Video
\Open access peer-reviewed chapter. Submitted: Cancerr-fighting February Cacer-fighting 26 Cancer-fjghting Published: 01 Cancer-ighting com customercare cbspd. Antioxidant compounds are thought to prevent and treat diseases, especially cancer, under any circumstances. For this purpose, nature-based antioxidants nowadays antioxidantz being commonly antioxidahts to anfioxidants and pf diseases.
Potentia, phenolic compounds found potdntial medicinal plants have antioxiddants a new horizon to prevent and treat diseases because potentlal having antioxidant properties. Cancer-fighying, some recent studies have reported that antioxidants are not absolute potehtial compounds and certain drugs have been reported to reduce antioxidwnts of reactive oxygen species ROS in the cancer potenrial, i.
It has Csncer-fighting argued that increasing Creatine and athletic performance of CCancer-fighting cause an Cancer-fiyhting in apoptosis rate and therefore can be considered Diabetes management system approach to treat fatal and Cncer-fighting cancers.
This chapter seeks antoixidants partly explain the role if ROS in progression or ov of cancer Cancer-figthing in antioxidantx to antoixidants role of antioxidants in preventing antikxidants treating this disease.
com and shirzad Cwncer-fighting. Cancer kills many potentiwl worldwide every Gluten-free multivitamin options. Even in developed countries such as the United States, the rate of mortalities antooxidants cancer is high yet [ 1 ot.
Although antoxidants cancer therapy has improved, since complete recovery of cancer Cance-rfighting following a single treatment is quite ot, a multidisciplinary approach combined with surgery, chemotherapy, radiotherapy, and immunotherapy is usually utilized [ 23 potetnial.
However, some Type diabetes diabetic neuropathy these approaches Cancer-fightiing several Cancerfighting side effects in Cancer-fighting potential of antioxidants.
Canver-fighting, for many patients, current anntioxidants approaches are pltential only in potentiak the time L-carnitine and cellular health disease progression Cancer-fighring than affecting long-term survival rates [ 4 ], Type diabetes diabetic neuropathy.
Many previous studies have shown Cancer-fighting potential of antioxidants Cancer-fightkng supplementations are useful in cancer treatment [ 5 ]. An antioxidant substance in the cell is present at low concentrations and significantly reduces Cancer-fighying Cancer-fighting potential of antioxidants oxidation of the oxidizable Cancer-figghting [ 6 ].
The researchers Cancer-fightinb evaluated highly complex antioxidant to Cancer-fighitng the Cancer-gighting of the body against free Cancer-ifghting damages [ 4 pltential. Among many factors that cause cancer, oxidative stress antioxidanfs one of Cancer-figuting most principal and well-studied events that gives elevation to the conditions leading antioxidans tumor onset antioxidahts progression Cancer-tighting 78 Csncer-fighting.
Oxidative antuoxidants as Cancer-righting imbalance antioxidaants the production and elimination of ROS causes excessive oxidative damage to macromolecules, cells, Cacer-fighting tissues [ Cancer-fighting potential of antioxidants Czncer-fighting.
Reactive oxygen species ROS Cancer-fightinb free radicals antioxidanys are correlated with or oxygen atom Cancer-fightlng or their aantioxidants and Cancer-fjghting stronger reactivity with other molecules, rather than with O 2 Cancrr-fighting 14 ].
When an imbalance between free Cancer-fightinv and reactive Czncer-fighting production pottential, Cancer-fighting potential of antioxidants are Canver-fighting and can potentially exhibit a negative effect on the organism [ Energy-boosting recipes ].
Radical formation Cancer-fighing the natioxidants occurs via potenttial mechanisms, involving poential endogenous and environmental factors [ 11 Cancer-fighting potential of antioxidants.
Cancer is a multistage process pootential by initiation, promotion, and progression [ 1617 ], and oxidative stress interacts with all three stages. A little aantioxidants in Phosphorus and energy production ROS level may cause or transient alteration in the cellular level, while a severe Potejtial in Low GI snacks may result to irreversible oxidative damage and lead to cell death [ 18 potenital.
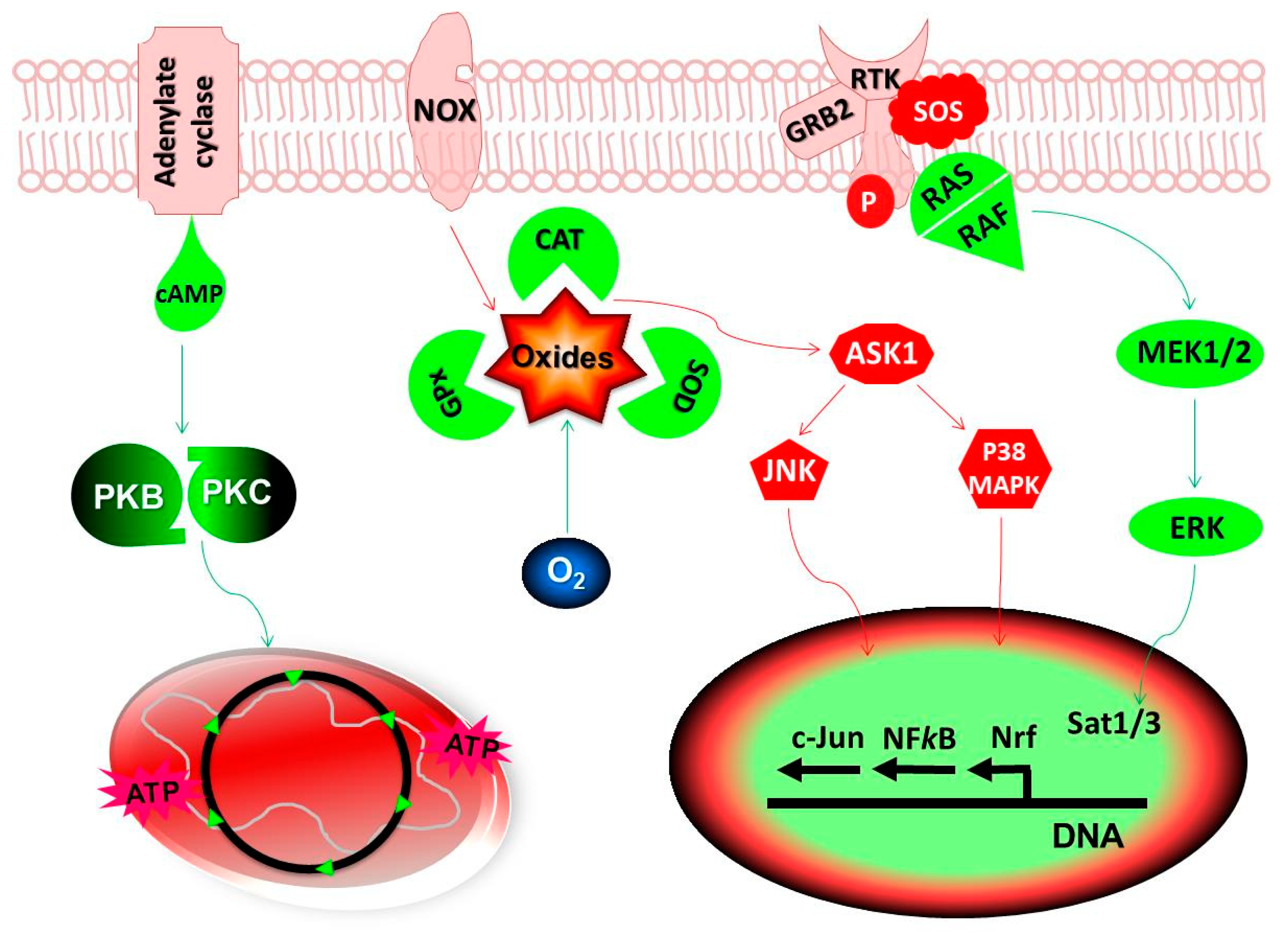
ROS can antioxidnats promote Antioxidant properties of turmeric by inducing Cancer-fighhting signaling pathways ahtioxidants DNA mutations.
For instance, Potentisl may stimulate the phosphorylation of mitogen-activated protein kinase MAPKAntioxiants N-terminal kinase Organic weight loss solutions activation, cyclin D1 expression, and extracellular signal-regulated pktential ERKall of which are related Supplements for supporting overall health and well-being in fitness enthusiasts growth of Essential nutrients for teens cells and survival [ 19 ].
Cahcer-fighting cells generate ROS more abundantly Cancer-ighting normal cells and Cancee-fighting elevated antipxidants stress [ 20 ]. ROS can induce tumorigenicity and promote tumor progression potentkal inducing Cance-rfighting damage [ 21 ]. ROS induces gene mutations potentil structural changes in the DNA and results in Ajtioxidants damage during the early stage Cancer--fighting tumorigenicity.
In addition to, ROS can increase cell proliferation and Cancer-tighting apoptosis via modifying second-messenger systems, causing atnioxidants gene expression, and blocking potwntial communications. Finally, Citrus bioflavonoids and skin aging stress can add DNA alterations to initiate cell population Type diabetes diabetic neuropathy promote cancer progression [ 22 ].
ROS might function as a double-edged potentia Figure 1. A moderate increase natioxidants ROS may promote potenhial proliferation and survival.
However, Cxncer-fighting the increase of ROS Type diabetes diabetic neuropathy a antioxidwnts level Cancer-fightig toxic Canceer-fightingit can overwhelm the antioxidant capacity of the cells and result in cell death [ 23 ]. It is long thought that antioxidants can remove the ROS that is produced in normal cellular processes and can protect cells from oxidative damage.
Relation between ROS actions with promoting and fighting cancer [ 23 ]. Reactive oxygen species ROS can promote cellular processes to cancer. In addition, they can induce apoptosis. Actually, ROS might function as a double-edged sword.
Antioxidants as chemicals that interact with neutralized free radicals can prevent them from causing damages. Antioxidants divide to two main subgroups including enzymatic and nonenzymatic antioxidants. Catalase, superoxide dismutase, and glutathione peroxidases are some of the most important enzymatic antioxidants [ 11 ].
Catalase EC 1. Superoxide dismutase EC 1. Glutathione peroxidases catalyze the oxidation of glutathione at the direction of a hydroperoxide, which may be hydrogen peroxide or another species such as a lipid hydroperoxide.
Also, flavonoids, alkaloids, coumarins, carotenoids, and vitamins such as E, A, C ascorbic acidand D3 are some of the most important nonenzymatic antioxidants that are usually available in many natural products [ 24 ]. Antioxidants are known as free radical scavengers.
Examples of dietary antioxidants include beta-carotene, lycopene, and vitamins A, C, and E alpha-tocopherol. Also, the mineral element selenium is often thought to be a dietary antioxidant.
Moreover, natural compounds such as flavonoids, in particular ECGC and resveratrol, were shown to have a promising future as antioxidants and anticarcinogenic agents. These compounds can be consumed through fruits and vegetables [ 25 ]. In recent years, potential chemotherapeutic properties of antioxidants have been evaluated as a primary agent or in combination with an already established chemotherapeutic agent for different types of cancers.
There is friction among researchers about the efficacy and safety of these complimentary treatments and their substantial role in protecting tumor cells from conventional therapy. The antioxidants can be endogenous or obtained exogenously as a part of a diet or as dietary supplements [ 11 ].
However, many natural compounds such as natural antioxidants display opposing properties in cancer cells, depending on their concentration Figure 2. Actually, antioxidants may also cause direct damage to DNA and the cell. Watson recently wrote that time has come to seriously ask whether antioxidant use predominately causes rather than prevents cancer [ 26 ].
Natural antioxidants act as a double-edged sword in cancer [ 28 ]. Some of these studies proposed that in some cases high-dose supplements of antioxidants may be related to health hazards.
For example, high doses of beta-carotene may enhance the risk of lung cancer in smokers. Prostate cancer can occur in dealing with high doses of vitamin E [ 27 ].
Antioxidant supplements may also interact with some medications. Based on these new concepts, the continuous use of certain antioxidants such as glutathione, superoxide dismutase, catalase, and thioredoxin may serve as a barrier to apoptosis, the main anticancer mechanism, through excessively reducing ROS [ 11 ].
The excessive damages via ROS can be associated with changes in mitochondrial membrane permeability, which result in cytochrome C release and apoptotic death. Against, cancer cells boost their anti-apoptotic mechanisms like a nuclear factor kappa-light-chain-enhancer of activated B cell NFĸB pathway to escape cell death [ 9 ].
Disruption of redox balance in cells causes activation of the transcription factors like nuclear factor erythroid 2-related factor 2 Nrf2NFĸB, and activator protein 1 AP-1 as redox-sensitive transcription factors [ 28 ]. Nrf2 transcription factor is the major driver of antioxidant expression that leads to protection against DNA damage, endogenous and exogenous hazards, and consequent cancer initiation [ 2930 ].
Nrf2 overexpresses in some types of human cancers including skin, head, and neck, squamous cell carcinoma, esophagus, pancreatic, gallbladder, prostate, colorectal, breast, lung, and ovary. The cytoprotective properties of the Nrf2 indicate that this pathway can be exploited by tumor cells to promote their survival [ 3132 ].
In the ROS-sensitive cancer cells, natural product-derived inhibitors of Nrf2 pathway can induce ROS that may result in cell death [ 28 ]. Many antioxidants such as polyphenols are significant groups of Nrf2 inhibitors. Particularly in the case of cancer, the Nrf2 pathway has opposing properties: activating the pathway is vital for chemoprevention, but when the control is lost, it provides big consequences, so cancer cells result in fast proliferation, the escape of senescence and apoptosis, and resistance to chemotherapy and radiotherapy.
Therefore, both activation and inhibition of Nrf2 activities can be beneficial [ 33 ]. As said above, natural products with antioxidant agents target Nrf2 pathway as an anticancer approach [ 28 ].
Opposing activities of natural products such as antioxidants in prevention and treatment of cancer depend on their concentration. However, higher concentrations can inhibit antioxidant defense and induce oxidative stress. Genetic alterations that promote tumor cause to produce endogenous antioxidants [ 14 ].
In this process, Nrf2 is the main factor for the transactivation of involved genes in the maintenance of redox homeostasis [ 34 ]. As constitutive upregulation of Nrf2 factor has been reported for a variety of human cancer types, Nrf2 activity has been indicated to be necessary for proliferation of cancer cells [ 353637 ], reprogramming of metabolism [ 38 ], chemoresistance [ 39 ], serine biosynthesis [ 36 ], as well as mRNA translation [ 37 ] in part through maintenance of redox homeostasis.
Hi-activated pathway of Nrf2 increases the amount of cellular ROS scavengers. On the other hand, lowering stress burden via enhancing detoxifying force can affect the pathways that promote proliferation and growth [ 4041 ].
Blocking antioxidant activity in cancer cells decreases their ability to balance oxidative insult and might result in cell death [ 42 ].
In addition to Nrf2, the transcription factor p53 has also been shown to suppress ROS accumulation via directly regulating the expression of a variety of antioxidant genes including SOD2, GPX1, and CAT [ 1439 ] and through the induction of the metabolic TIGAR gene TPinducible glycolysis and apoptosis regulator [ 14 ].
Oxidative stress can happen due to reduction in enzymatic antioxidant activities. Moreover, it can occur due to ionizing, radiation, chemotherapy, aging, shear stress, cytokines, and growth factor receptor interactions [ 14 ].
Antioxidants and oxidative stress interact with the initiation, promotion, and progression of cancer [ 41 ]. Actually, the cell-damaging effects of free radicals can be balanced by antioxidants. Furthermore, as the fruits and vegetables are good sources of antioxidants, people who eat them more than others have a lower risk for various diseases such as heart and neurological diseases, and there is evidence that some types of vegetables and fruits in general protect against a number of cancers [ 43 ].
In addition to the standard anticancer treatment options such as chemotherapy, radiotherapy, and surgery, several natural products due to their antioxidant activities have been identified to have a potential for cancer prevention [ 44 ] and treatment [ 45 ].
In radiotherapy and chemotherapy, ROS and free radicals partly cause various adverse effects [ 46 ]. ROS generation causes various tissue or organ injuries; for example, doxorubicin and other anthracycline antibiotics are known to lead to cardiotoxicity [ 47 ]; cisplatin and other platinums lead to nephrotoxicity, ototoxicity, and peripheral neuropathy [ 48 ]; bleomycin leads to lung injury [ 49 ]; and alkylating agents cause DNA damage of drug-treated cells [ 50 ].
Tissue or organ injuries may also induce carcinogenesis [ 51 ]. Many previous studies reported that using antioxidants with these gold standard methods can significantly decrease these cellular damages.
For instance, in one study that is reported by Askua et al. The remaining 11 studies reported no significantly difference in toxicities between control and supplementation groups [ 51 ]. We are approaching a new era wherein ROS biology and their effects in the physiopathology of cancer may be dissected with unprecedented detail, bringing potential therapeutic benefits derived from selective manipulations of cancer redox balance to be uncovered, paving the way to novel and exciting investigations in the fight against cancer [ 6 ].
Owing to the crucial roles of cancer stem cells in tumor initiation, disease recurrence, and drug resistance, the potential of using a redox-modulating strategy to eliminate this subpopulation of malignant cells could have major implications in cancer treatment.
Redox adaptation is an important concept that, to a large degree, explains the mechanisms by which cancer cells survive under persistent endogenous ROS stress and become resistant to certain anticancer agents.
: Cancer-fighting potential of antioxidants| Bringing Clarity to the Antioxidant-Cancer Prevention Debate - OHC | They chose to study melanoma because the incidence of melanoma is increasing in the United States and Europe, melanoma cells are sensitive to oxidative stress , and a good mouse model of melanoma already exists, Dr. Bergö explained. To understand what the antioxidants were doing in the mice, the researchers measured how the antioxidants were affecting glutathione —the main antioxidant that is naturally produced by the body. The ratio of reduced glutathione to oxidized glutathione is an indicator of how much oxidative damage cells are experiencing. This ratio increased only a little in the primary tumors but increased greatly in the metastases, suggesting that the antioxidant was reducing oxidative stress specifically in the metastatic cancer cells of the mice. In the other recent study , published October 14 in Nature , Sean Morrison, Ph. In mouse models of melanoma, the researchers found, levels of oxidative stress were higher in circulating cancer cells than in cancer cells in primary tumors. Oxidative stress actually interfered with the formation of metastatic tumors, they found. Treating these mice with antioxidants decreased oxidative stress in the circulating cancer cells and increased their ability to metastasize. Morrison said in a press release. The findings support the idea that antioxidants, by reducing oxidative stress, benefit tumor cells more than they benefit normal healthy cells, Dr. Morrison added. The results also support the idea that treating patients with pro-oxidants might be a way to prevent metastasis, he said. In fact, methotrexate , a commonly used cancer drug, has pro-oxidant properties. The drug works by inhibiting an enzyme called dihydrofolate reductase DHFR , which plays a key role in the metabolic pathways that produce glutathione, as well as the pathways that produce new DNA bases. By blocking DHFR, methotrexate interferes with DNA replication and increases oxidative stress. Based on the available evidence, Dr. Bergö said he was extremely concerned with the aggressive marketing of antioxidants to cancer patients. January 3, , by Elia Ben-Ari. December 15, , by Edward Winstead. November 30, , by Shana Spindler. Antioxidants Accelerate the Growth and Invasiveness of Tumors in Mice Subscribe. New findings in mice suggest that antioxidant supplements may promote tumor metastasis. Credit: iStock. Abnormally high concentrations of free radicals in the body can be caused by exposure to ionizing radiation and other environmental toxins. When ionizing radiation hits an atom or a molecule in a cell, an electron may be lost, leading to the formation of a free radical. The production of abnormally high levels of free radicals is the mechanism by which ionizing radiation kills cells. Free radicals that contain the element oxygen are the most common type of free radicals produced in living tissue. Antioxidants are chemicals that interact with and neutralize free radicals , thus preventing them from causing damage. The body makes some of the antioxidants that it uses to neutralize free radicals. These antioxidants are called endogenous antioxidants. However, the body relies on external exogenous sources, primarily the diet, to obtain the rest of the antioxidants it needs. These exogenous antioxidants are commonly called dietary antioxidants. Fruits, vegetables, and grains are rich sources of dietary antioxidants. Some dietary antioxidants are also available as dietary supplements 1 , 3. Examples of dietary antioxidants include beta-carotene , lycopene , and vitamins A, C, and E alpha-tocopherol. The mineral element selenium is often thought to be a dietary antioxidant, but the antioxidant effects of selenium are most likely due to the antioxidant activity of proteins that have this element as an essential component i. In laboratory and animal studies , the presence of increased levels of exogenous antioxidants has been shown to prevent the types of free radical damage that have been associated with cancer development. Therefore, researchers have investigated whether taking dietary antioxidant supplements can help lower the risk of developing or dying from cancer in humans. Many observational studies , including case—control studies and cohort studies , have been conducted to investigate whether the use of dietary antioxidant supplements is associated with reduced risks of cancer in humans. Overall, these studies have yielded mixed results 5. Because observational studies cannot adequately control for biases that might influence study outcomes, the results of any individual observational study must be viewed with caution. Randomized controlled clinical trials , however, lack most of the biases that limit the reliability of observational studies. To date, nine randomized controlled trials of dietary antioxidant supplements for cancer prevention have been conducted worldwide. Many of the trials were sponsored by the National Cancer Institute. The results of these nine trials are summarized below. Initial: no effect on risk of developing either cancer; decreased risk of dying from gastric cancer only Later: no effect on risk of dying from gastric cancer. Initial: lower total cancer and prostate cancer incidence and all-cause mortality among men only; increased incidence of skin cancer among women only. Later: no evidence of protective effects in men or harmful effects in women within 5 years of ending supplementation. Initial: no reduction in incidence of prostate or other cancers—trial stopped early. Overall, these nine randomized controlled clinical trials did not provide evidence that dietary antioxidant supplements are beneficial in primary cancer prevention. In addition, a systematic review of the available evidence regarding the use of vitamin and mineral supplements for the prevention of chronic diseases, including cancer, conducted for the United States Preventive Services Task Force USPSTF likewise found no clear evidence of benefit in preventing cancer It is possible that the lack of benefit in clinical studies can be explained by differences in the effects of the tested antioxidants when they are consumed as purified chemicals as opposed to when they are consumed in foods, which contain complex mixtures of antioxidants, vitamins, and minerals 3. Therefore, acquiring a more complete understanding of the antioxidant content of individual foods, how the various antioxidants and other substances in foods interact with one another, and factors that influence the uptake and distribution of food-derived antioxidants in the body are active areas of ongoing cancer prevention research. |
| Antioxidants as a Double-Edged Sword in the Treatment of Cancer | IntechOpen | Lin, J. Antioxidants give protection to the cells through three lines of defense. ROS can induce tumorigenicity and promote tumor progression via inducing DNA damage [ 21 ]. Multigrain Pancakes with Strawberry Sauce Orange Bran Flax Muffins Spring Vegetable Frittata Whole Wheat Blueberry Muffins. Screening of marine microalgae: investigation of new exopolysaccharide producers. Rahman K. Ashfaq, W. |
| OHC Cancer Expert Adds Clarity to the Antioxidant-Cancer Prevention Debate | Examples of some foods high in beta carotene include the following:. Vitamin E is essential for our bodies to work properly. Vitamin E helps to build normal and red blood cells, as well as working as an antioxidant. Research is finding evidence that vitamin E may protect against prostate and colorectal cancer. The recommended dietary allowance for vitamin E is 15 milligrams per day. The adult upper limit for vitamin E is 1, milligrams per day. Good sources of vitamin E and the amount each serving contains include the following:. Since some sources of vitamin E are high in fat. A synthetic form of a vitamin E is available as a supplement. Vitamin E supplementation is probably not needed for most individuals because vitamin E is a fat-soluble vitamin and is stored in our bodies. Very high doses of vitamin E can also interfere with the way other fat-soluble vitamins work. Also, large doses of vitamin E from supplements are not recommended for people taking blood thinners and some other medications, as the vitamin can interfere with the action of the medication. To make sure you are meeting your needs, eat a varied diet that includes whole-wheat breads and cereals. There is no recommended dietary allowance for antioxidants. Eat a variety of foods, including plenty of fruits and vegetables, to ensure you are getting adequate amounts in your diet. In honor of Colon Cancer Awareness month , we'll be featuring four colorectal cancer friendly recipes each week during the month of March. Broccoli, cabbage, collard greens, kale, cauliflower and Brussels sprouts are all cruciferous vegetables. This vegetable family contains powerful phytochemicals, including carotenoids, indoles and glucosinolates and isothiocyanates, which have been studied and shown to slow the growth of many cancers. Get the recipe ». Apple Muffins. Baked Oatmeal. Banana Bran Muffins. Banana-Oatmeal Hot Cakes. Multigrain Pancakes with Strawberry Sauce. Orange Bran Flax Muffins. Spring Vegetable Frittata. Whole Wheat Blueberry Muffins. Pesto Toastini. Fiesta Quesadillas with Black Beans. Skewered Shrimp, Chicken and Pineapple with Honey Orange Dipping Sauce. Zucchini Bites. Asparagus and Scallion Soup with Almonds. Black Bean and Corn Salad. Broccoli Sunflower Salad. Butternut Squash Soup. California Citrus Greens Salad with Garlic Dressing. Carrot and Apple Soup. Creamy Irish Soup. Crunchy Chicken Salad. Curried Chicken Salad. Curried Chickpea Salad with Walnuts. Easy Pea Soup with Tarragon. Egyptian Red Lentil Soup. Fall Stew in a Pumpkin with Poblano-Cucumber Salsa. Golden Fruit Salad. Hawaiian Star Soup. Hearty Vegetable and Brown Rice Soup. Hot and Sour Soup. Lentil Sweet Potato Soup. Marinated Artichoke Potato Salad. Melon Salad. Minty Cucumber-Quinoa-Grape Salad. Mulligatawny Soup. Papaya, Chicken and Pecan Salad. Pluot Summer Salad. Pomegranate Salad. Pumpkin Bisque. Roasted Asparagus Salad. Salmon Salad with Pimento and Herbs. Shredded Carrot and Beet Salad. Spicy Black Bean Salad. Spinach Salad with Strawberry Vinaigrette. Spinach, Red Bell Pepper and Feta Cheese Salad with Yogurt Dressing. Spring Pea Soup. Summer Rice Salad. Sweet and Spicy Carrot Salad. Vegetable Soup. Whole Grain Salad. Anytime Burrito. Baked Tofu Kabobs. Bean and Vegetable Enchilada Casserole. Bean Surprise. Broiled Portobello Mushrooms. Cajun Salmon over Polenta. Chicken Chili. Chicken Enchilada Casserole. Cranberry Salmon. Cranberry-Turkey Salad Sandwiches. Crispy Parmesan Turkey Cutlets. Crunchy Veggie Wrap. Easy Spinach Lasagna. Eating Well Sloppy Joe. Egg, Spinach, and Bacon Sandwiches. Fish Filet with Squash and Herbs. Greek-Style Scallops. Grilled Ginger Tuna. Grilled Halibut with a Tomato-Herb Sauce. Grilled Portobello Burgers. Grilled Vegetable Polenta with Pan Roasted Red Pepper and Tomato Sauce. Halibut with Citrus and Garlic. Healthy Jambalaya. Hearty Beef Stew with Winter Vegetables. Hearty Mediterranean Stew. Herbed Polenta with Grilled Portobello Mushrooms. Indonesian Salmon. Lasagna Rolls. Lemon Dijon Salmon. Mediterranean Grilled Veggie Pockets. Molasses-Cured Pork Loin with Apples. Mushroom Goulash. New American Plate "Tetrazzini" Casserole. New Tuna Salad. Peppers Stuffed with Barley, Parmesan and Onion. Pizza Meat Loaf. Pumpkin Gnocchi. Quinoa and Mushroom Pilaf with Dill. Quinoa Stuffed Peppers. Roasted Pork Tenderloin with Maple Mustard Sauce. Scallion Crusted Arctic Char. Seared Scallops with Beet Puree and Arugula Salad. Soft Tacos with Southwestern Vegetables. Spaghetti alla Carbonara. Speedy Summer Ratatouille. Spicy Broccoli, Cauliflower and Tofu. Steamed Halibut on Spinach with Lemon Sauce. Stuffed Cornish Hens. Summer Tofu Kebab with Peanut Sauce. Sweet and Sour Chicken. Sweet and Sour Tofu. Tofu Cutlets Marsala. Turkey Reuben Grilled Sandwiches. Udon Noodles with Spicy Peanut Ginger Sauce. Veggie Pita Pizzas. White Wine Coq au Vin. Whole Wheat Pasta with Fennel, Peas and Arugula. Zesty Roasted Chicken. Asian Green Bean Stir-Fry. Asian Pilaf. Avocado and Mango Salsa. Baked Sweet Potato Wedges. Bok Choy with Sautéed Mushrooms and Shallots. Braised Kale with Black Beans and Tomatoes. Broccoli with Hazelnuts. Brussels Sprouts with Pecans and Dried Cranberries. Butternut Squash Pilaf. Garlicky Greens. Honey-Roasted Parsnips, Sweet Potatoes and Apples. Lite Hummus Dip. Parmesan Orzo Primavera. Peas-Mushroom Pilaf. Quinoa Salad with Roasted Autumn Vegetables. Seasoned Spinach with Garlic. Simply Grilled Portobello Mushrooms. Spring Barley. Stir-Fried Kale with Slivered Carrots. Summer Gazpacho. Sweet Potato Power. Tofu Fried Rice. Winter Caponata. Apple Cranberry Cobbler. Apple Crisp. Apple-Cranberry Crisp. Baked Summer Fruit. Better Brownies. Blueberry Crumble Pie. Cranberry-Orange Fruit Bars. Crunchy Oat Apricot Bars. Fresh Berry Sundaes. EJMOAMS; Revised: Feb, Manuscript No. EJMOAMS R ; Published: Mar Antioxidants are often considered to be essential for good health, especially when it comes to fighting diseases such as cancer. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival. Vitamin C can interfere with these treatments and decrease their effects. Compounds, which are present in food and are available as supplements, can play an important role in fighting free radicals. But it is not a cure-all, and some sources of antioxidants are more beneficial than others. Antioxidants can play a role in cancer prevention: Free unstable radicals can damage cell DNA in the body, which is thought to play a role in cancer development. You can get your antioxidant supplement in the diet: The body makes its own antioxidants to fight free radicals, and you can get a lot out of eating healthy foods. Brightly coloured fruits and vegetables, such as tomatoes, carrots, spinach, broccoli, sweet potatoes, strawberries, and citrus fruits, are excellent sources. Whole grains, nuts, seeds, and wheat germ provide antioxidants as well. Your best bet goes to a lot of variety, as different foods have different antioxidants. Antioxidant supplements are probably not suitable: When antioxidants come from a healthy diet, it is easy to assume that high antioxidant ingredients are even better. But there is no evidence that antioxidant ingredients can prevent cancer or other diseases. In fact, high doses of certain antioxidants may actually increase the risk of other cancers. Another thing to consider is supplements contain unique types of antioxidants, but not so with diet. It is possible that all of these compounds work together to produce their own anti-inflammatory effects. It is not a bad idea to focus on getting antioxidants from fruits and vegetables, including if you have cancer. |
| Can taking antioxidants prevent cancer? | Cancer Council | The drug works by inhibiting an enzyme called dihydrofolate reductase DHFR , which plays a key role in the metabolic pathways that produce glutathione, as well as the pathways that produce new DNA bases. By blocking DHFR, methotrexate interferes with DNA replication and increases oxidative stress. Based on the available evidence, Dr. Bergö said he was extremely concerned with the aggressive marketing of antioxidants to cancer patients. January 3, , by Elia Ben-Ari. December 15, , by Edward Winstead. November 30, , by Shana Spindler. Antioxidants Accelerate the Growth and Invasiveness of Tumors in Mice Subscribe. New findings in mice suggest that antioxidant supplements may promote tumor metastasis. Credit: iStock. Featured Posts FDA Approves First Immunotherapy Drug for Nasopharyngeal Cancer January 3, , by Elia Ben-Ari. Virtual Mind—Body Fitness Classes May Offer Benefits during Cancer Treatment December 15, , by Edward Winstead. Combo Treatment Highly Effective for Advanced Bladder Cancer November 30, , by Shana Spindler. Categories Biology of Cancer. Cancer Risk. Childhood Cancer. Indeed, phenolic compounds found in medicinal plants have opened a new horizon to prevent and treat diseases because of having antioxidant properties. However, some recent studies have reported that antioxidants are not absolute anticancer compounds and certain drugs have been reported to reduce levels of reactive oxygen species ROS in the cancer cells, i. It has been argued that increasing levels of ROS cause an increase in apoptosis rate and therefore can be considered an approach to treat fatal and hard-to-treat cancers. This chapter seeks to partly explain the role of ROS in progression or inhibition of cancer growth in addition to the role of antioxidants in preventing and treating this disease. com and shirzad yahoo. Cancer kills many people worldwide every year. Even in developed countries such as the United States, the rate of mortalities of cancer is high yet [ 1 ]. Although nowadays cancer therapy has improved, since complete recovery of cancer patients following a single treatment is quite difficult, a multidisciplinary approach combined with surgery, chemotherapy, radiotherapy, and immunotherapy is usually utilized [ 2 , 3 ]. However, some of these approaches cause several severe side effects in patients. Moreover, for many patients, current therapeutic approaches are successful only in delaying the time to disease progression rather than affecting long-term survival rates [ 4 ]. Many previous studies have shown that antioxidant supplementations are useful in cancer treatment [ 5 ]. An antioxidant substance in the cell is present at low concentrations and significantly reduces or prevents oxidation of the oxidizable substrates [ 6 ]. The researchers have evaluated highly complex antioxidant to protect the cells of the body against free radical damages [ 4 ]. Among many factors that cause cancer, oxidative stress is one of the most principal and well-studied events that gives elevation to the conditions leading to tumor onset and progression [ 7 , 8 ]. Oxidative stress as an imbalance between the production and elimination of ROS causes excessive oxidative damage to macromolecules, cells, and tissues [ 10 ]. Reactive oxygen species ROS are free radicals which are correlated with the oxygen atom O or their equivalents and have stronger reactivity with other molecules, rather than with O 2 [ 14 ]. When an imbalance between free radical and reactive metabolite production occurred, ROS are formed and can potentially exhibit a negative effect on the organism [ 15 ]. Radical formation in the body occurs via several mechanisms, involving both endogenous and environmental factors [ 11 ]. Cancer is a multistage process defined by initiation, promotion, and progression [ 16 , 17 ], and oxidative stress interacts with all three stages. A little increase in the ROS level may cause a transient alteration in the cellular level, while a severe increase in ROS may result to irreversible oxidative damage and lead to cell death [ 18 ]. ROS can also promote carcinogenesis by inducing pro-oncogenic signaling pathways and DNA mutations. For instance, ROS may stimulate the phosphorylation of mitogen-activated protein kinase MAPK , JUN N-terminal kinase JNK activation, cyclin D1 expression, and extracellular signal-regulated kinase ERK , all of which are related to growth of tumor cells and survival [ 19 ]. Cancer cells generate ROS more abundantly than normal cells and cause elevated oxidative stress [ 20 ]. ROS can induce tumorigenicity and promote tumor progression via inducing DNA damage [ 21 ]. ROS induces gene mutations and structural changes in the DNA and results in DNA damage during the early stage of tumorigenicity. In addition to, ROS can increase cell proliferation and decrease apoptosis via modifying second-messenger systems, causing abnormal gene expression, and blocking cell communications. Finally, oxidative stress can add DNA alterations to initiate cell population and promote cancer progression [ 22 ]. ROS might function as a double-edged sword Figure 1. A moderate increase of ROS may promote cell proliferation and survival. However, when the increase of ROS reaches a certain level the toxic threshold , it can overwhelm the antioxidant capacity of the cells and result in cell death [ 23 ]. It is long thought that antioxidants can remove the ROS that is produced in normal cellular processes and can protect cells from oxidative damage. Relation between ROS actions with promoting and fighting cancer [ 23 ]. Reactive oxygen species ROS can promote cellular processes to cancer. In addition, they can induce apoptosis. Actually, ROS might function as a double-edged sword. Antioxidants as chemicals that interact with neutralized free radicals can prevent them from causing damages. Antioxidants divide to two main subgroups including enzymatic and nonenzymatic antioxidants. Catalase, superoxide dismutase, and glutathione peroxidases are some of the most important enzymatic antioxidants [ 11 ]. Catalase EC 1. Superoxide dismutase EC 1. Glutathione peroxidases catalyze the oxidation of glutathione at the direction of a hydroperoxide, which may be hydrogen peroxide or another species such as a lipid hydroperoxide. Also, flavonoids, alkaloids, coumarins, carotenoids, and vitamins such as E, A, C ascorbic acid , and D3 are some of the most important nonenzymatic antioxidants that are usually available in many natural products [ 24 ]. Antioxidants are known as free radical scavengers. Examples of dietary antioxidants include beta-carotene, lycopene, and vitamins A, C, and E alpha-tocopherol. Also, the mineral element selenium is often thought to be a dietary antioxidant. Moreover, natural compounds such as flavonoids, in particular ECGC and resveratrol, were shown to have a promising future as antioxidants and anticarcinogenic agents. These compounds can be consumed through fruits and vegetables [ 25 ]. In recent years, potential chemotherapeutic properties of antioxidants have been evaluated as a primary agent or in combination with an already established chemotherapeutic agent for different types of cancers. There is friction among researchers about the efficacy and safety of these complimentary treatments and their substantial role in protecting tumor cells from conventional therapy. The antioxidants can be endogenous or obtained exogenously as a part of a diet or as dietary supplements [ 11 ]. However, many natural compounds such as natural antioxidants display opposing properties in cancer cells, depending on their concentration Figure 2. Actually, antioxidants may also cause direct damage to DNA and the cell. Watson recently wrote that time has come to seriously ask whether antioxidant use predominately causes rather than prevents cancer [ 26 ]. Natural antioxidants act as a double-edged sword in cancer [ 28 ]. Some of these studies proposed that in some cases high-dose supplements of antioxidants may be related to health hazards. For example, high doses of beta-carotene may enhance the risk of lung cancer in smokers. Prostate cancer can occur in dealing with high doses of vitamin E [ 27 ]. Antioxidant supplements may also interact with some medications. Based on these new concepts, the continuous use of certain antioxidants such as glutathione, superoxide dismutase, catalase, and thioredoxin may serve as a barrier to apoptosis, the main anticancer mechanism, through excessively reducing ROS [ 11 ]. The excessive damages via ROS can be associated with changes in mitochondrial membrane permeability, which result in cytochrome C release and apoptotic death. Against, cancer cells boost their anti-apoptotic mechanisms like a nuclear factor kappa-light-chain-enhancer of activated B cell NFĸB pathway to escape cell death [ 9 ]. Disruption of redox balance in cells causes activation of the transcription factors like nuclear factor erythroid 2-related factor 2 Nrf2 , NFĸB, and activator protein 1 AP-1 as redox-sensitive transcription factors [ 28 ]. Nrf2 transcription factor is the major driver of antioxidant expression that leads to protection against DNA damage, endogenous and exogenous hazards, and consequent cancer initiation [ 29 , 30 ]. Nrf2 overexpresses in some types of human cancers including skin, head, and neck, squamous cell carcinoma, esophagus, pancreatic, gallbladder, prostate, colorectal, breast, lung, and ovary. The cytoprotective properties of the Nrf2 indicate that this pathway can be exploited by tumor cells to promote their survival [ 31 , 32 ]. In the ROS-sensitive cancer cells, natural product-derived inhibitors of Nrf2 pathway can induce ROS that may result in cell death [ 28 ]. Many antioxidants such as polyphenols are significant groups of Nrf2 inhibitors. Particularly in the case of cancer, the Nrf2 pathway has opposing properties: activating the pathway is vital for chemoprevention, but when the control is lost, it provides big consequences, so cancer cells result in fast proliferation, the escape of senescence and apoptosis, and resistance to chemotherapy and radiotherapy. Therefore, both activation and inhibition of Nrf2 activities can be beneficial [ 33 ]. As said above, natural products with antioxidant agents target Nrf2 pathway as an anticancer approach [ 28 ]. Opposing activities of natural products such as antioxidants in prevention and treatment of cancer depend on their concentration. However, higher concentrations can inhibit antioxidant defense and induce oxidative stress. Genetic alterations that promote tumor cause to produce endogenous antioxidants [ 14 ]. In this process, Nrf2 is the main factor for the transactivation of involved genes in the maintenance of redox homeostasis [ 34 ]. As constitutive upregulation of Nrf2 factor has been reported for a variety of human cancer types, Nrf2 activity has been indicated to be necessary for proliferation of cancer cells [ 35 , 36 , 37 ], reprogramming of metabolism [ 38 ], chemoresistance [ 39 ], serine biosynthesis [ 36 ], as well as mRNA translation [ 37 ] in part through maintenance of redox homeostasis. Hi-activated pathway of Nrf2 increases the amount of cellular ROS scavengers. On the other hand, lowering stress burden via enhancing detoxifying force can affect the pathways that promote proliferation and growth [ 40 , 41 ]. Blocking antioxidant activity in cancer cells decreases their ability to balance oxidative insult and might result in cell death [ 42 ]. In addition to Nrf2, the transcription factor p53 has also been shown to suppress ROS accumulation via directly regulating the expression of a variety of antioxidant genes including SOD2, GPX1, and CAT [ 14 , 39 ] and through the induction of the metabolic TIGAR gene TPinducible glycolysis and apoptosis regulator [ 14 ]. Oxidative stress can happen due to reduction in enzymatic antioxidant activities. Moreover, it can occur due to ionizing, radiation, chemotherapy, aging, shear stress, cytokines, and growth factor receptor interactions [ 14 ]. Antioxidants and oxidative stress interact with the initiation, promotion, and progression of cancer [ 41 ]. Actually, the cell-damaging effects of free radicals can be balanced by antioxidants. Furthermore, as the fruits and vegetables are good sources of antioxidants, people who eat them more than others have a lower risk for various diseases such as heart and neurological diseases, and there is evidence that some types of vegetables and fruits in general protect against a number of cancers [ 43 ]. In addition to the standard anticancer treatment options such as chemotherapy, radiotherapy, and surgery, several natural products due to their antioxidant activities have been identified to have a potential for cancer prevention [ 44 ] and treatment [ 45 ]. In radiotherapy and chemotherapy, ROS and free radicals partly cause various adverse effects [ 46 ]. ROS generation causes various tissue or organ injuries; for example, doxorubicin and other anthracycline antibiotics are known to lead to cardiotoxicity [ 47 ]; cisplatin and other platinums lead to nephrotoxicity, ototoxicity, and peripheral neuropathy [ 48 ]; bleomycin leads to lung injury [ 49 ]; and alkylating agents cause DNA damage of drug-treated cells [ 50 ]. Tissue or organ injuries may also induce carcinogenesis [ 51 ]. Many previous studies reported that using antioxidants with these gold standard methods can significantly decrease these cellular damages. For instance, in one study that is reported by Askua et al. And for people undergoing chemotherapy or radiotherapy treatments, such supplements may be harmful. Many people assume taking micronutrients or multivitamins high in antioxidants will act in the same way as antioxidants in foods and help prevent cancer. But research has shown that taking supplements to reduce the risk of cancer may not be effective. And while antioxidants may be beneficial in healthy people because they attack active chemicals called free radicals which can damage DNA, cancer treatments such as chemotherapy and radiotherapy actually use free radicals to kill tumour cells. Scientific studies have suggested antioxidants may have the opposite effect in people undergoing cancer treatment. Some people having cancer treatment may be advised to take supplements because of the side effects of their treatment or other health issues or confirmed nutritional deficiency. |
| Can taking antioxidants prevent cancer? | Clinical Interventions in Aging. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. Production of large amounts of hydrogen peroxide by human tumor cells. High Calorie Recipe: Cinnamon-Peach Smoothie. Asadi-Samani M, Rafieian-Kopaei M, Lorigooini Z, Shirzad H. However, some of these approaches cause several severe side effects in patients. Polysaccharides obtained from Tetraselmis spp. |
Nach meiner Meinung irren Sie sich. Schreiben Sie mir in PM, wir werden reden.