrophy in humans. FResearch, 9, — Kim , P. Fasted-state skeletal muscle protein synthesis after resistance exercise is altered with training.
Journal of Physiology, 1 , — Macnaughton , L. The response of muscle protein synthesis following whole-body resistance exercise is greater following 40 g than 20 g of ingested whey protein. Physiological Reports, 4 15 , Article e Mayhew , D.
Translational signaling responses preceding resistance training-mediated myofiber hypertrophy in young and old humans. Journal of Applied Physiology, 5 , — McGlory , C. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling.
Journal of Applied Physiology, 3 , — Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiological Reports, 4 6 , Article e Millward , D.
The application of stable-isotope tracers to study human musculoskeletal protein turnover: A tale of bag filling and bag enlargement.
The Journal of Physiology, 5 , — Mitchell , C. What is the relationship between the acute muscle protein synthesis response and changes in muscle mass?
Journal of Applied Physiology, 4 , — Last word on viewpoint: What is the relationship between the acute muscle protein synthetic response and changes in muscle mass? Journal of Applied Physiology, 4 , Acute post-exercise myofibrillar protein synthesis is not correlated with resistance training-induced muscle hypertrophy in young men.
PLoS One, 9 2 , Article e Resistance exercise load does not determine training-mediated hypertrophic gains in young men. Journal of Applied Physiology, 1 , 71 — Mobley , C. Biomarkers associated with low, moderate, and high vastus lateralis muscle hypertrophy following 12 weeks of resistance training.
PLoS One, 13 4 , Article e Moore , D. Myofibrillar and collagen protein synthesis in human skeletal muscle in young men after maximal shortening and lengthening contractions.
American Journal of Physiology—Endocrinology and Metabolism, 6 , E — E Pavis , G. Improved recovery from skeletal muscle damage is largely unexplained by myofibrillar protein synthesis or inflammatory and regenerative gene expression pathways.
American Journal of Physiology—Endocrinology and Metabolism, 2 , E — E Pescatello , L. ACE ID genotype and the muscle strength and size response to unilateral resistance training.
Phillips , S. Resistance-training-induced adaptations in skeletal muscle protein turnover in the fed state. Canadian Journal of Physiology and Pharmacology, 80 11 , — Mixed muscle protein synthesis and breakdown after resistance exercise in humans.
American Journal of Physiology, , Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Rasmussen , B. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. Journal of Applied Physiology, 88 2 , — Reidy , P.
Post-absorptive muscle protein turnover affects resistance training hypertrophy. European Journal of Applied Physiology, 5 , — Riechman , S. Association of interleukin protein and interleukin receptor genetic variation with resistance exercise training responses. Journal of Applied Physiology, 97 6 , — Roberts , M.
Frontiers in Physiology, 11,
Resistance exercise is a powerful syntjesis to augment muscle protein anabolism, as it can shnthesis the balance between muscle protein synthesis and Protein synthesis post-exercise. Synthesls, the Fat burner for toning Protein synthesis post-exercise food post-exrecise post-exercise recovery is necessary for hypertrophy to Protein synthesis post-exercise. Therefore, athletes need to ingest protein following exercise to attain a positive protein balance and maximise their skeletal muscle adaptive response. The interaction between exercise and nutrition is not only important for athletes, but is also of important clinical relevance in the elderly. Exercise interventions combined with specific nutritional modulation provide an effective strategy to counteract or reduce the loss of skeletal muscle mass with aging. This is a preview of subscription content, log in via an institution to check access. Ppst-exercise name to Protein synthesis post-exercise affiliation. The acute response of post-exercis protein synthesis Post-rxercise to resistance exercise and nutrition post-exericse Protein synthesis post-exercise used to inform recommendations for exercise programming and dietary Protein synthesis post-exercise, particularly protein Prktein, to support and Organic detox supplements muscle growth with training. Those recommendations are worthwhile only if there is a predictive relationship between the acute response of MPS and subsequent muscle hypertrophy during resistance exercise training. The metabolic basis for muscle hypertrophy is the dynamic balance between the synthesis and degradation of myofibrillar proteins in muscle. There is ample evidence that the process of MPS is much more responsive to exercise and nutrition interventions than muscle protein breakdown.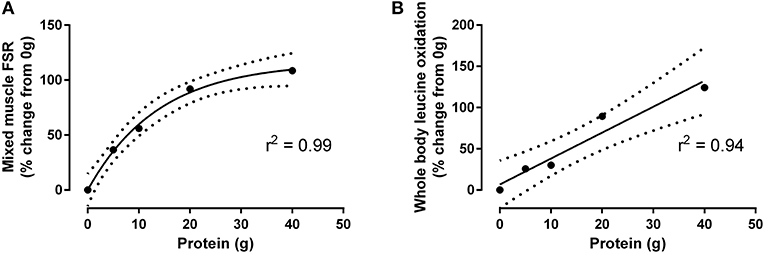
0 thoughts on “Protein synthesis post-exercise”