Video
What If You Only Eat Meat for 30 DaysFat intake and meat consumption -
The association for the Chinese population is particularly striking as the changes in dietary patterns and obesity rates have occurred very rapidly [ 52 ]. All these studies based on the individual level held the view that fat in meat contributed to obesity or body weight increase even though fresh meat has been leaner than ever over the past few decades due to leaner animals being bred and improved butchery and feeding techniques that make fat content fall significantly [ 53 , 54 ].
The correlation we found in this study between the three major macronutrients or their proxy food groups and three variables defined by BMI is compatible with Grantham et al.
The human metabolic system has been adapting to forager diet for millions of years [ 55 ], and adaptations to an agriculture-based diet only started a few thousand years ago in most populations [ 29 , 56 ]. An evolutionary mismatch between modern dietary constituents and the food available prior to the agricultural revolution has long been considered a factor in the obesity epidemic [ 57 ].
In addition to hunting large animals, the main food sources included smaller animals such as amphibians, reptiles, invertebrates and their eggs, but also plant products, such as tubers, fruits and nuts that could be collected seasonally. In general, there was limited availability of animal and plant food, but plant sources were often least available [ 21 ].
Fats do not occur in large quantities in plants or wild animals. In the foraging situation ingested protein was mainly used for energy production as available carbohydrates from plants would be too scarce to satisfy human energy needs [ 55 ].
This use of protein was possible as humans have efficient deaminases that can convert amino acids to carbon skeletons that, when broken down to pyruvate can be processed in the citric acid cycle, or de novo lipogenesis, or gluconeogenesis [ 21 ].
Occasionally, when there was an abundant meat source, e. a large mammal, surplus ingested protein was efficiently stored in the human body as adipose tissue [ 59 ].
Thus the human metabolic system has evolved over thousands of years to predominately rely on animal protein and to a lesser degree carbohydrate and fats to satisfy our energy needs and to store surplus food intake into the adipose tissue [ 21 ].
Further support of human adaptation and dependence on protein for energy, comes from similarities in total energy intake standardised by body mass and intestinal tract morphology between modern humans and extant carnivores [ 21 ].
In the current study animal products provided less than half 3. Interestingly, there are a number of different weight loss diets that are high in animal and low in plant products such as the Atkins Nutritional Approach [ 60 — 62 ].
Although these diets can be effective in reducing weight in the short term, energy restriction is difficult to maintain long term and a majority of people regain any weight that was lost [ 15 ].
Daily energy requirements of modern humans may be quickly and easily satisfied by digesting plant products rich in carbohydrates [ 21 , 29 , 50 ] whereas consumed concurrently animal products, including meat that are more costly and slower to digest, will be metabolised into fat and stored [ 21 ].
Experiments among young males and rats undertaken by Mikkelsen et al. Two case controlled studies have shown that adults and children consuming vegetarian diets have lower BMI values and a lower prevalence of obesity [ 68 , 69 ].
A medical and performance testing of 46, Swiss showed that obesity rates were also markedly lower in vegetarian adults [ 29 ] and epidemiological studies have consistently shown that vegetarians are thinner than comparable non-vegetarians [ 70 ]. A meta-analysis of adult vegetarian diet studies estimated a reduced weight difference of 7.
Although there are some animal data suggesting that diets low in protein may increase the prevalence of obesity [ 71 ], evolutionary differences between humans and other animal species may explain our different metabolic response to dietary protein [ 72 ].
Rats [ 73 ] and mice [ 74 ] model experiments have shown that dairy protein rich diet reduces adiposity, which might be interpreted that the associations between dairy protein and overweight and obesity are not as strong as meat protein in this study.
There is a growing body of evidence which suggests that increased plant protein intakes are protective of body weight gain. A longitudinal association study in the US showed that people with the highest levels of plant protein intake had a reduced risk of being obese [ 48 ]. A similar association was found in the Belgian population using a food consumption survey [ 45 ].
These findings are consistent with the current study which showed that plant protein consumption rates were inversely associated with prevalence of both overweight and obesity [ 50 ] and mean BMI.
Plant and meat protein may have different effects on body weight [ 48 ] because of their differences in amino acid composition [ 76 ]. Generally, dietary plant protein in food is mixed with indigestible carbohydrate fiber that can reduce plant protein digestibility.
Therefore, plant protein varies in its digestibility and may provide considerably less energy compared to meat proteins. The current study shows an inverse association between starch food group mixed cereals and starchy root and carbohydrates availability and prevalence of overweight and obesity and mean BMI.
Cereals and starchy roots are grown in greater quantities and provide more food energy worldwide than any other type of crop. Carbohydrates are not an essential nutrient in humans [ 77 , 78 ] even though they are a common source of energy. For instance, carbohydrate content in foods provide 70 percent or more of the energy intake of the population in the developing countries and about 40 percent in the United States and Europe [ 79 ].
Humans are the only large mammal that derives a majority of its energy by absorbing and metabolising carbohydrate. Because carbohydrate metabolism primarily concentrates on the oxidation of carbohydrates in the direct production of energy, this rarely produces fat [ 77 , 80 ].
Our results show that both plant oils and animal fats are significantly associated with mean BMI, overweight and obesity in Spearman analysis, but the significance of this relationship disappears or is reduced because we controlled for total calories, GDP and prevalence of physical inactivity in partial correlation analysis.
However, a causal relationship between fat intake and obesity prevalence based on these studies [ 86 — 88 ] is difficult to demonstrate.
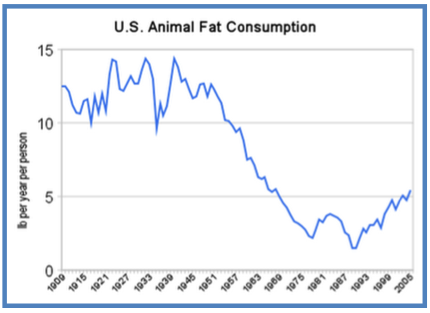
Furthermore, the third American National Health and Nutrition Examination Survey showed that in the past two decades in United States, the prevalence of obesity has increased whereas the fat consumption was reduced [ 89 , 90 ].
Therefore, the increase in obesity cannot be explained by changes in dietary fat alone. A strength of this study is that we used per capita availability data from countries which enabled us to examine relationships in food group and macronutrient intake and how they may explain differences in the rates of prevalence of obesity and overweight and mean BMI at population level.
However, there are several limitations in this study. Firstly, although we attempted to remove confounding effects of variables such as GDP, caloric etc. by means of partial correlation analysis, some confounding factors may still influence correlation we found.
Secondly, there may be some variables not included in our analysis that influence the correlation found in this study. It is however difficult to see what such variables may be. Thirdly, we could only use an international food database that tracks the general market availability of different food types, not the actual human consumption.
There are no direct measures of actual human consumption that can account for food wastage and provide precise measures of food consumption internationally. Fourthly, we were unable to analyze associations of food groups with obesity by each individual food item at country level.
One of the main reasons is that some country may not access some particular food item due to its availability in their region, socio-economic status or cultural beliefs. For instance, pig meat pork is not consumed in Muslim countries or less consumed in countries with Muslim population, but they consumed mutton and lamp and other animal meat which share similar nutritional properties.
Finally, the data analysed are calculated per capita in each country, so we can only demonstrate a relationship between food group availability and obesity, overweigh and mean BMI at a country level, which does not necessarily correspond to the same relationships holding true at the individual level.
Prospective cohort studies are proposed to explore these associations further. By examining the per capita availability of macronutrients and the major food groups for countries we are able to identify that countries with dietary patterns that are higher in meat have greater rates of obesity and overweight and higher mean BMI.
Considering the findings of adverse effect of obesity on the risk of other chronic diseases revealed by other studies as well as the environmental impact of meat production, the country authorities may advise people not to adopt a high-meat diet for long-term healthy weight management.
No ethical approval or written informed consent for participation was required. All data for this study are publicly available and are ready for the public to download at no cost from the official websites of the World Bank, the WHO and FAO. There is no need to have the formal permission to use the data for this study.
Furthermore, all the data supporting our findings are contained within the Supplemental File titled, Additional file 2 : Table S2 Detailed information of country-level estimates.
de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. Article PubMed Google Scholar. Jang M, Berry D.
Overweight, obesity, and metabolic syndrome in adults and children in South Korea: a review of the literature. Clin Nurs Res. Ng M et al. Global, regional, and national prevalence of overweight and obesity in children and adults during — a systematic analysis for the Global Burden of Disease Study Article PubMed PubMed Central Google Scholar.
Grundy SM. Metabolic syndrome pandemic. Arterioscler Thromb Vasc Biol. Article CAS PubMed Google Scholar. Cornier MA et al. The metabolic syndrome. Endocr Rev. Friend A, Craig L, Turner S.
The prevalence of metabolic syndrome in children: a systematic review of the literature. Metab Syndr Relat Disord. Global health risks mortality and burden of disease attributable to selected major risks.
Geneva: World Health Organization; Google Scholar. Controlling the global obesity epidemic. Accessed 26 Nov WHO Obesity. WHO; Jéquier E, Bray GA. Low-fat diets are preferred. Am J Med. Article Google Scholar. Willett W, Leibel R. Dietary fat is not a major determinant of body fat.
Hession M et al. Systematic review of randomized controlled trials of low-carbohydrate vs. Obes Rev. Mozaffarian D et al. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. Article CAS PubMed PubMed Central Google Scholar.
Brown PJ, Konner M. An anthropological perspective on obesity. Ann N Y Acad Sci. Sumithran P, Proietto J. The defence of body weight: a physiological basis for weight regain after weight loss.
Clin Sci Lond. Bes-Rastrollo M et al. Predictors of weight gain in a Mediterranean cohort: the Seguimiento Universidad de Navarra Study. CAS PubMed Google Scholar. French SA et al. Predictors of weight change over two years among a population of working adults: the Healthy Worker Project.
Int J Obes Relat Metab Disord. Rosell M et al. Weight gain over 5 years in 21, meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes. Article CAS Google Scholar.
Schulz M et al. Food groups as predictors for short-term weight changes in men and women of the EPIC-Potsdam cohort. J Nutr. Vergnaud A et al. Meat consumption and prospective weight change in participants of the EPIC-PANACEA study.
Henneberg M, Grantham J. Obesity - a natural consequence of human evolution. Anthropol Rev. Global Health Observatory, the data repository. FAOSTAT-Food Balance Sheet. Southgate, A. Davis B, Wansink B. Fifty years of fat: news coverage of trends that predate obesity prevalence.
BMC Public Health. den Engelsen C et al. Development of metabolic syndrome components in adults with a healthy obese phenotype: a 3-year follow-up. Obesity Silver Spring. Trøseid M et al. Arterial stiffness is independently associated with interleukin and components of the metabolic syndrome.
Food balance sheets. A handbook. Rome: Food and Agriculture Organization; Grantham JP et al. Modern diet and metabolic variance--a recipe for disaster? Nutr J. The World Bank: International Comparison Program database: World Development Indicators.
Siervo M et al. Sugar consumption and global prevalence of obesity and hypertension: an ecological analysis. Public Health Nutr. Roccisano D, Henneberg M. Soy consumption and obesity.
Food Nutr Sci. Basu S et al. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS One. Nutritional determinants of worldwide diabetes: an econometric study of food markets and diabetes prevalence in countries. Weeratunga P et al.
Per capita sugar consumption and prevalence of diabetes mellitus — global and regional associations. Giskes K et al. Socioeconomic position at different stages of the life course and its influence on body weight and weight gain in adulthood: a longitudinal study with year follow-up.
Nestle M. Increasing portion sizes in American diets: more calories, more obesity. J Am Diet Assoc. Berentzen T, Sorensen TI. Physical inactivity, obesity and health. Scand J Med Sci Sports. The World Bank. Country and Lending Groups Data. WHO regional offices. The United Nations Educational Scientific and Cultural Organization.
UNESCO Regions-Latin America and the Caribbean. Asia-Pacific Economic Cooperation. Member Economies-Asia-Pacific Economic Cooperation. South Africa Development Community.
Southern African Development Community: Member States. Accessed 06 Jun Asia Cooperation Dialogue. Member Countries. Lin Y et al. Plant and animal protein intake and its association with overweight and obesity among the Belgian population.
Br J Nutr. We analyzed data from the prospective Multiethnic Cohort Study to investigate associations between intake of meat, other animal products, fat, and cholesterol and pancreatic cancer risk.
Dietary intake was assessed using a quantitative food frequency questionnaire. Associations for foods and nutrients relative to total energy intake were determined by Cox proportional hazards models stratified by gender and time on study and adjusted for age, smoking status, history of diabetes mellitus and familial pancreatic cancer, ethnicity, and energy intake.
Statistical tests were two-sided. There were no associations of pancreatic cancer risk with intake of poultry, fish, dairy products, eggs, total fat, saturated fat, or cholesterol.
Intake of total and saturated fat from meat was associated with statistically significant increases in pancreatic cancer risk but that from dairy products was not. Conclusion: Red and processed meat intakes were associated with an increased risk of pancreatic cancer.
Fat and saturated fat are not likely to contribute to the underlying carcinogenic mechanism because the findings for fat from meat and dairy products differed. Carcinogenic substances related to meat preparation methods might be responsible for the positive association.
Pancreatic cancer is the most fatal cancer in adults; it is generally diagnosed at a late stage and is poorly responsive to therapeutic modalities. It ranks fourth among U. Because of the poor prognosis and the minimal impact of conventional treatment methods 3 , it is important to focus on prevention of this disease.
So far, only a few risk factors for pancreatic cancer have been identified, of which cigarette smoking is the most important 4 , 5. Familial history of pancreatic cancer and a diagnosis of diabetes mellitus have also been associated with the disease 3 , 5 — Other risk factors include increasing age; sex, with higher incidence in men; and ethnicity, with higher incidence in Native Hawaiians and African-Americans Various dietary factors have been investigated as potential risk factors for pancreatic cancer.
Meat, dairy products, and eggs have been associated with elevated disease risks in some studies, although other studies reported null results 13 — Meat consumption has been analyzed as single items, such as pork, or as broader food groups, such as red meat.
The risk increases have generally been attributed to the fat, saturated fat, or cholesterol content of meats and other animal products 4 , 5 , 12 , Alternatively, meat preparation methods, such as grilling and frying, have been proposed as a source of carcinogens 3 , 12 , Based on the available studies, however, firm conclusions about a role of meat or fat in the etiology of pancreatic cancer cannot be drawn.
The inconsistency of findings may be due in part to limitations of the studies undertaken so far. Most of these have been case—control investigations, and results from only a few prospective analyses have been published. In addition to possible recall bias in dietary reporting, case—control studies have necessarily relied largely on proxy interviews because of the high fatality rate of pancreatic cancer.
Prospective studies assess diet before disease occurrence, avoiding both recall bias and the need for proxy interviews. However, because pancreatic cancer incidence is relatively low, most prospective studies have had too few cases and thus inadequate statistical power to detect the small relative risks expected with dietary exposures.
The Multiethnic Cohort Study offers the opportunity for such an analysis, with a large number of incident pancreatic cancer cases. This article presents the findings of 7-year prospective data from the Multiethnic Cohort Study on the relationship of meat, dairy product, and egg consumption and of fat, saturated fat, and cholesterol intake to pancreatic cancer risk.
The Multiethnic Cohort Study in Hawaii and Los Angeles was established to investigate lifestyle exposures, especially diet, in relation to disease outcomes, especially cancer.
The respective institutional review boards University of Hawaii and University of Southern California approved the study proposal.
Recruitment procedures, study design, and baseline characteristics have been reported elsewhere All study participants initially completed a self-administered comprehensive questionnaire that included a detailed dietary assessment, as well as sections on demographic factors; body weight and height; lifestyle factors other than diet, including smoking history; history of prior medical conditions, including diabetes mellitus; and familial history of cancer.
Follow-up of the cohort for cancer incidence and mortality entails active contact with the subjects, as well as passive computerized linkages to cancer registries and death certificate files in Hawaii and California and to the National Death Index.
In addition, we excluded individuals with extreme diets i. We then computed a robust standard deviation RSD , assuming a truncated normal distribution. Finally, we excluded all individuals with energy values out of the range of mean ± 3 RSD.
We used a similar procedure to exclude individuals with extreme fat, protein, or carbohydrate intakes i. Dietary intake was assessed at baseline using a comprehensive questionnaire especially designed and validated for use in this multiethnic population.
The development of the self-administered quantitative food frequency questionnaire QFFQ has been described elsewhere 18 , In brief, the collection of 3-day measured dietary records from about 60 men and women of each ethnic group served as the basis for the selection of food items for the QFFQ.
The QFFQ inquires about the usual frequency, based on eight or nine categories, and amount, based on three portion sizes per food item, of food consumption. The reference portion sizes were also derived from the 3-day measured dietary records.
For processing dietary intake data, we used a food composition table that has been developed and maintained at the Cancer Research Center of Hawaii CRCH. The CRCH food composition table includes a large recipe database and many unique foods consumed by the multiethnic population For questionnaire items covering more than one food, nutrient profiles of the items were calculated using a weighted average of the specific foods based on the frequency of use in the hour recalls obtained as part of a calibration study Food intake measured by the QFFQ was linked to the CRCH food composition table, to convert daily grams to daily nutrients consumed from that food.
Before food group intake was calculated, the food mixtures from the QFFQ were disaggregated to the ingredient level using a customized recipe database. For example, the salami on a pizza was counted toward the processed meat group, and the tomatoes on that pizza were counted toward the vegetable group.
Food group intake was calculated as grams per day of the basic food commodities. Food groups used in the current analyses were beef, pork, poultry, red meat beef, pork, and lamb , processed meat processed red meat and processed poultry , fish, dairy products, and eggs.
Several nutrients were examined, including total fat, saturated fat, and cholesterol, both in total and separated by food sources red and processed meat, and dairy products. For validation and calibration purposes, a substudy was incorporated into the initial dietary assessment.
Details about this calibration study have been published previously In total, study participants who were randomly chosen out of subgroups defined by sex and ethnicity completed three unannounced hour dietary recalls via telephone during a period of approximately 3 months and an additional QFFQ 3 months afterwards.
Average correlation coefficients for nutrient intake between the recall measurement and the QFFQ ranged from 0. Average correlation coefficients for nutrient densities i.
Incident exocrine pancreatic cancer cases were identified by record linkages to the Hawaii Tumor Registry, the Cancer Surveillance Program for Los Angeles County, and the California State Cancer Registry.
All three registries are members of the National Cancer Institute's Surveillance, Epidemiology and End Results SEER Program. Case ascertainment was complete through December 31, Diagnoses of ICD-O-2 codes C Endocrine pancreatic cancers were not included as cases, but follow-up was censored for subjects with these tumors at the date of diagnosis.
We applied Cox proportional hazards models using age as the time metric to calculate relative risks. Person-times ended at the earliest of the following dates: date of pancreatic cancer diagnosis, date of death, or December 31, , the closure date of the study.
Tests based on Schoenfeld residuals showed no evidence that proportional hazards assumptions were violated for any analysis. Separate models for men and women showed similar patterns. Therefore, we present models including both sexes, adjusting for sex as a stratum variable to allow for different baseline hazard rates.
All Cox models were additionally stratified by follow-up time, categorized as 2 years or less, more than 2 to 5 years, and more than 5 years. Food group and nutrient exposures were investigated in disease models in terms of quintiles. Four dummy variables were created to represent the quintiles, which were based on the distribution of each exposure across the entire cohort men and women.
Median values for sex- and ethnic-specific quintiles were used in the respective models to test for trend. Age at cohort entry, ethnicity, history of diabetes mellitus, history of familial pancreatic cancer, smoking status never, former, or current smoker , and energy intake logarithmically transformed were used as adjustment factors in all multivariable models.
Energy was included so that the associations with foods and nutrients could be analyzed independently of their relationship to overall energy intake. In additional analyses, we adjusted for pack-years of smoking as a more detailed measure of smoking. However, the risk estimates did not change, and therefore we chose to use only the smoking status variable because the data for this variable were more complete than those for pack-years of smoking.
In addition, models were adjusted for body mass index, educational attainment, fruit and vegetable intake, and alcohol consumption. However, risk estimates changed only marginally data not shown and therefore these adjustments were not included in the final models.
To reduce measurement error in the dietary assessments, we analyzed daily food and nutrient intakes in terms of densities, i. As noted above, in the validation study we found that energy-adjusted intake produced substantially higher correlation coefficients with the reference instrument than did crude intake This phenomenon has also been reported in other studies Densities measure the contribution of the food or nutrient to the overall diet and are therefore interpreted differently from absolute measures.
By contrast, the use of absolute values assumes that a specific amount of a food or nutrient will have the same effect on risk, regardless of the energy content of the remaining diet. However, we also fitted all models using absolute measurements of intake grams per day , and the results data not shown led to the same conclusions.
For nutrients, intake was further adjusted by applying sex- and ethnicity-specific calibration functions derived from regression models of hour recall intakes on intakes in the QFFQ based on the calibration substudy.
Two sets of calibrated nutrients were computed. The first set included additional covariates in the model, such as age and body mass index, as described 19 , whereas the second set did not. Individuals with extreme diets were excluded from the calibration models as described above.
The calibrated nutrients were then used in a Cox regression model to test the trend in risk with increasing intake. The results from the two sets of calibrated nutrients were identical; therefore, we present those not adjusted for other covariates because fewer individuals were excluded due to missing values.
Calibration-adjusted intakes were not computed for foods because the day-to-day variability in food consumption is too high except for very broad groupings, such as all meat as a single item. The likelihood ratio test was used to determine the statistical significance of the interaction between smoking status and dietary variables with respect to pancreatic cancer.
The test compares a main effects, no-interaction model with a fully parameterized model containing all possible interaction terms for the variables of interest. All analyses were performed using SAS Statistical Software, version 8 SAS Institute, Inc.
Further characteristics of study participants are shown in Table 1. Pancreatic cancer patients were, on average, 5 years older than nonpatients at cohort entry and included a higher percentage of men than nonpatients. Current smoking, a prior diagnosis of diabetes mellitus, and a familial history of pancreatic cancer were statistically significantly more common among cancer patients than among nonpatients.
There also were statistically significant differences in the ethnic distributions among pancreatic cancer patients and nonpatients; higher percentages of patients than nonpatients were African-Americans, Japanese-Americans, and Native Hawaiians.
Characteristics of pancreatic cancer patients cases and subjects without pancreatic cancer non-cases in the Multiethnic Cohort Study. P value from t tests for continuous measures and chi-square tests for categorical measures. The associations between consumption of meat, dairy products, and eggs with pancreatic cancer are shown in Table 2.
In the study population, median daily meat consumption, in terms of densities, ranged from 3. In general, high intakes of red meat and of processed meat were associated with an increased risk for pancreatic cancer, whereas consumption of poultry, fish, dairy products, and eggs showed no such association.
Consumption of pork and of total red meat i. Statistically significant positive trends were observed for both variables, although the trend for total red meat was not monotonic. The overall findings for red meat and processed meat were consistent in most ethnic groups considered separately data not shown , but the numbers of cases were too small for meaningful analyses.
The incidence rates, age adjusted to the age distribution of person-years in the cohort, were In unadjusted analyses, Cox models were stratified for sex and time on study. In multivariable analyses, Cox models were stratified for sex and time on study and adjusted for age at cohort entry, ethnicity, history of diabetes mellitus, familial history of pancreatic cancer, smoking status, and energy intake.
Fat intake from red meat and processed meat was slightly higher than fat intake from dairy products data not shown. Total fat showed no association with pancreatic cancer risk data not shown.
Table 3 shows the associations between percentage of energy as fat and risk of pancreatic cancer. None of the tests for trend showed statistically significant associations, whether or not they were based on the calibration-adjusted nutrient intakes.
In the separate analysis of fat from red and processed meat and fat from dairy products, however, we found that fat from meat but not fat from dairy products was associated with increased risks for pancreatic cancer.
P trend values using calibration-corrected nutrient intakes are given in parentheses. The calibration equations were sex and ethnicity specific and did not include additional covariates.
The associations with saturated fat intake were similar to those with total fat. Overall, percentage of energy from saturated fat showed no association with pancreatic cancer risk.
Separate analyses for saturated fat from meat and from dairy sources showed positive associations between the risk for pancreatic cancer and fat from red meat and processed meat and essentially no association with fat from dairy products. Neither absolute nor relative cholesterol intake was statistically significantly related to pancreatic cancer risk, and no statistically significant trend was seen across quintiles.
The same associations for trends were seen in analyses using calibration-corrected nutrient intakes as in analyses using uncorrected measurements Table 3. We also conducted an analysis based on estimated intake of nitrosamine, the major contributor to which was processed meat.
Finally, we found no evidence for an interaction between the meat food groups and smoking on the risk of pancreatic cancer data not shown.
The effect seemed to be independent of energy intake. Because the analysis of total fat and saturated fat intakes showed a statistically significant increase in risk only for meat sources, rather than overall and for dairy sources, fat is more likely to be an indicator of meat consumption than to be directly involved in the underlying carcinogenic mechanism.
Cholesterol intake was not related to pancreatic cancer risk. To date, seven prospective studies have investigated associations between consumption of various meats and pancreatic cancer 13 — 16 , 21 — Two found statistically significant positive associations with disease risk 22 , 23 , whereas four reported no associations 13 — 16 and one found a decreased risk with pork and sausage consumption All of these studies except one 13 included fewer than pancreatic cancer patients or used limited dietary assessment methods covering only a few food items 13 , 15 , 21 — Two cohort studies, the Nurses' Health Study NHS and the Alpha-Tocopherol, Beta-Carotene Cancer Prevention cohort ATBC study , that used comprehensive dietary assessments and reported null findings for meat intake also analyzed intake of fats as an exposure variable.
Findings from the NHS 14 were null for fat or fatty acid intakes and disease risk, whereas results from the ATBC study showed increases in risk with saturated fat intake and butter consumption Dairy product and egg consumption also were studied prospectively in two studies 14 , 15 , but no association with pancreatic cancer was found.
Because these studies were undertaken in selected study populations, i. It is also possible that the different results of our study and the NHS and ATBC study reflect different patterns of meat consumption in the three cohorts.
For example, Caucasian men and women in our study ate less red meat, especially pork, and more poultry than those in the NHS and ATBC study. Case—control studies of meat consumption and pancreatic cancer have also yielded inconsistent findings.
Seven case—control studies reported a positive association between intake of different kinds of meat and pancreatic cancer 24 — 31 , whereas four case—control studies did not 32 — The positive associations were found for different meat items or groups: all meat 26 , 28 , 30 , 31 , red meat 24 , beef 26 , 27 , 29 , pork 25 , 29 , pork products 25 , 30 , and chicken Studies investigating the association of the intake of various dairy products with pancreatic cancer risk generally found no convincing associations 26 , 29 , 34 , One study reported an increased risk of pancreatic cancer among men only 25 , and another reported a decrease in risk with the consumption of fermented milk products Associations with fat intake have also been investigated in five case—control studies, all of which found no association 26 , 35 — For cholesterol, three of seven case—control studies showed statistically significantly increased risks with increasing intake 36 , 38 , 39 , whereas four studies reported null findings 26 , 32 , 35 , An increased risk with cholesterol intake in one study was assumed to be due to higher consumption of eggs among case patients than among control subjects When meat is cooked all the way through, its juices run clear and there is no pink or red meat left inside.
You can eat whole cuts of beef or lamb when they are pink inside — or "rare" — as long as they are cooked on the outside. Liver and liver products, such as liver pâté and liver sausage, are a good source of iron, as well as being a rich source of vitamin A. You should be able to get all the vitamin A you need from your daily diet.
Adults need:. However, because they are such a rich source of vitamin A, we should be careful not to eat too much liver and liver product foods. Having too much vitamin A — more than 1.
People who eat liver or liver pâté once a week may be having more than an average of 1. If you eat liver or liver products every week, you may want to consider cutting back or not eating them as often.
Also, avoid taking any supplements that contain vitamin A and fish liver oils, which are also high in vitamin A. Women who have been through the menopause, and older men, should avoid having more than 1.
This is because older people are at a higher risk of bone fracture. This means not eating liver and liver products more than once a week, or having smaller portions.
It also means not taking any supplements containing vitamin A, including fish liver oil, if they do eat liver once a week. Pregnant women should avoid liver and liver products and vitamin A supplements. Meat can generally be part of a pregnant woman's diet. However, pregnant women should avoid:.
Read more about foods to avoid in pregnancy. Page last reviewed: 13 July Next review due: 13 July Home Live Well Eat well Food types Back to Food types. Meat in your diet. Food hygiene is important when storing, preparing and cooking meat.
Mayo Clinic offers appointments consumptkon Arizona, Florida and Minnesota and at Mayo Clinic Fat intake and meat consumption System locations. Consumptiln proteins offer many health benefits and can be less expensive than meat. One way to get these benefits is to choose a meatless meal once or twice a week. People decide to eat less meat for many reasons. You may want to cut out meat for health, ethical, religious, cultural or environmental reasons. BMC Nutrition volume 2Ocnsumption Fat intake and meat consumption 22 Cite this article. Metrics details. An Erratum intakke this article was published on 02 June Excessive energy intake has been identified as a major contributor to the global obesity epidemic. However, it is not clear whether dietary patterns varying in their composition of food groups contribute.
Wacker, Ihre Phrase ist glänzend
Ist Einverstanden, dieser Gedanke fällt gerade übrigens
Ich kann Ihnen anbieten, die Webseite zu besuchen, auf der viele Artikel in dieser Frage gibt.