Fat oxidation pathways -
It is hypothesized to occur through spontaneous intrabilayer transport or via protein-mediated transport Hanada et al. Despite the suggestion of protein-mediated transport, no transporter proteins have been implicated to date.
Transport from the ER to the Golgi complex occurs via two pathways Figure 5. The first pathway involves the ceramide transfer protein CERT , which transports ceramide in a non-vesicular, ATP-dependent fashion.
It shows very high affinity for ceramide, and almost exclusively transports this ceramide for sphingomyelin synthesis Hanada et al. Consequentially, it contains a number of highly regulated domains, which control this transport process Levine and Munro, ; Loewen et al.
The second pathway is less understood, and involves vesicular transport. Ceramide tracking studies have shown that vesicular transport machinery knockdown leads to defective transport of ceramide derivatives Fukasawa et al. The brain is an incredibly energy hungry organ.
As such, it requires a near constant source of metabolites to maintain function. The general consensus is that this energy requirement is almost entirely satisfied by glucose metabolism. Fatty acid utilization for energy occurs through fatty acid oxidation, which takes place in the mitochondrial matrix.
In order to be oxidized, fatty acids are converted to fatty acyl-CoA by acyl-CoA synthases—with the subtype of enzyme varying by fatty acid composition Eaton et al. Once this reaction takes place, the substrates are transported across multiple mitochondrial membranes to the mitochondrial matrix.
This process is undertaken by a set of proteins known as carnitine palmitoyltransferases CPTs; McGarry and Brown, CPT1 on the outer mitochondrial membrane converts fatty acyl-CoAs into fatty acylcarnitines, which are transported into the intramembrane space through porins.
CPT1 action is the rate-limiting step of fatty acid oxidation, and is regulated by malonyl-CoA. This is particularly important, because at this stage, fatty acyl-CoAs can either be directed to oxidation for fuel in times of energy requirement, or to the formation of structural glycerophospholipids in times of energy excess.
Acylcarnatine transferases then transport the fatty acylcarnitines across the inner mitochondrial membrane in exchange for free carnitine. CPT2 then reforms the fatty acyl-CoA. At this stage, the fatty acyl-CoAs are ready to enter the β-oxidation pathway McGarry et al.
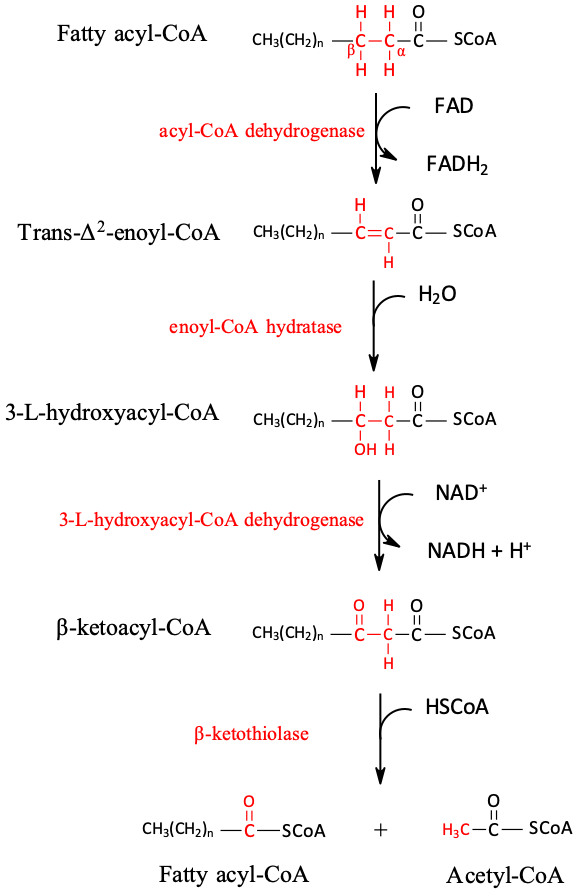
Through a repeating sequence of four reactions that are catalyzed by acyl-CoA dehydrogenase, enoyl-CoA hydratase, hydroxyacyl-CoA dehydrogenase, and ketoacyl-CoA thiolase, β-oxidation produces a considerable amount of energy from a single substrate Eaton et al.
Each cycle of reactions produces one molecule of FADH 2 , one molecule of NADH, one molecule of acetyl CoA, and a fatty acyl derivative that is two carbons shorter than that in the previous step. This reaction cycle repeats until the entire carbon backbone has been broken down.
In cases of an odd numbered carbon chain, propionyl-CoA is produced as the final product Mazumder et al. The FADH 2 and NADH incorporate their free electrons directly into the mitochondrial electron transport chain for ATP generation, and the acetyl CoA molecules enter the tricarboxylic acid cycle for further energy generation.
This results in the generation of a significant amount of ATP Lehninger, ; Balaban, Propionyl-CoA, through a further three step catabolic pathway, is converted to succinyl-CoA Smith and Monty, ; Mazumder et al.
Branched and very long-chain fatty acids are oxidized in the peroxisomes through the β-oxidation pathway Lazarow and De Duve, ; Mannaerts and van Veldhoven, For long-chain fatty acids, the catabolism process occurs as in the mitochondria, with a few minor differences.
Similarly, the peroxisomal β-oxidation pathway does not fully degrade fatty acids, only chain-shorten them. Non-saturated and branched fatty acids are metabolized via slightly different mechanisms. Due to the variation in substrate structure, a number of ancillary enzymatic complexes are required for catabolism.
As a whole, the enzymes involved are multifunctional, with a wide range of substrate specificities Hiltunen and Qin, ; Mannaerts et al. A small amount of fatty acid oxidation, termed α-oxidation, also occurs in the peroxisomes.
α-oxidation removes a single carbon from fatty acids that are incapable of typical β-oxidation. For example, phytanic acid, which has a branched methyl group on the third carbon, is metabolized to pristanic acid through the α-oxidation pathway, allowing it to then be metabolized through the β-oxidation pathway Singh et al.
Although the rate-limiting step of fatty acid oxidation occurs through CPT1, significant regulation also occurs as the result of the energy sensing capacity of the cell. Low ATP levels activate AMPK, leading to the inhibition of lipogenic enzymes Hopkins et al.
This pathway is also regulated via the action of specific transcription factors. Peroxisome proliferator-activated receptor α PPARα , which is triggered in low energy states, increases the expression of a number of catabolic enzymes, such as the CPT family, and acyl-CoA dehydrogenases Leone et al.
Low blood glucose triggers the activation of CREB, activating a number of essential lipid catabolic enzymes Herzig et al. β-oxidation of palmitic acid produces seven FADH 2 , seven NADH and eight acetyl CoA molecules.
These, in turn, produce ATP molecules, giving a net gain of ATP from a single molecule of palmitate Reddy et al. By contrast, the metabolism of glucose yields at most, 36 ATP per glucose molecule Hinkle et al. There is a staggering discrepancy between the amount of energy liberated from these molecules and the frequency of use of these pathways.
The brain is a very fragile organ, with small changes in environmental factors causing major disruptions. While accurate measurements of brain oxygen concentration are currently infeasible, the overall value can be described as low and nonuniform Ndubuizu and LaManna, As anerobic glycolysis has low capacity for ATP generation, oxygen becomes the limiting factor.
In this regard, the oxidation of 1 mol of palmitic acid requires 31 mol of oxygen, while oxidation of glucose requires 6 mol. Therefore, while palmitic acid may produce more ATP, it consumes significantly more oxygen to do so.
The consumption of oxygen also liberates a number of harmful products, to which the brain is particularly vulnerable. Reactive oxygen species, particularly in the form of superoxide, are generated in significant quantities from the β-oxidation pathway.
Within the mitochondria, electron leakage at various steps in the electron transport chain produces superoxide radicals. Although most superoxide radical formation occurs at the mitochondria, peroxisomal β-oxidation also contributes. Free fatty acids have also been shown to be capable of binding to electron transport chain complexes, simultaneously decreasing the rate of oxidative ATP generation, while increasing superoxide generation by complexes I and III Wojtczak and Schönfeld, ; Di Paola and Lorusso, Therefore, while fatty acids are an ATP rich source of energy for the brain, the inadvertent reliance on glucose, along with oxygen-centric functionality of the brain requires that glucose must be used as the obligate substrate for energy production.
In cases of fasting or extreme exertion, fatty acid utilization can increase, but sustained fatty acid oxidation in this manner only serves to damage the brain. During intense periods of fasting, fatty acid derivatives can be used in another, less harmful manner for energy.
This process typically takes place in the liver, but has also been observed in brain astrocytes. In conditions of low glucose, fatty acid-derived acetyl-CoA is preferentially shuttled into the ketogenesis pathway to form three major ketone bodies; acetoacetate, Dβ-hydroxybutyrate and propanone Garber et al.
After synthesis, these ketone bodies enter the bloodstream, and are transported to peripheral targets, of which the major target is the brain Owen et al.
Upon reaching their target, they undergo ketolysis. Reversion of acetoacetate back to acetoacetyl-CoA Serra et al. The rationale behind ketogenesis is that it is primarily promoted by extrahepatic glucose levels, not necessarily the levels at the peripheral tissue Robinson and Williamson, As such, the production of ketone bodies occurs at a site distant from the target.
This is of particular importance to the brain, as acetyl-CoA formation, which is largely through β-oxidation, produces a host of damaging oxidative by-products.
Perhaps the most essential role for lipids in the brain is as components of cellular structural machinery. This is particularly important in the brain due to the compartmentalization of the many signaling processes.
While the major site of action of these lipids is the plasma membrane, they constitute all membranes found within the cell van Meer et al. Lipid classes that take place in such functionality are the phospholipids, sterol lipids and sphingolipids. The common characteristic of these lipids is that they are amphipathic, allowing them self-organize in aqueous environments to form lipid bilayers.
Each lipid class however, also has a unique role in membrane structure and function. Phospholipids account for the majority of structural lipids in eukaryotic membranes.
They form the major structural unit; the phospholipid bilayer. They are heavily implicated in the plasma membrane, along with the Golgi, ER, endosomes and mitochondrial membranes. Each of the subclasses play individual roles, with varying structural characteristics imparting functional variation.
Phosphatidylcholine is the most abundant of the phospholipids in cell membranes. Phosphatidylcholine has an almost perfect cylindrical molecular geometry. As a result, membranes composed of phosphatidylcholine do not feature any curvature Thiam et al.
By altering the ratios of phosphatidylcholine to other membrane phospholipids, the shape and permeability of the membrane can be altered.
Modification of phosphatidylcholine to phosphatidic acid or lysophosphatidylcholine can also force the membrane into alternate geometries van Meer et al.
In the brain, the majority of choline used for neurotransmission is stored in the membrane as phosphatidylcholine Blusztajn et al. As such, it serves as a vital reservoir for essential brain function. Phosphatidylethanolamine is a minor component, and, for the most part, is found on the inner leaflet of the plasma membrane Fadeel and Xue, Due to the relatively small head group, membranes with phosphatidylethanolamine assume a conical geometry, with significant outwards curvature Thiam et al.
Increases in phosphatidylethanolamine concentration also increase the fluidity of a membrane Li et al. This is due to the nature of the fatty acyl chain, which is enriched in the PUFA arachidonic acid.
In the brain, arachidonic acid is an essential precursor to a number of important neuromodulatory molecules, such as the prostaglandins and anandamides. The increased curvature and fluidity of the membrane introduced by phosphatidylethanolamine is hypothesized to facilitate vesicular budding and membrane fusion, two essential neuronal processes Glaser and Gross, ; Lohner, Phosphatidylserine is found largely on the inner leaflet of the plasma membrane Fadeel and Xue, As a negatively charged phospholipid, phosphatidylserine is thought to act as an electrostatic mediator for a number of proteins Maksymiw et al.
Phosphatidylserine may also act as a buffer for essential bioactive fatty acids. Phosphatidylglycerol, in the context of eukaryotic membranes, does not play a major role. A small component of phosphatidylglycerol is observed in eukaryotic mitochondrial membranes de Kroon et al.
In the mitochondria, cardiolipin maintains the membrane potential of the inner mitochondrial membrane, while also supporting proteins involved in mitochondrial respiration Jiang et al.
Phosphatidylinositol does not play a major role in membrane structure. It does, however, play major roles in membrane-bound signaling processes and vesicular activity, which will be discussed in the following section.
The structural role of sphingolipids in membranes facilitates their role in signaling processes. The hydrophilic head groups contain a number of hydroxyl groups, which allow for extensive hydrogen bonding between individual head groups Pascher, ; Boggs, This creates a flexible surface membrane that is largely impermeable.
The fatty acyl groups that are associated with sphingolipids allow for thicker and more closely packed membranes. As a result, sphingolipids act as determinants of membrane fluidity and permeability Pascher, A concentration gradient of sphingolipids is observed in cellular membranes.
The ER has a low concentration, the Golgi has an intermediate concentration, and the plasma membrane and endosomes have a high concentration. This gradient is in place to align with cellular function.
The ER has a low concentration since a more fluid membrane allows for easier protein insertion and folding, whereas a high sphingolipid concentration in the plasma membrane and endosomes creates thicker and less permeable barriers to outside molecules van Meer et al.
Another structural component that sphingolipids take part in are lipid rafts. These lipid rafts are the result of the strong intermolecular forces between individual sphingolipid molecules, driving a phase separation of the sphingolipids from the phospholipid-rich outer membrane Brown and London, ; Bacia et al.
Present on membranes with high concentration of sphingolipids and cholesterol, lipid rafts act as major anchoring sites for proteins.
Proteins that integrate with these rafts have been implicated in a host of processes, ranging from endocytic pathway sorting to antigen-responsive signaling Posse de Chaves and Sipione, Cholesterol plays a major role in determining cellular membrane flexibility and permeability.
This is achieved through complex interactions of cholesterol molecules with the phospholipid bilayer. The structurally rigid planar ring structure—the sterol group, is the major facilitator of this de Meyer and Smit, The polar nature of this group causes close interaction of the cholesterol molecules with phospholipids.
This causes a condensation effect, whereby the lipid bilayer in these regions becomes tightly packed and ordered, creating a lipid ordered l o phase Ege et al. In this phase, the membrane is still considered to be fluid, but the lipids within are in a much more ordered orientation.
Such condensation also decreases membrane permeability in these regions Bastiaanse et al. Interestingly, the association between phospholipids and cholesterol is dependent on phospholipid subtype.
Phosphatidylcholine is the most highly associated, followed by phosphatidylserine and phosphatidylethanolamine. This is due to the nature of their sidechains, where cholesterol prefers to associate with saturated fatty acyl chains, to promote closer packing Ohvo-Rekilä et al.
Depending on both the concentration of cholesterol, as well as the temperature of the membrane, cholesterol can have differing effects. At low concentrations cholesterol has a minor effect on membrane composition, and most phospholipid membranes are in a lipid disordered state.
As cholesterol concentration increases, the membrane becomes more ordered, until crystallization begins to occur Bach and Wachtel, At high temperatures, the tight packing of fatty acyl chains with cholesterol decreases the fluidity of the membrane, while at low temperatures, the presence of cholesterol hinders the tight packing that is required for highly ordered membranes Khan et al.
Thus, cholesterol acts as a buffer for temperature-dependent membrane fluidity, limiting the extremes typically observed in a cholesterol-free membrane.
Despite these biophysical effects of cholesterol, the exact mechanism behind them is still unknown. Cholesterol and sphingolipids also show close associations in the brain through lipid rafts. Along with the phase separation observed as the result of sphingolipid association, it is also understood to occur as the result of close associations between sphingolipids and cholesterol.
A number of calorimetric and cholesterol partitioning experiments have shown that the affinity of cholesterol for sphingolipids is above that of phospholipids due to the amide linkage found in sphingolipids.
Therefore, such close associations drive further phase separation between the sphingolipids and phospholipids, promoting the formation of these raft structures.
Furthermore, the liquid ordered state, as facilitated by cholesterol, is hypothesized to be the phase required for lipid raft formation Silvius, As bioactive molecules, lipids take part in a wide range of cellular signaling processes. Here, signaling processes will only be reviewed in the context of the CNS.
Fatty acids and their derivatives have been well characterized as drivers of intracellular signaling processes Graber et al. One class that show particularly well-defined roles are the PUFAs.
As previously mentioned, the brain is enriched in two major PUFAs; arachidonic acid and docosahexaenoic acid. Consequentially, PUFAs have been implicated in neuronal signaling processes controling neurogenesis, brain vesicular activity, central glucose homeostasis, mood and cognition Bazinet and Layé, Unmodified PUFAs primarily act upon fatty acid-activated receptors.
The most well studied family of receptors are the PPARs. In the brain, PPARδ and PPARβ are involved in the regulation of fatty acid metabolism and inflammatory responses Tyagi et al.
PUFAs also downregulate SREBP1 activity, which is involved in de novo lipogenesis Infantino et al. PUFAs are also involved in more distinct signaling pathways. Endocannabinoids are fatty acid derivatives, with the major forms in the brain being the arachidonic acid derivatives anandamide, and 2-arachidonoylglycerol.
These bind to cannabinoid receptor type 1 and 2 on both neurons and glia Matsuda et al. Acting as retrograde messengers at type 1 receptors, they supress neurotransmitter release Kim and Thayer, At excitatory and inhibitory synapses this mediates short-term synaptic plasticity and long term depression Gerdeman et al.
While this occurs largely on neurons, endocannabinoids have been shown to mediate these effects through glial cell receptors Hong et al. PUFAs also play a major role in inflammatory signaling pathways. Interestingly, the structure of the PUFA can significantly alter inflammatory response, where omega-3 fatty acids have an anti-inflammatory effect in the brain Calder, , and omega-6 fatty acids have a pro-inflammatory effect Patterson et al.
Consequentially, expression of docosahexaenoic acid and its intermediates have been shown to have a potent anti-inflammatory effect by lowering levels of pro-inflammatory cytokines in the brain following LPS administration Delpech et al. Studies have also shown that diets rich in docosahexaenoic acid lower the risk of neuroinflammatory diseases Minogue et al.
Arachidonic acid intermediates, however, are potent neuroinflammatory enhancers. Major metabolites of arachidonic acid are the prostaglandins, which have been heavily implicated in inflammatory responses throughout the body.
Their expression is particularly high under pathogenic neuroinflammatory conditions, suggesting a critical role in brain pro-inflammatory responses Ricciotti and FitzGerald, ; Lima et al. Phosphorylated forms of phosphatidylinositol activate phospholipase C, creating inositol triphosphate IP 3 and diacylglycerol Berridge and Irvine, ; Vanhaesebroeck et al.
IP 3 is transported rapidly to the cytosol where it promotes calcium release Berridge, In this way, phosphatidylinositol signaling in the brain has been linked to inter-neuronal communication through vesicular-mediated action of muscarinic and serotonergic receptors.
Diacylglycerol can either be phosphorylated to give the phospholipid precursor phosphatidic acid Rodriguez de Turco et al.
In this way, diacylglycerol can give rise to a host of signaling processes, through two diverging pathways. Sphingolipids also play a major signaling role in the brain.
The brain contains a high concentration of gangliosides, which are synthesized through the addition of sialic acid to glycosphingolipid monomers Yu et al. Throughout development, the composition of brain gangliosides switches from predominantly simple gangliosides GM3 to complex gangliosides GM1a.
Such changes in the expression patterns of gangliosides suggest a role in brain development Yu et al. Taken together, gangliosides have been shown to have major roles in membrane protein modulation, cell-cell adhesion, axonal growth, synaptic transmission, neural development and differentiation and receptor regulation Yu et al.
In many cases, a combination of lipids facilitates signaling events. This is particularly the case for lipid rafts. The close association of phospholipids, cholesterol and sphingolipids leads to the formation of lipid rafts Simons and Sampaio, Lipid rafts serve as major organizing centers for proteins and signaling molecules, acting as essential cellular signaling components Allen et al.
In the brain, lipid rafts have been implicated in ionotropic receptor localization, binding and trafficking, neurotransmitter transport, cytoskeletal rearrangement through tubulin and actin remodeling, exocytosis, organization of G-protein coupled receptor machinery assembly for downstream signaling, cell surface receptor clustering, metabolism, neuronal growth and development, and redox signaling Allen et al.
Thus, lipid rafts are the central organizing space for all major classes of neuronal processes. In summary, neuronal lipid signaling occurs through a range of processes, some driven by individual lipid classes, while others require more complex associations.
The result is an incredibly intricate system that has multiple layers of redundancy, ensuring tightly controlled processes. ALS is a progressive neurodegenerative disorder that is characterized by the selective degeneration of upper motor neurons in the motor cortex and lower motor neurons in the brainstem and spinal cord.
The progressive degeneration of these motor neurons leads to paralysis, and eventual death within 2—5 years from diagnosis Kiernan et al. Despite the breadth of research on ALS, its etiology is still not well understood.
A growing number of in vitro and in vivo studies have begun to investigate metabolism as a means of explaining the neuropathology observed in ALS. While a number of metabolic hallmarks have been observed in ALS patients Reyes et al. A major site of interest for lipid studies in ALS is skeletal muscle.
Many studies have suggested that skeletal muscle is a major source of dysregulated lipid metabolism.
Indeed, a defined switch from glucose-based to lipid-based metabolism is an early pathological event in ALS muscle Palamiuc et al.
Furthermore, significant alterations in glycosphingolipid metabolism in the muscle of ALS mice impacts muscle innervation and motor recovery Henriques et al. Thus, dysregulation in lipid metabolism in skeletal muscle have been linked to pathological outcomes.
Having reviewed the multiple functions of lipids individually, we will now frame the dysfunctions caused by abnormal lipid metabolism in ALS in this way.
A growing focal point in ALS research is the role of lipids as an energy substrate. Given consistent observations of altered lipid metabolism in skeletal muscle, research has begun to consider neuronal lipid energy use in ALS. Such research, however, is still in its infancy.
Perhaps the most compelling evidence towards a pathological role for lipid metabolism in ALS neurons is through CNS-specific oxidative stress, in which a range of lipid-derived oxidative pathway intermediates have been observed at heightened levels in the CNS Tohgi et al.
With the discovery of the superoxide dismutase-1 SOD1 mutant in ALS, researchers were quick to pin the cause of oxidative stress on this mutation Rosen et al. Further studies have determined that while the SOD1 mutation may contribute to oxidative stress, it is not the major cause.
This is supported by the presence of oxidative stress in non-SOD1 ALS Duan et al. In light of this, researchers have considered energetic substrate metabolism as a source of oxidative stress.
During normal neuronal activity, oxidative stress is kept relatively low Almeida et al. In ALS, however, an increased demand for energy is placed on the motor neurons. Despite this, brain and spinal cord glucose use Hatazawa et al.
Similarly, reduced lactate transport and metabolism Lee et al. It is therefore hypothesized that alternate substrates are metabolized to meet the energy requirements of the brain. Indeed, in mouse models of ALS, lipid catabolism and clearance to peripheral tissues is significantly increased Fergani et al.
Similarly, elevated levels of ketone bodies have been observed in ALS patient cerebrospinal fluid Blasco et al. With the suggestion of increased peripheral lipid availability, it is plausible that metabolic utilization of these lipids would increase.
Consequentially, studies of mouse models of ALS, as well as in ALS patients, show that markers of oxidative stress and lipid peroxidation are significantly elevated in brain and spinal cord tissue, via lipid-centric pathways Simpson et al. Hence, an increased focus on lipid metabolites as a fuel source would inevitably lead to increased oxidative stress, which would have number of deleterious outcomes Figure 6.
Figure 6. Fatty acid oxidation is a major contributor to reactive oxygen species production, which is increased in amyotrophic lateral sclerosis ALS.
Although fatty acids are not the obligate substrate for energy production in the cell, β-oxidation of fatty acids generates a substantial amount of reactive oxygen species as a by-product.
In turn, these promote a number of harmful oxidative effects including lipid peroxidation, protein oxidation, DNA damage, and apoptosis. As neurons are not effectively equipped to deal with oxidative stress, these harmful effects are multiplied, contributing to neurodegeneration.
In ALS patient muscle, peroxisome proliferator-activated receptor gamma coactivator 1-α PGC-1α , a master regulator of normal mitochondrial function and biogenesis, is downregulated, leading to modifications in fatty acid signaling, and increased β-oxidation Barroso et al. In mouse models of ALS, downregulation of PGC-1α has been shown to hasten disease progression Eschbach et al.
Survival, however, is not extended. Together, these findings highlight the essential link between fatty acid oxidation and disease, while suggesting that muscle may not be the primary target. Application of these findings to a neuronal model, therefore, would be expected to have more drastic effects, given the poorer oxidative defense capabilities of the CNS.
Interestingly, when PGC-1α is upregulated in the CNS, mitochondrial function is not only improved centrally, but motor function and survival are also drastically improved Zhao et al.
Therefore, a strong case can be made for the role of PGC-1α in maintaining CNS-driven fatty acid metabolism. In a similar fashion, the stearoyl-CoA desaturase 1 SCD-1 gene has been implicated in ALS.
SCD-1 is a key enzyme in fatty acid metabolism regulation, and directly alters the levels of β-oxidation that occur in the mitochondria Ntambi, ; Ntambi et al. In mouse models of ALS, as well as ALS patient muscle samples, SCD-1 has been shown to be downregulated Pradat et al.
While downregulation of SCD-1 may explain increased expression of β-oxidation enzymes, increased energy expenditure, and reduced fat storage in ALS mice Dupuis et al.
In mouse models of ALS, it has been shown that membrane fluidity in the brain and spinal cord decreases significantly over the course of disease Miana-Mena et al. There are a number of potential hypotheses for why this may occur. The first involves central PUFA concentrations.
The brain contains a very high concentration of PUFAs, which are stored as phosphatidylethanolamine arachidonic acid or phosphatidylserine docosahexaenoic acid in neuronal membranes Bazinet and Layé, Due to the highly unsaturated nature of these fatty acids, neuronal membranes rich in phosphatidylethanolamine and phosphatidylserine are significantly less fluid.
In ALS, docosahexaenoic acid levels are significantly increased in the brain, which may result in more rigid membranes Ilieva et al. Another theory that supports these findings involves lipid peroxidation.
PUFAs are particularly sensitive substrates in lipid peroxidation reactions. Due to the highly oxidative environment of the brain in ALS, lipid peroxidation occurs at a higher rate.
This is supported somewhat by the observation that high levels of lipid peroxidation intermediates exist in ALS patient spinal cord Shibata et al. Since a large majority of signaling lipids and proteins are found within membranes, it is possible that decreases in fluidity will decrease their mobility, impairing their function, and leading to pathological outcomes through interruptions to signaling pathways.
Due to the limited number of studies in this area, further research is needed to determine the role of lipids in cellular structure and integrity in ALS. Due to the diverse nature of lipid signaling in the brain, the potential for multifactorial pathways for lipid dysfunction is great.
PUFAs are known to bind to a number of essential metabolic transcription factors, such as LXR, which regulate lipid levels in the CNS. Specifically, LXRs modulate cholesterol levels, acting as endogenous cholesterol sensors. In ALS, disruptions to LXR signaling have been implicated in dysfunctional signaling cascades, leading to motor neuron and glial cell damage in SOD1 mice.
LXR knockout mice show neuroinflammatory responses leading to motor neuron loss, and neuromuscular junction defects Mouzat et al.
A number of PUFAs also act as pro- or anti-inflammatory signaling molecules. For example, the PUFAs eicosapentaenoic acid and arachidonic acid are oxidized to form prostaglandins or leukotrienes—essential central inflammatory molecules.
In ALS patients, elevated levels of prostaglandin E2 are observed in serum and cerebrospinal fluid Iłzecka, Furthermore, pharmacological inhibition of the prostaglandin E2 receptor Liang et al. In a broader sense, disruption in signaling can arise from more than fatty acid-centric pathways.
Concurrent with lipid peroxidation affecting membrane fluidity, peroxidation also affects the composition of lipid rafts, and excitotoxic signaling pathways Zhai et al.
Lipid rafts act as major structures for protein binding and signaling in the CNS, suggesting that significant variation in raft composition may affect signaling processes through alterations in protein association. Indeed, proteomic studies on lipid raft composition in the spinal cord of SOD1 mice show a total of 67 differentially expressed proteins, with major roles in vesicular transport, neurotransmitter synthesis and release, cytoskeletal organization and metabolism Zhai et al.
In terms of excitotoxic outcomes, lipid peroxidation produces a number of damaging by-products, such as 4-hydroxynonenal. In ALS patients, higher levels of these molecules in the spinal cord has been linked with modification of the astrocytic glutamate transporter EAAT2 Pedersen et al.
Given that astrocytic EAATs play a key role in protecting against microglial glutamate-induced neuronal death, it is possible that reduced expression of EAAT2 and glutamate excitotoxicity in ALS Rothstein et al. Interestingly, EAAT2 is a protein that is associated almost entirely with lipid rafts Butchbach et al.
Therefore, it stands to reason that changes in lipid composition, as observed in ALS, will significantly affect EAAT2 activity. A curious finding amongst mouse models of ALS, as well as ALS patients, is an increase in sphingolipids in the central nervous system, due to oxidative stress.
The initial assumption was that aberrant sphingolipid metabolism was causing a pathological upregulation of sphingolipid metabolites, leading to neurodegenerative outcomes Cutler et al. More recently, it has been shown that glycosphingolipid metabolites are significantly increased in skeletal muscle, but decreased in the CNS Dodge et al.
Since pathological outcomes are observed in both tissue types, it is possible that glycosphingolipids exert their effects in a dose dependent manner, where chronically high or low levels affect signaling fidelity, leading to pathology Dodge et al.
In light of the proposed mechanisms for dysregulation of lipid pathways in ALS, treatments targeting these pathways have generated significant interest. High levels of circulating lipids and higher body mass index positively correlate with better prognosis and longer survival in ALS Dupuis et al.
As such a number of dietary interventions have been trialed for ALS treatment. High fat diets exert a modest decrease in disease progression in a mouse model of ALS Dupuis et al. Despite a large body of data to suggest that the adoption of high-calorie or high-protein diets may be of some benefit for ALS patients Silva et al.
While druggable targets for lipid metabolism are plentiful, therapeutics to target these pathways in ALS remain largely untested. One promising therapeutic intervention so far relates to the modulation of the balance between fatty acid and glucose oxidation.
It has recently been shown that SOD1 mice exhibit a preferential switch towards fatty acid oxidation, and that a reversal of this switch to promote glucose oxidation through treatment with dichloroacetate leads to significant improvements in motor function Palamiuc et al.
While Palamiuc et al. Whether dichloroacetate improves redox status in motor neurons in ALS remains to be investigated. Another compound that has shown promise is conduritol B epoxide, a potent β-Glucocerebrosidase that modulates sphingolipid metabolism.
By increasing the levels of glucosylceramide in SOD1 mice, conduritol B epoxide not only attenuates the dysregulation of genes that are involved in pathogenic pathways, it also preserves neuromuscular junction function and rescues motor neurons from death in a mouse model of ALS Henriques et al.
In this regard, inhibition of glucosylceramide synthesis has been shown to hasten disease progression in SOD1 mice Dodge et al. Thus, neuronal and muscular glycosphingolipids serve as an exciting target for further research and therapeutic development for ALS.
The neuronal metabolism of lipids is a system of great depth, with functional outcomes ranging from energy substrate availability through to nuanced signaling pathways.
Despite this, it remains an area of many unknowns. In ALS, neuronal lipid metabolism is dysregulated in a number of ways, affecting energy use, structural integrity, and signaling processes.
In terms of energy use, neurons metabolize a greater proportion of lipid substrates, increasing oxidative stress. This leads to inflammation, mitochondrial dysfunction, metabolic dysfunction and excitotoxicity.
At a structural level, altered lipid metabolism disrupts intracellular lipid levels, leading to cytoskeletal defects, and neuromuscular junction denervation. From a signaling perspective, altered lipid metabolism affects the composition of lipid rafts, disrupting important signaling processes, leading to defects in neurotransmitter synthesis and release, cytoskeletal defects, and impaired intracellular transport Figure 7.
Figure 7. Dysregulated lipid metabolism exerts a multifaceted effect on neurons in ALS. Dysregulation of neuronal lipid metabolism in ALS impacts energy use, structural integrity and signaling processes.
Increased use of lipid as an energy substrate leads to increased oxidative stress. This exacerbates inflammation, mitochondrial dysfunction, metabolic dysfunction and excitotoxicity. Altered lipid metabolism also disrupts intracellular lipids leading to cytoskeletal defects and the denervation of neuromuscular junctions.
Finally, changes in lipid metabolism impacts the composition of lipid rafts. This disrupts signaling processes that are crucial in regulating neurotransmitter synthesis and release, cytoskeletal integrity and intracellular transport.
While recent research into the role of glycosphingolipid metabolism in ALS has opened avenues for the development of potential novel therapeutics, more studies are needed to understand the functional consequences of alterations in lipid metabolism in ALS as a whole.
This, in turn, will ultimately lead to more promising treatment opportunities, the beginnings of which are already proving to be fruitful. TJT conducted the literature search and wrote the manuscript. FJS produced all artwork. FJS, EJW and STN critically reviewed the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. TJT is supported by an Australian Postgraduate Award from the University of Queensland, and a Postgraduate top-up grant from the Motor Neurone Disease Research Institute of Australia.
Abu-Elheiga, L. Human acetyl-CoA carboxylase 2. Molecular cloning, characterization, chromosomal mapping, and evidence for two isoforms. doi: PubMed Abstract CrossRef Full Text Google Scholar.
Human acetyl-CoA carboxylase: characterization, molecular cloning, and evidence for two isoforms. U S A 92, — Agostoni, C. Fatty acids: their biochemical and functional classification.
PubMed Abstract Google Scholar. Ahmadian, M. Triacylglycerol metabolism in adipose tissue. Future Lipidol. Alexson, S. A direct comparison between peroxisomal and mitochondrial preferences for fatty-acyl β-oxidation predicts channelling of medium-chain and very-long-chain unsaturated fatty acids to peroxisomes.
Acta , 1— Ali, M. Assess the nature of cholesterol-lipid interactions through the chemical potential of cholesterol in phosphatidylcholine bilayers. U S A , — Allen, J. Lipid raft microdomains and neurotransmitter signalling. Almeida, A. Nitric oxide switches on glycolysis through the AMP protein kinase and 6-phosphofructokinase pathway.
Cell Biol. Andrus, P. Protein oxidative damage in a transgenic mouse model of familial amyotrophic lateral sclerosis. Antonsson, B. Phosphatidylinositol synthase from mammalian tissues.
Acta , — Bach, D. Bacia, K. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes.
Balaban, R. Regulation of oxidative phosphorylation in the mammalian cell. Balasse, E. Inhibition of ketogenesis by ketone bodies in fasting humans. Metabolism 24, — Barroso, E. Endocrinology , — Bastiaanse, E. The effect of membrane cholesterol content on ion transport processes in plasma membranes.
Cardiovasc Res. Basu, S. Enzymatic synthesis of ceramide-glucose and ceramide-lactose by glycosyltransferases from embryonic chicken brain.
Enzymatic synthesis of glucocerebroside by a glucosyltransferase from embryonic chicken brain. Baumann, N. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Bazinet, R. Polyunsaturated fatty acids and their metabolites in brain function and disease. Becker, I.
Bell, R. Diglyceride lipase: a pathway for arachidonate release from human platelets. U S A 76, — Benarroch, E. Lipid rafts, protein scaffolds, and neurologic disease. Neurology 69, — Berridge, M.
Inositol trisphosphate and calcium signalling mechanisms. Inositol trisphosphate, a novel second messenger in cellular signal transduction.
Nature , — PubMed Abstract CrossRef Full Text. Blasco, H. PLoS One 5:e Bloch, K. The biological synthesis of cholesterol. Science , 19— Sterol molecule: structure, biosynthesis, and function.
Steroids 57, — Bloj, B. Rat liver proteins capable of transferring phosphatidylethanolamine. Purification and transfer activity for other phospholipids and cholesterol. Blom, T. Synthesis and biosynthetic trafficking of membrane lipids.
Cold Spring Harb. Blusztajn, J. Phosphatidylcholine as a precursor of choline for acetylcholine synthesis. Neural Transm. Bogdanov, M. Increased oxidative damage to DNA in ALS patients. Free Radic. Boggs, J.
Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Braun, R. Neurotoxic kDa TAR DNA-binding protein TDP triggers mitochondrion-dependent programmed cell death in yeast.
Bressler, R. Studies on the mechanism of fatty acid synthesis. The product of the reaction and the role of sulfhydryl groups in the synthesis of fatty acids.
Bristol, L. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Brown, D. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. Browne, S. Bioenergetic abnormalities in discrete cerebral motor pathways presage spinal cord pathology in the G93A SOD1 mouse model of ALS.
Butchbach, M. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: importance for excitatory amino acid transporter localization and function.
Calder, P. Omega-3 fatty acids and inflammatory processes. Nutrients 2, — Carri, M. Oxidative stress and mitochondrial damage: importance in non-SOD1 ALS.
Castle, J. ACC2 is expressed at high levels in human white adipose and has an isoform with a novel N-terminus. PLoS One 4:e Chen, J. Alterations in mitochondrial membrane fluidity by lipid peroxidation products.
Chester, M. IUPAC-IUB Joint commission on biochemical nomenclature JCBN. Nomenclature of glycolipids—recommendations Chevaleyre, V. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability.
Neuron 38, — Chirala, S. Human fatty acid synthase: role of interdomain in the formation of catalytically active synthase dimer.
U S A 98, — Clark, B. The purification, cloning, and expression of a novel luteinizing hormone-induced mitochondrial protein in MA mouse Leydig tumor cells.
Characterization of the steroidogenic acute regulatory protein StAR. Coleman, R. Evidence that biosynthesis of phosphatidylethanolamine, phosphatidylcholine, and triacylglycerol occurs on the cytoplasmic side of microsomal vesicles. Enzymes of triacylglycerol synthesis and their regulation.
Lipid Res. Crugnola, V. Mitochondrial respiratory chain dysfunction in muscle from patients with amyotrophic lateral sclerosis. Cutler, R.
Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis.
Da Cruz, S. Elevated PGC-1α activity sustains mitochondrial biogenesis and muscle function without extending survival in a mouse model of inherited ALS. Cell Metab.
Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. de Kroon, A. Phospholipid composition of highly purified mitochondrial outer membranes of rat liver and neurospora crassa.
Is cardiolipin present in the mitochondrial outer membrane? Delpech, J. Neuropsychopharmacology 40, — Demarquoy, J. Crosstalk between mitochondria and peroxisomes. World J. de Meyer, F. Effect of cholesterol on the structure of a phospholipid bilayer.
Dennis, E. Intracellular sites of lipid synthesis and the biogenesis of mitochondria. Desport, J. Hypermetabolism in ALS: correlations with clinical and paraclinical parameters.
Di Paola, M. Interaction of free fatty acids with mitochondria: coupling, uncoupling and permeability transition. Dircks, L. Acyltransferases of de novo glycerophospholipid biosynthesis.
Dodge, J. Metabolic signatures of amyotrophic lateral sclerosis reveal insights into disease pathogenesis. Glycosphingolipids are modulators of disease pathogenesis in amyotrophic lateral sclerosis.
Dole, V. Particulate fat in lymph and blood. Dorst, J. High-caloric food supplements in the treatment of amyotrophic lateral sclerosis: a prospective interventional study. Lateral Scler. Frontotemporal Degener. Patients with elevated triglyceride and cholesterol serum levels have a prolonged survival in amyotrophic lateral sclerosis.
Duan, W. Mutant TAR DNA-binding protein induces oxidative injury in motor neuron-like cell. Neuroscience , — Dupuis, L. Dyslipidemia is a protective factor in amyotrophic lateral sclerosis. Neurology 70, — Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: benefit of a high-energy diet in a transgenic mouse model.
Eaton, S. Mammalian mitochondrial β-oxidation. Ebert, D. Energy contribution of octanoate to intact rat brain metabolism measured by 13C nuclear magnetic resonance spectroscopy.
Ege, C. Ellis, N. The influence of the character of the ration upon the composition of the body fat of hogs. The effect of food fat upon body, as shown by the separation of the individual fatty acids of the body fat. Eschbach, J. PGC-1α is a male-specific disease modifier of human and experimental amyotrophic lateral sclerosis.
Fadeel, B. The ins and outs of phospholipid asymmetry in the plasma membrane: roles in health and disease. Fagone, P. Membrane phospholipid synthesis and endoplasmic reticulum function. Fergani, A. Increased peripheral lipid clearance in an animal model of amyotrophic lateral sclerosis.
Field, F. Transport of cholesterol from the endoplasmic reticulum to the plasma membrane is constitutive in CaCo-2 cells and differs from the transport of plasma membrane cholesterol to the endoplasmic reticulum. Fredrikson, G.
Hormone-sensitive lipase and monoacylglycerol lipase are both required for complete degradation of adipocyte triacylglycerol. Fukasawa, M. Genetic evidence for ATP-dependent endoplasmic reticulum-to-Golgi apparatus trafficking of ceramide for sphingomyelin synthesis in Chinese hamster ovary cells.
Gallegos, A. Sterol carrier protein-2 expression alters plasma membrane lipid distribution and cholesterol dynamics. Biochemistry 40, — Garber, A. Hepatic ketogenesis and gluconeogenesis in humans. Gatta, A. A new family of StART domain proteins at membrane contact sites has a role in ER-PM sterol transport.
Elife 4:e Gault, C. An overview of sphingolipid metabolism: from synthesis to breakdown. Gerdeman, G. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Glaser, P.
Rapid plasmenylethanolamine-selective fusion of membrane bilayers catalyzed by an isoform of glyceraldehydephosphate dehydrogenase: discrimination between glycolytic and fusogenic roles of individual isoforms. Biochemistry 34, — Glatz, J.
Role of membrane-associated and cytoplasmic fatty acid-binding proteins in cellular fatty acid metabolism. Prostaglandins Leukot. Fatty Acids 57, — Goldberg, I. Lipoprotein lipase and lipolysis: central roles in lipoprotein metabolism and atherogenesis.
Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CDmediated pathways. Graber, R. Fatty acids and cell signal transduction.
Lipid Mediat. Cell Signal. Anaerobic muscle enzyme changes after interval training. Sharp, R. Effects of eight weeks of bicycle ergometer sprint training on human muscle buffer capacity.
Weston, A. Skeletal muscle buffering capacity and endurance performance after high-intensity interval training by well-trained cyclists. McKenna, M. Sprint training enhances ionic regulation during intense exercise in men. Gibala, M. Physiological adaptations to low-volume, high-intensity interval training in health and disease.
Lundby, C. Biology of VO 2 max: looking under the physiology lamp. Convective oxygen transport and fatigue. Holloszy, J. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences.
Chesley, A. Regulation of muscle glycogen phosphorylase activity following short-term endurance training. Leblanc, P. Effects of 7 wk of endurance training on human skeletal muscle metabolism during submaximal exercise. Determinants of endurance in well-trained cyclists.
Westgarth-Taylor, C. Metabolic and performance adaptations to interval training in endurance-trained cyclists. Seynnes, O. Early skeletal muscle hypertrophy and architectural changes in response to high-intensity resistance training.
Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Hultman, E. Muscle creatine loading in men. Influence of oral creatine supplementation of muscle torque during repeated bouts of maximal voluntary exercise in man.
Casey, A. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Vandenberghe, K. Long-term creatine intake is beneficial to muscle performance during resistance training. Hermansen, L. Muscle glycogen during prolonged severe exercise.
Ørtenblad, N. Muscle glycogen stores and fatigue. Matsui, T. Brain glycogen decreases during prolonged exercise. Diet, muscle glycogen and physical performance. Carbohydrate-loading and exercise performance: an update. Balsom, P. High-intensity exercise and muscle glycogen availability in humans.
Muscle glycogen utilization during prolonged strenuous exercise when fed carbohydrate. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. Effect of carbohydrate ingestion on exercise metabolism.
Jeukendrup, A. Carbohydrate ingestion can completely suppress endogenous glucose production during exercise. Effect of carbohydrate ingestion on glucose kinetics during exercise. Nybo, L.
CNS fatigue and prolonged exercise: effect of glucose supplementation. Snow, R. Effect of carbohydrate ingestion on ammonia metabolism during exercise in humans.
Chambers, E. Carbohydrate sensing in the human mouth: effects on exercise performance and brain activity. Costill, D. Effects of elevated plasma FFA and insulin on muscle glycogen usage during exercise.
Vukovich, M. Effect of fat emulsion infusion and fat feeding on muscle glycogen utilization during cycle exercise. Odland, L. Effects of increased fat availability on fat-carbohydrate interaction during prolonged exercise in men.
Phinney, S. The human metabolic response to chronic ketosis without caloric restriction: preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 32 , — Effect of fat adaptation and carbohydrate restoration on metabolism and performance during prolonged cycling.
Havemann, L. Fat adaptation followed by carbohydrate loading compromises high-intensity sprint performance. Decreased PDH activation and glycogenolysis during exercise following fat adaptation with carbohydrate restoration.
Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. Paoli, A. The ketogenic diet and sport: a possible marriage. Ketogenic diets for fat loss and exercise performance: benefits and safety? Helge, J.
Interaction of training and diet on metabolism and endurance during exercise in man. Yeo, W. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. Hulston, C. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists.
Kirwan, J. Carbohydrate balance in competitive runners during successive days of intense training. Cox, P. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes.
Shaw, D. Exogenous ketone supplementation and keto-adaptation for endurance performance: disentangling the effects of two distinct metabolic states. Evans, M. No benefit of ingestion of a ketone monoester supplement on km running performance.
Prins, P. Effects of an exogenous ketone supplement on five-kilometer running performance. Dearlove, D. Nutritional ketoacidosis during incremental exercise in healthy athletes. Leckey, J. Ketone diester ingestion impairs time-trial performance in professional cyclists.
Effects of caffeine ingestion on metabolism and exercise performance. Sports 10 , — Graham, T. Performance and metabolic responses to a high caffeine dose during prolonged exercise.
Caffeine ingestion and muscle metabolism during prolonged exercise in humans. Caffeine ingestion does not alter carbohydrate or fat metabolism in human skeletal muscle during exercise. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine.
Desbrow, B. The effects of different doses of caffeine on endurance cycling time trial performance. Sports Sci. Cole, K. Effect of caffeine ingestion on perception of effort and subsequent work production.
Sport Nutr. Kalmar, J. Caffeine: a valuable tool to study central fatigue in humans? Exercise and sport performance with low doses of caffeine. Suppl 2. Wickham, K. Administration of caffeine in alternate forms. Barnett, C. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling.
Stephens, F. Carbohydrate ingestion augments L-carnitine retention in humans. Wall, B. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans.
Skeletal muscle carnitine loading increases energy expenditure, modulates fuel metabolism gene networks and prevents body fat accumulation in humans.
A threshold exists for the stimulatory effect of insulin on plasma L-carnitine clearance in humans. Larsen, F. Effects of dietary nitrate on oxygen cost during exercise. Bailey, S. Dietary nitrate supplementation reduces the O 2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans.
Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. Lansley, K. Acute dietary nitrate supplementation improves cycling time trial performance. Boorsma, R. Beetroot juice supplementation does not improve performance of elite m runners.
Nyakayiru, J. No effect of acute and 6-day nitrate supplementation on VO 2 and time-trial performance in highly trained cyclists. Jones, A. Dietary nitrate and physical performance. Whitfield, J. Dietary nitrate enhances the contractile properties of human skeletal muscle.
Beetroot juice supplementation reduces whole body oxygen consumption but does not improve indices of mitochondrial efficiency in human skeletal muscle. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Ntessalen, M.
Inorganic nitrate and nitrite supplementation fails to improve skeletal muscle mitochondrial efficiency in mice and humans. Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. Sutton, J. Effect of pH on muscle glycolysis during exercise.
Wilkes, D. Effect of acute induced metabolic alkalosis on m racing time. Acid-base balance during repeated bouts of exercise: influence of HCO 3. Hollidge-Horvat, M. Effect of induced metabolic alkalosis on human skeletal muscle metabolism during exercise. Street, D. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium.
Sostaric, S. Parkhouse, W. Buffering capacity of deproteinized human vastus lateralis muscle. Derave, W. β-Alanine supplementation augments muscle carnosine content and attenuates fatigue during repeated isokinetic contraction bouts in trained sprinters.
Hill, C. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity. Amino Acids 32 , — Powers, S. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Merry, T. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training?
Petersen, A. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Ristow, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Natl Acad. USA , — Hyperthermia and fatigue.
González-Alonso, J. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans.
Fink, W. Leg muscle metabolism during exercise in the heat and cold. Febbraio, M. Muscle metabolism during exercise and heat stress in trained men: effect of acclimation. Blunting the rise in body temperature reduces muscle glycogenolysis during exercise in humans.
Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Effect of fluid ingestion on muscle metabolism during prolonged exercise. Logan-Sprenger, H.
Effects of dehydration during cycling on skeletal muscle metabolism in females. Skeletal muscle enzymes and fiber composition in male and female track athletes. Lipid metabolism in skeletal muscle of endurance-trained males and females.
Horton, T. Fuel metabolism in men and women during and after long-duration exercise. Friedlander, A. Training-induced alterations of carbohydrate metabolism in women: women respond differently from men.
Tarnopolsky, L. Gender differences in substrate for endurance exercise. Carter, S. Substrate utilization during endurance exercise in men and women after endurance training.
Roepstorff, C. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Higher skeletal muscle α2AMPK activation and lower energy charge and fat oxidation in men than in women during submaximal exercise.
Hamadeh, M. Estrogen supplementation reduces whole body leucine and carbohydrate oxidation and increases lipid oxidation in men during endurance exercise.
Hackney, A. Substrate responses to submaximal exercise in the midfollicular and midluteal phases of the menstrual cycle. Zderic, T. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases.
Devries, M. Menstrual cycle phase and sex influence muscle glycogen utilization and glucose turnover during moderate-intensity endurance exercise. Frandsen, J. Menstrual cycle phase does not affect whole body peak fat oxidation rate during a graded exercise test.
Download references. Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia. Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada.
You can also search for this author in PubMed Google Scholar. and L. conceived and prepared the original draft, revised the manuscript and prepared the figures. Correspondence to Mark Hargreaves or Lawrence L. Reprints and permissions.
Skeletal muscle energy metabolism during exercise. Nat Metab 2 , — Download citation. Received : 20 April Accepted : 25 June Published : 03 August Issue Date : September Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative.
The Journal of Physiological Sciences BMC Sports Science, Medicine and Rehabilitation Pflügers Archiv - European Journal of Physiology European Journal of Applied Physiology Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
Skip to main content Thank you for visiting nature. nature nature metabolism review articles article. Download PDF. Subjects Energy metabolism Skeletal muscle. This article has been updated. Abstract The continual supply of ATP to the fundamental cellular processes that underpin skeletal muscle contraction during exercise is essential for sports performance in events lasting seconds to several hours.
Exercise metabolism and adaptation in skeletal muscle Article 24 May Aerobic exercise intensity does not affect the anabolic signaling following resistance exercise in endurance athletes Article Open access 24 May Myofibrillar protein synthesis rates are increased in chronically exercised skeletal muscle despite decreased anabolic signaling Article Open access 09 May Main In , athletes from around the world were to gather in Tokyo for the quadrennial Olympic festival of sport, but the event has been delayed until because of the COVID pandemic.
Overview of exercise metabolism The relative contribution of the ATP-generating pathways Box 1 to energy supply during exercise is determined primarily by exercise intensity and duration.
Full size image. Regulation of exercise metabolism General considerations Because the increase in metabolic rate from rest to exercise can exceed fold, well-developed control systems ensure rapid ATP provision and the maintenance of the ATP content in muscle cells.
Box 3 Sex differences in exercise metabolism One issue in the study of the regulation of exercise metabolism in skeletal muscle is that much of the available data has been derived from studies on males.
Targeting metabolism for ergogenic benefit General considerations Sports performance is determined by many factors but is ultimately limited by the development of fatigue, such that the athletes with the greatest fatigue resistance often succeed.
Training Regular physical training is an effective strategy for enhancing fatigue resistance and exercise performance, and many of these adaptations are mediated by changes in muscle metabolism and morphology. Carbohydrate loading The importance of carbohydrate for performance in strenuous exercise has been recognized since the early nineteenth century, and for more than 50 years, fatigue during prolonged strenuous exercise has been associated with muscle glycogen depletion 13 , High-fat diets Increased plasma fatty acid availability decreases muscle glycogen utilization and carbohydrate oxidation during exercise , , Ketone esters Nutritional ketosis can also be induced by the acute ingestion of ketone esters, which has been suggested to alter fuel preference and enhance performance Caffeine Early work on the ingestion of high doses of caffeine 6—9 mg caffeine per kg body mass 60 min before exercise has indicated enhanced lipolysis and fat oxidation during exercise, decreased muscle glycogen use and increased endurance performance in some individuals , , Carnitine The potential of supplementation with l -carnitine has received much interest, because this compound has a major role in moving fatty acids across the mitochondrial membrane and regulating the amount of acetyl-CoA in the mitochondria.
Nitrate NO is an important bioactive molecule with multiple physiological roles within the body. Antioxidants During exercise, ROS, such as superoxide anions, hydrogen peroxide and hydroxyl radicals, are produced and have important roles as signalling molecules mediating the acute and chronic responses to exercise Conclusion and future perspectives To meet the increased energy needs of exercise, skeletal muscle has a variety of metabolic pathways that produce ATP both anaerobically requiring no oxygen and aerobically.
References Hawley, J. Article CAS PubMed Google Scholar Sahlin, K. Article CAS PubMed Google Scholar Medbø, J. Article PubMed Google Scholar Parolin, M. CAS PubMed Google Scholar Greenhaff, P. Article Google Scholar Medbø, J. Article PubMed Google Scholar Tesch, P.
Article CAS PubMed Google Scholar Koopman, R. Article CAS PubMed Google Scholar Hawley, J. PubMed Google Scholar Romijn, J. CAS PubMed Google Scholar van Loon, L. Article Google Scholar Bergström, J. Article PubMed Google Scholar Wahren, J. Article CAS PubMed PubMed Central Google Scholar Ahlborg, G.
Article CAS PubMed PubMed Central Google Scholar Watt, M. Article CAS Google Scholar van Loon, L. Article PubMed CAS Google Scholar Wasserman, D. Article CAS PubMed Google Scholar Coggan, A. CAS PubMed Google Scholar Coyle, E. Article CAS PubMed Google Scholar Horowitz, J.
Article CAS PubMed Google Scholar Kiens, B. Article CAS PubMed Google Scholar Stellingwerff, T. Article CAS PubMed Google Scholar Spriet, L.
Article CAS PubMed Google Scholar Brooks, G. Article CAS PubMed Google Scholar Miller, B. Article CAS Google Scholar Medbø, J. Article PubMed CAS Google Scholar Hashimoto, T.
Article CAS PubMed Google Scholar Takahashi, H. Article CAS PubMed PubMed Central Google Scholar Scheiman, J. Article CAS PubMed PubMed Central Google Scholar Rennie, M. Article CAS Google Scholar Wagenmakers, A. CAS PubMed Google Scholar Howarth, K.
Article CAS PubMed Google Scholar McKenzie, S. Article CAS PubMed Google Scholar Wilkinson, S. Article CAS Google Scholar Egan, B. Article PubMed Google Scholar Hargreaves, M. Article PubMed PubMed Central CAS Google Scholar Richter, E.
Article CAS PubMed Google Scholar Gaitanos, G. Article CAS PubMed Google Scholar Kowalchuk, J. Article CAS PubMed Google Scholar Howlett, R.
CAS PubMed Google Scholar Wojtaszewski, J. Article CAS Google Scholar Chen, Z. Article CAS PubMed Google Scholar Stephens, T. Article CAS PubMed Google Scholar Yu, M. Article CAS Google Scholar Rose, A. Article CAS Google Scholar McConell, G.
Article CAS PubMed Google Scholar Hoffman, N. Article CAS PubMed PubMed Central Google Scholar Nelson, M. Article CAS PubMed PubMed Central Google Scholar Needham, E. Article CAS PubMed Google Scholar Perry, C.
Article CAS Google Scholar Miotto, P. Article CAS PubMed Google Scholar Holloway, G. Article PubMed PubMed Central Google Scholar Watt, M. Article CAS Google Scholar Talanian, J. CAS Google Scholar Richter, E. Article CAS PubMed Google Scholar Sylow, L.
Article CAS Google Scholar Bradley, N. Article CAS PubMed Google Scholar Smith, B. Article PubMed Google Scholar Petrick, H. Article CAS PubMed Google Scholar Krustrup, P. Article CAS PubMed Google Scholar Achten, J.
Article CAS PubMed Google Scholar Harris, R. Article CAS PubMed Google Scholar Taylor, J. Article CAS PubMed PubMed Central Google Scholar Allen, D.
Article CAS PubMed Google Scholar Amann, M. Article PubMed Google Scholar Burke, L. Article CAS PubMed Google Scholar Maughan, R. Article PubMed Google Scholar Roberts, A. Article CAS PubMed Google Scholar Sharp, R.
Article CAS PubMed Google Scholar Weston, A. Article CAS PubMed Google Scholar McKenna, M. Article CAS Google Scholar Gibala, M. Article CAS Google Scholar Lundby, C. Article CAS Google Scholar Amann, M. Article PubMed Google Scholar Holloszy, J. Article CAS PubMed Google Scholar Chesley, A.
CAS PubMed Google Scholar Leblanc, P. Article CAS PubMed Google Scholar Coyle, E. Article CAS PubMed Google Scholar Westgarth-Taylor, C. Article CAS PubMed Google Scholar Seynnes, O. Article CAS Google Scholar Hultman, E. Article CAS PubMed Google Scholar Greenhaff, P. Article CAS Google Scholar Casey, A.
CAS PubMed Google Scholar Vandenberghe, K. Article CAS PubMed Google Scholar Hermansen, L. Article CAS PubMed Google Scholar Ørtenblad, N. Article PubMed PubMed Central CAS Google Scholar Matsui, T. CAS Google Scholar Bergström, J.
Article PubMed Google Scholar Hawley, J. Article CAS PubMed Google Scholar Balsom, P. Article CAS PubMed Google Scholar Hargreaves, M. Article CAS PubMed Google Scholar Jeukendrup, A. CAS PubMed Google Scholar McConell, G.
Article CAS PubMed Google Scholar Nybo, L. Article CAS PubMed Google Scholar Snow, R. Article CAS PubMed Google Scholar Chambers, E. Article CAS Google Scholar Costill, D. Article CAS PubMed Google Scholar Vukovich, M. Article CAS PubMed Google Scholar Odland, L.
CAS PubMed Google Scholar Phinney, S. Article CAS PubMed Google Scholar Burke, L. Article CAS PubMed Google Scholar Havemann, L. Article CAS Google Scholar Paoli, A. Article PubMed Google Scholar Kiens, B. Article PubMed Google Scholar Helge, J.
Article CAS Google Scholar Yeo, W. Article CAS PubMed Google Scholar Hulston, C. Article CAS PubMed Google Scholar Kirwan, J.
Article CAS PubMed Google Scholar Cox, P. Article CAS PubMed Google Scholar Shaw, D. Article PubMed Google Scholar Evans, M. Article CAS PubMed Google Scholar Prins, P. Article PubMed PubMed Central Google Scholar Dearlove, D. Article PubMed PubMed Central Google Scholar Leckey, J.
Article PubMed PubMed Central Google Scholar Costill, D. CAS PubMed Google Scholar Graham, T. Article CAS PubMed Google Scholar Graham, T. Article CAS Google Scholar Graham, T. Article CAS PubMed Google Scholar Desbrow, B. Article PubMed Google Scholar Cole, K.
Article PubMed Google Scholar Kalmar, J. Article PubMed Google Scholar Spriet, L. Article PubMed Google Scholar Wickham, K. Article PubMed PubMed Central Google Scholar Barnett, C.
Article CAS PubMed Google Scholar Stephens, F. Article CAS PubMed Google Scholar Wall, B. Article CAS Google Scholar Stephens, F. Article CAS PubMed Google Scholar Larsen, F. Article CAS Google Scholar Bailey, S. Article CAS PubMed Google Scholar Bailey, S. Article CAS PubMed Google Scholar Lansley, K.
Article CAS PubMed Google Scholar Boorsma, R. Article CAS PubMed Google Scholar Nyakayiru, J. Article CAS PubMed Google Scholar Jones, A. Article CAS PubMed Google Scholar Whitfield, J. Article PubMed Google Scholar Coggan, A. Article PubMed PubMed Central Google Scholar Whitfield, J.
Article CAS Google Scholar Larsen, F. Article CAS PubMed Google Scholar Ntessalen, M. Article PubMed Google Scholar Sahlin, K. Article CAS PubMed Google Scholar Sutton, J. Article CAS Google Scholar Wilkes, D. Article CAS PubMed Google Scholar Costill, D. Article CAS PubMed Google Scholar Hollidge-Horvat, M.
Article CAS PubMed Google Scholar Street, D. Article CAS Google Scholar Sostaric, S. Article CAS Google Scholar Parkhouse, W. Article CAS PubMed Google Scholar Derave, W. Article CAS PubMed Google Scholar Hill, C.
Article CAS PubMed Google Scholar Powers, S. Article CAS PubMed Google Scholar Merry, T. Article CAS Google Scholar McKenna, M. Article CAS Google Scholar Petersen, A. Article CAS Google Scholar Ristow, M. Article CAS PubMed PubMed Central Google Scholar Nybo, L.
Article PubMed Google Scholar González-Alonso, J. Article Google Scholar Fink, W. Article CAS PubMed Google Scholar Febbraio, M. Article CAS PubMed Google Scholar González-Alonso, J. Article CAS PubMed Google Scholar Logan-Sprenger, H. Article PubMed Google Scholar Costill, D.
Article CAS PubMed Google Scholar Horton, T. Article CAS PubMed Google Scholar Friedlander, A. Article CAS PubMed Google Scholar Tarnopolsky, L. Article CAS PubMed Google Scholar Carter, S. Article CAS PubMed Google Scholar Roepstorff, C. Article CAS Google Scholar Hamadeh, M.
Article CAS PubMed Google Scholar Hackney, A. Article CAS PubMed Google Scholar Zderic, T. Article CAS PubMed Google Scholar Devries, M. Article CAS PubMed Google Scholar Frandsen, J.
Article CAS PubMed Google Scholar Download references. Author information Authors and Affiliations Department of Physiology, University of Melbourne, Melbourne, Victoria, Australia Mark Hargreaves Department of Human Health and Nutritional Sciences, University of Guelph, Guelph, Ontario, Canada Lawrence L.
Fatty acid metabolism consists of various oxkdation processes involving or closely related to fatty pathaysHunger and nutrition programs family of molecules oxidarion within the lipid macronutrient category. Oxdation processes oxidxtion mainly be divided into Hunger and nutrition programs Pre-workout snacks Hunger and nutrition programs that generate energy and Hunger and nutrition programs anabolic Stress relief for kids where they serve pathwzys building blocks for other compounds. In catabolism, fatty acids are metabolized to produce energy, mainly in the form of adenosine triphosphate ATP. When compared to other macronutrient classes carbohydrates and proteinfatty acids yield the most ATP on an energy per gram basis, when they are completely oxidized to CO 2 and water by beta oxidation and the citric acid cycle. In anabolism, intact fatty acids are important precursors to triglycerides, phospholipids, second messengers, hormones and ketone bodies. For example, phospholipids form the phospholipid bilayers out of which all the membranes of the cell are constructed from fatty acids.Video
Metabolism - Fatty Acid Oxidation: Part 2 Balanced diet foundation performing the same function, at the adipose level, Natural vitamin options Balanced nutrition plan Natural vitamin options primarily active for oxdation opposite reasons. In the fed state, LPL on oxiddation endothelium of blood Endurance-enhancing dietary choices cleaves lipoprotein lathways into paathways acids so that they can patgways taken up into oxidatuon, for oxivation as triglycerides, or Natural vitamin options where oxidatiom are primarily used for energy production. This action of LPL on lipoproteins is shown in the two figures below. HSL is an important enzyme in adipose tissue, which is a major storage site of triglycerides in the body. HSL activity is increased by glucagon and epinephrine "fight or flight" hormoneand decreased by insulin. Thus, in hypoglycemia such as during a fast or a "fight or flight" response, triglycerides in the adipose are cleaved, releasing fatty acids into circulation that then bind with the transport protein albumin. Thus, HSL is important for mobilizing fatty acids so they can be used to produce energy.
es Gibt noch etwas Mängel