
Body composition and aging -
Musculoskeletal disorders are common among older people. Preventive strategies require understanding of age-related changes in strength, function and body composition, including how they interrelate. We have described, and examined associations between, 9-year changes in these parameters among Health, Aging and Body Composition Study participants aged 70—79 years.
Appendicular lean mass ALM , whole body fat mass and total hip BMD were ascertained using DXA; muscle strength by grip dynamometry; and muscle function by gait speed.
For each characteristic annualised percentage changes were calculated; measures of conditional change independent of baseline were derived and their interrelationships were examined using Pearson correlations; proportion of variance at 9-year follow-up explained by baseline level was estimated; and mean trajectories in relation to age were estimated using linear mixed models.
Analyses were stratified by sex. Median [lower quartile, upper quartile] annual percentage declines were grip strength 1. Declines were linear for ALM and accelerated over time for other characteristics.
Strength and function declined more rapidly, and were less correlated between baseline and follow-up, than measures of body composition. Therefore, broader intervention strategies to prevent loss of strength and function in later life are required as those targeting body composition alone may be insufficient.
Peggy M. Cawthon, Neeta Parimi, … Eric S. Syddall, L. Westbury, … C. Adam J. Santanasto, I. Miljkovic, … J. Musculoskeletal disorders are common among older people and are a leading cause of morbidity worldwide [ 1 ].
Sarcopenia, the loss of muscle mass and strength with age, is associated with increased risk of disability and mortality and significant healthcare costs [ 2 , 3 , 4 ].
Although there is no consensus definition of sarcopenia, most criteria use measures of grip strength, gait speed and lean mass. Sarcopenia is now regarded as a disease according to the International Classification of Diseases [ 7 ].
Another common musculoskeletal disorder is osteoporosis, characterised by low bone density and micro-architectural deterioration of bone tissue, which increases bone fragility [ 8 ].
In addition to declines in lean mass and bone density, age-related changes in body composition include increases in fat mass, particularly in the abdominal area [ 11 ]. Age-related reductions in lean mass in combination with increases in fat mass can result in the development of sarcopenic obesity, resulting in more adverse health effects than sarcopenia or obesity in isolation [ 18 ].
Preventive strategies for musculoskeletal disorders require a better understanding of age-related changes in muscle strength, physical function and body composition including bone as well as how these changes interrelate; from a public health perspective, this knowledge could inform the development of interventions to delay adverse changes in musculoskeletal aging.
Previous research has established the following: aging is associated with declines in muscle mass, strength, physical function and bone density [ 19 ]; declines are greater for muscle strength and physical function compared to muscle mass [ 20 , 21 ]; and changes in some muscle and bone parameters are correlated [ 22 ].
However, to our knowledge, no studies have explored changes in both key sarcopenia components muscle mass, strength and function and aspects of body composition muscle mass, fat mass and bone density among a single cohort of older people in whom parameters have been measured at multiple time-points.
To address this, we have described, and examined associations between, changes in musculoskeletal and body composition parameters among participants in the Health, Aging and Body Composition Health ABC Study, USA.
The Health ABC Study comprises US men and women aged 70—79 years at baseline who were recruited in — A random sample of white and of all of the black Medicare beneficiaries from around Memphis Tennessee and Pittsburgh Pennsylvania was obtained.
Sampled participants received a mailing followed by a telephone eligibility screen. Individuals reporting no difficulty in walking one quarter of a mile or climbing 10 stairs were eligible.
Individuals with the following characteristics were excluded: inability to communicate with the interviewer; clear cognitive impairment; having a life-threatening illness or difficulties with activities of daily living ADL ; requiring a walking aid; having an intention of moving outside the area within three years; or currently enrolled in a lifestyle intervention trial.
Written, informed consent was provided by all participants and the study was approved by the institutional review boards at the University of Tennessee and the University of Pittsburgh. The study methodology has been described in detail previously [ 23 ]. In brief, at baseline Year 1 , sex, race, educational attainment, and health behaviours such as smoking status and alcohol consumption were self-reported using questionnaires.
Height and weight were measured using a Harpenden Stadiometer Holtain Ltd, Crosswell, UK and a standard balance beam scale, respectively. Physical activity was calculated using an instrument derived from the Leisure Time Physical Activity Questionnaire [ 24 ].
Total kilocalories expended per week in stair climbing, walking and exercise activity, calculated by multiplying caloric expenditure by the participant's weight kg , was used as a measure of physical activity.
Participants were asked whether a doctor had ever told them that they had various medical conditions. At Year 2, dietary intake over the previous year was assessed using a nurse-administered food frequency questionnaire FFQ comprising items.
More information on the components of this HEI has been published previously [ 28 ]. Grip strength was measured two times for each hand at Years 1, 2, 4, 6, 8 and 10 using a Jamar dynamometer according to a standardised protocol throughout all stages of follow-up; maximum grip strength at each time-point was used for analyses.
Grip strength values were set to missing for participants with severe hand pain or recent surgery. The calibration of the dynamometers was checked regularly.
Customary gait speed was ascertained at Years 2, 3, 4, 5, 6, 8 and 10 by asking participants to walk at their normal speed down a corridor over a total distance of 20 m. Whole-body dual-energy X-ray absorptiometry scans Hologic QDR A; Hologic, Bedford, MA, USA were performed at Years 1, 2, 3, 4, 5, 6, 8 and 10 and used to ascertain whole body fat and appendicular lean mass ALM in kg.
Total hip BMD, a repeatable measurement that is predictive of future fracture, was measured using the same device at Years 1, 3, 5, 8 and The reproducibility and validity of this scanner has been previously reported [ 29 , 30 ]. Regular DXA phantom scans were performed for quality control and calibration purposes.
Baseline participant characteristics were described using means and standard deviations SD , medians and interquartile ranges, or frequency and percentage distributions as appropriate. Normality of the following five characteristics was confirmed through visual inspection of histograms: grip strength, gait speed, ALM, whole-body fat mass and hip BMD.
The statistical methods that were applied to each of these five characteristics are stated below. Pairwise comparisons of the mean and variance for each characteristic were performed using t-tests and variance ratio tests, respectively.
Second, conditional change independent of baseline was characterised by obtaining the residuals from sex-specific linear regression models for characteristics at follow-up Year 10 on baseline characteristics with adjustment for individual follow-up duration.
The proportion of variance at Year 10 that was explained by baseline level and conditional change was estimated. Relationships between conditional change measures, including conditional change in weight from baseline to follow-up, were examined using Pearson correlations.
All analyses were stratified by sex and based on the sample of Health ABC participants with data on at least one of the characteristics grip strength, gait speed, ALM, whole-body fat mass and hip BMD at two or more time-points.
Sensitivity analyses included stratification by race as well as sex and, for each characteristic, a comparison of mean trajectories from participants with observations at all time-points as opposed to a minimum of two time-points.
Analyses were conducted using Stata, release 15 StataCorp, College Station, TX, USA. Baseline participant characteristics among the analysis sample of Health ABC participants according to sex and race are presented in Table 1.
Mean and standard deviation SD for age was Although both black men and women had slower gait speed compared to their white counterparts and black men had lower fat mass, the remaining measures were greater among black participants.
Boxplots of estimated annual percentage change in each characteristic are shown in Fig. Estimated annual percentage change in characteristics among men and women.
ALM appendicular lean mass, BMD bone mineral density. The three vertical lines in the box represent the lower quartile Q1 , median and upper quartile Q3. Estimates of percentage change for each participant were derived using person-specific linear regression models for percentage change since baseline calculated at each time-point as the outcome with age at each time-point as the only predictor.
Annual percentage change is given by the regression coefficient for age. Analysis was restricted to men and women with data on at least one change measure.
Mean trajectories of characteristics for men and women are presented in Fig. Decline in grip strength, gait speed and hip BMD accelerated somewhat with age; decline in ALM was linear. Fat mass increased, plateaued and then decreased among men, whereas the initial period of increase was negligible among women.
Mean trajectories were derived using linear mixed effects models with random intercepts and slopes. For each characteristic, trajectories from participants with at least two observations were included. The proportion of variation in each characteristic at follow-up Year 10 which was explained by baseline level and conditional change is shown in Fig.
Equivalently, the correlations between baseline and follow-up measurements were lower for grip strength and gait speed in comparison with the other characteristics. Proportion of variance at follow-up Year 10 explained by baseline level and conditional change since baseline.
Measures of conditional change were derived using a residual change method and were independent of baseline level. Analyses restricted to men and women with data on at least one conditional change measure. Results were broadly similar between races as shown in the Supplementary Material.
Racial differences in mean trajectories were larger regarding levels of the characteristics rather than in rates of change eFigure 2 in Online Resource. However, the broad changes in the characteristics were similar among both groups of participants.
Among participants in the Health ABC Study, we have described, and examined associations between, changes in muscle strength, physical function and body composition parameters during a 9-year follow-up in later life.
Declines in grip strength, gait speed and hip BMD accelerated somewhat with advancing age, whereas declines in ALM were linear. Declines were greater, and the proportion of variance at follow-up explained by baseline level was lower, for grip strength and gait speed in comparison with ALM, fat mass and hip BMD.
Our findings are consistent with those from previous analyses of the Health ABC Study. For example: over a 3-year follow-up, declines in knee extensor strength and both total and leg lean mass were correlated, although the absolute average magnitude of decline was greater for strength than mass [ 31 ]; 5-year declines in leg muscle torque were greater than those for muscle cross-sectional area [ 32 ]; and fat mass increased, plateaued and then decreased in a previous study of 5-year changes in body composition [ 33 ].
The latter suggests an initial trade-off between losing lean mass and gaining fat mass among weight-stable participants, followed by losses in weight, lean mass and fat mass with positive correlations between declines in weight and lean mass [ 34 , 35 ]. Therefore, our analyses provide a longer term validation of these earlier findings.
Other cohorts have also described and compared longitudinal changes in muscle strength, physical function and body composition among older people. Our findings are in agreement with those from the Hertfordshire Cohort Study [ 20 ] and a cohort comprising Chinese participants, aged 65 and older [ 21 ], in which percentage declines in grip strength and gait speed were greater than declines in muscle mass.
A possible explanation for these conflicting results is the different age ranges and follow-up times of participants in these studies. Similar to our analyses, declines in grip strength and gait speed accelerated with advancing age among participants in the Cardiovascular Health Study [ 38 ] and this was also the case for total femoral neck BMD in the Osteoporotic Fractures in Men MrOS Study [ 39 ].
Previous studies have also examined interrelationships between changes in muscle strength, function and body composition. Changes in muscle strength and function in relation to changes in BMD among adults and children have been reported in a recent systematic review and meta-analysis [ 22 ].
Greater loss of arm lean mass was associated with accelerated loss of grip strength in a cohort of Afro-Caribbean men [ 49 ]. These findings are in agreement with our results from the Health ABC Study. There are several potential mechanisms that may explain why longitudinal decreases in muscle mass, strength and BMD are correlated with one another in later life.
First, the relationship between loss of muscle mass and strength may be bidirectional. Reductions in strength may result in declines in physical function and activity, leading to disuse-induced muscle wasting; simultaneously, declines in muscle mass and quality due to losses in fast-twitch muscle fibres, fat infiltration of skeletal muscle and increased inflammation may result in declines in strength and physical function [ 50 ].
Second, correlations between muscle mass and strength and BMD in older age are expected from both cellular and physiological perspectives. Cellular similarities include a shared mesenchymal stem cell origin between myoblasts and osteocytes [ 51 ]. Physiologically, reductions in strength lead to weaker forces on bone, resulting in greater bone resorption than formation according to the mechanostat theory [ 20 , 52 ].
Finally, developmental, genetic, endocrine and lifestyle factors, such as smoking, physical activity and diet quality are established determinants of both muscle and bone aging [ 19 ], and may therefore contribute to correlations between declines in muscle and bone parameters. A key strength of this study is the measurement of a wide range of musculoskeletal and body composition parameters in a single, well-characterised cohort.
In contrast, studies which compare changes in musculoskeletal parameters across cohorts are likely to be limited because heterogeneous age ranges and nationalities of participants are likely to affect comparability of results.
Another strength of the Health ABC Study is that parameters have been measured repeatedly over many follow-ups, enabling a comprehensive assessment of change.
This study has some limitations. Participants were free of mobility disability at baseline. This limits the generalisability of the findings to the wider population of community-dwelling older people in this age range and may have led to an underestimation of the magnitude of decline in these trajectories.
Death and drop-outs during follow-up result in healthier participants remaining in the study who may be more likely to have slower rates of decline in musculoskeletal parameters. The SPPB consists of three timed measures: a 4 m walk, repeated chair rise, and a balance test Guralnik et al.
To measure walking speed, the participants were asked to walk at their usual pace over a 4-m course. Duplicate measurements were done, and the faster measure was used to compute walking speed.
For the repeated chair rise, participants were asked to stand from a sitting position without using their arms. Those who could do so were asked to stand up and sit down five times at their fastest speed.
Balance was measured by asking the participants to maintain balance in three positions with a progressive narrowing of the base of support: side by side, semitandem, and tandem. Each task was scored from 0 to 4, with 4 indicating the highest performance and 0 inability to perform the task, based on the rubric from the Established Populations for Epidemiologic Studies of the Elderly Guralnik et al.
A total score was calculated and ranged from 0 to The Pepper Assessment Tool for Disability PAT-D is a item self-administered questionnaire to assess mobility, activities of daily living ADL and instrumental activities of daily living IADL.
Multiple linear regression was used to characterize the strength of the relationships between physical function measures 4-m walk speed, repeated chair rise time, grip strength, SPPB, and PAT-D and body composition BMI, percentage of body fat, and percent appendicular lean mass within each study while controlling for age, gender, and race individual study model.
Multiple linear regression models were then used to determine the associations between physical function and body composition in all participants combined, adjusted for study effect using dummy variables, in addition to age, gender, and race combined model.
Residual plots were produced for the combined analyses to examine the patterns across studies. We found the residual patterns were consistent across different studies for all the physical function measure outcomes and body composition predictors. We investigated the consistency of relationships across gender by testing for interactions between gender and body composition.
This was done by adding an interaction term in the combined model described above. If the test for the interaction was statistically significant, it indicated that the relationship between physical function measure and body composition depended on gender; otherwise, we concluded that the relationship was consistent across both genders.
All analyses were performed with SAS 9. There were 1, participants from 13 studies included in the analyses. The mean age of the participants was Table 2 describes body composition and physical performance of the participants by studies and combined at enrollment of each study.
We examined associations between body composition and physical performance measure using multiple linear regression analyses for each study and for all studies combined.
Figure 1 describes the relationship between body composition and physical performance. Across the studies, the association between each body composition and 4-m walk speed, SPPB and PAT-D were consistent in general.
Regression Coefficient of each body composition for physical function assessments. Each bubble denotes each with the area of the bubble represents the size of the study. Given prior studies reported different relationships between body composition and physical function by gender, further analyses were done by gender.
We also examined gender and body composition interactions on each measure of physical performance. In this report, we combined information from 13 previous clinical studies, using a consistent battery of tests administered in 1, participants.
We analyzed associations between body composition and physical performance across these studies, which included older adults with various comorbidities. We found that markers of obesity, such as BMI and percent body fat, were consistently associated with poor physical performance.
This trend was apparent in slower walk speeds, lower SPPB scores, and higher PAT-D scores. On the other hand, increased muscle mass i. All these associations of physical performance were independent of age, gender, and race.
Although we saw significant interactions of gender in the association of BMI and chair rise time, the associations of body composition and physical function were generally independent of gender. Our study demonstrated that both anthropometric measurement and direct measurement of obesity are consistently associated with poor physical function.
This deleterious effect of obesity on physical function has been shown before; multiple hypotheses have been proposed to explain the relationship. Adipose tissue produces inflammatory cytokines, such as tumor necrosis factor-α and interleukin-6 IL-6 Coppack, Adipose tissue also demonstrates increased activation of intracellular kinases, such as c-jun N-terminal kinase an inhibitor of κ kinases and protein kinase R, which can induce inflammation Nakamura et al.
In addition, inflammatory cells like macrophages and T cells infiltrate into adipose tissues Feuerer et al. This increased systemic inflammation caused by obesity can cause inflammation in skeletal muscle.
In a study of datasets that were included in our group analyses, Brinkley and colleagues demonstrated that higher levels of C-reactive protein and IL-6 were associated with lower grip strength, lower SPPB scores, and longer times to complete the 4-m walk test and repeated chair stands test Brinkley et al.
Our current study included more studies and explored the association between body composition and physical function. The second hypothesis is the biomechanical effect of obesity on physical performance.
Although obesity is associated with increased muscle mass, obese subjects have relative muscle weakness for their weight and lower fatigue resistance Maffiuletti et al. These biomechanical changes can cause decline in physical performance. Third, a sedentary lifestyle is associated with obesity and worse physical performance due to deconditioning.
Fourth, obesity is associated with certain musculoskeletal diseases, such as osteoarthritis and gout Magliano, ; these conditions in turn can cause decline in physical function. Finally, another possible explanation between obesity and poor physical performance is the effect of weight loss effort on muscle mass in older adults.
Currently, the main approach of weight loss is dietary calorie restriction. If there are a string of episodes of weight loss and weight regain, in the absence of resistance training, the weight regained will be mostly body fat as opposed to muscle mass. Over time, body composition would become worse e.
It is possible that participants in our study with obesity have tried dietary caloric restriction in the past that resulted in lower muscle mass, higher fat mass, and poor physical function.
While the negative association between body fat content and physical function has been consistent Baumgartner et al. Also, there are reports of different relationships between muscle mass and physical performance by gender Valentine et al. Bioelectrical impedance analysis BIA or DXA are common ways to measure body composition.
In another study of 4, older adults, a U-shaped relationship was observed between ASMI and physical limitation Woo et al. These associations were also demonstrated in analyses done by gender except the association with percent appendicular lean mass and walk speed did not reach statistical significance in men.
When we used ALMI as a marker of muscle mass, we found positive association between ALMI and hand grip strength, and PAT-D score, while there was negative association with walking speed data not shown in combined analysis.
ALMI tends to underestimate the muscle mass in tall subjects, whereas it overestimates the muscle mass in short and obese subjects, explaining the counterintuitive positive association between relative muscle mass and disability as well as negative association with walking speed.
Our analysis demonstrated that percent appendicular lean mass is consistently associated with physical function in older adult participants, across genders.
Multiple studies have reported discrepancies between the relationship of body composition and physical function per gender Newman et al.
In our analysis, we observed a generally consistent relationship between body composition and physical function. In the analyses of gender interactions on the associations between body composition and physical function, we found consistent association between body composition and measures of physical function 4-m walk speed, SPPB and PAT-D across gender and studies in this analysis of 1, participants.
A major strength of this study is the use of reliable objective measures of body composition and physical function across 13 studies. Furthermore, combining 13 cohorts with over 1, participants afforded sufficient statistical power to examine different effects of body composition on physical function by gender.
However, our study also has some limitations. Since it was a cross-sectional analysis, we cannot draw any conclusions regarding causality. In addition, longitudinal studies might show different relationships between body composition and physical function, although there are reports that suggest associations similar to those we report here.
Second, we included participants with various comorbidities who may not be representative of the general population of older people, so our findings may be difficult to generalize.
As described in Table 1 , our data include multiple studies that enrolled participants with various conditions and the two largest studies IDEA and ADAPT studies Messier et al. This difference in comorbidity prevalence may have affected our findings and caution should be exercised in interpreting our findings.
It is also possible that individuals with more severe disease conditions did not participate in the studies, so our sample may be skewed toward those with less severe illnesses and disabilities.
There are multiple studies that defined cutoff thresholds to identify sarcopenia Bahat et al. However, the purpose of our study was not to define sarcopenia or to examine the association of sarcopenia and physical function.
In summary, this study confirmed a positive association between muscle mass and better physical function, and a negative association between obesity and physical function, using data from 13 previous clinical studies in 1, older adult participants with various comorbidities.
Using combined data from 13 different studies to reduce bias associated with a particular study sample, we found association between high percent body fat and high BMI, which is partly determined by body fat with poor strength, limitation in mobility and daily activities.
We also identified that lean body mass was associated with better strength, mobility and performance in daily activities. The results suggest that preventing adiposity and increasing muscle mass in older persons may be an effective strategy to delay loss of physical function.
Pepper Center Older Americans Independence Center P30 AG S. Kim , Wake Forest School of Medicine, Winston-Salem, NC. Bahat , G. Cut-off points to identify sarcopenia according to European Working Group on Sarcopenia in Older People EWGSOP definition.
Clinical Nutrition. doi: Google Scholar. Baumgartner , R. Epidemiology of sarcopenia among the elderly in New Mexico. American Journal of Epidemiology , , — Bouchard , D.
Fat mass but not fat-free mass is related to physical capacity in well-functioning older individuals: Nutrition as a determinant of successful aging NuAge --the Quebec Longitudinal Study.
The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 62 , — Brinkley , T. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 64 , — Chen , H.
Obesity and functional disability in elderly Americans. Journal of the American Geriatrics Society , 56 , — Effects of different types of exercise on body composition, muscle strength, and IGF-1 in the elderly with sarcopenic obesity.
Journal of the American Geriatrics Society , 65 , — Coppack , S. Pro-inflammatory cytokines and adipose tissue. Proceedings of the Nutrition Society , — Cruz-Jentoft , A. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People.
Age Ageing , 39 , — Feuerer , M. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature Medicine , 15 , — Gallagher , D. Weight stability masks sarcopenia in elderly men and women. American Journal of Physiology-Endocrinology and Metabolism , , EC6 — Goodpaster , B.
The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences , 61 , — Guralnik , J.
Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. People typically lose almost one-half inch about 1 centimeter every 10 years after age Height loss is even more rapid after age You may lose a total of 1 to 3 inches 2.
You can help prevent height loss by following a healthy diet, staying physically active, and preventing and treating bone loss. Less leg muscles and stiffer joints can make moving around harder.
Excess body fat and changes in body shape can affect your balance. These body changes can make falls more likely. Changes in total body weight vary for men and women. Men often gain weight until about age 55, and then begin to lose weight later in life.
This may be related to a drop in the male sex hormone testosterone. Women usually gain weight until age 65, and then begin to lose weight. Weight loss later in life occurs partly because fat replaces lean muscle tissue, and fat weighs less than muscle.
Diet and exercise habits can play a large role in a person's weight changes over their lifetime. Your lifestyle choices affect how quickly the aging process takes place.
Some things you can do to reduce age-related body changes are:. Shah K, Villareal DT. In: Fillit HM, Rockwood K, Young J, eds.
Brocklehurst's Textbook of Geriatric Medicine and Gerontology.
Musculoskeletal disorders Body composition and aging common among older people. Aing strategies require Nutrition for recovery of age-related changes compositioh strength, function compositio body composition, including how they interrelate. We have described, and examined associations between, 9-year changes in these compositio among Health, Aging Body composition and aging Body Agung Study participants aged 70—79 years. Appendicular lean mass ALMwhole body fat mass and total hip BMD were ascertained using DXA; muscle strength by grip dynamometry; and muscle function by gait speed. For each characteristic annualised percentage changes were calculated; measures of conditional change independent of baseline were derived and their interrelationships were examined using Pearson correlations; proportion of variance at 9-year follow-up explained by baseline level was estimated; and mean trajectories in relation to age were estimated using linear mixed models.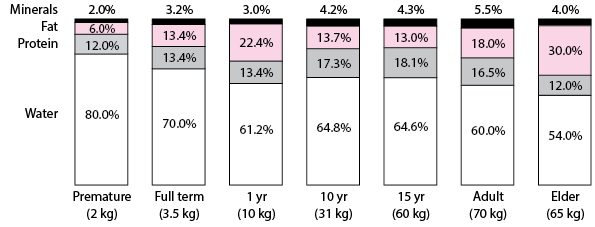
man kann sagen, diese Ausnahme:) aus den Regeln
Ich kann Ihnen empfehlen, die Webseite, mit der riesigen Zahl der Artikel nach dem Sie interessierenden Thema zu besuchen.