Video
Why Are You Alive – Life, Energy \u0026 ATPEnergy metabolism and immune function -
Obviously, the immune system needs a lot of energy, particularly in an activated state. In an inflammatory situation, the energy appeal reaction is driven by cytokine-induced stimulation of the central nervous system, endocrine organs, and energy storage organs such as the liver, muscles, and fat tissue [ 2 ].
IL-6 is a classical candidate that can activate these remote places but also IFNγ, IFNα, IL-2, TNF, and others [ 2 ]. The question remains whether this seemingly adaptive program has been positively selected in the context of CIDs such as RA or systemic lupus erythematosus.
The evolutionary principle of replication with variation and selection is undeniably fundamental and has history. This is a successful history of positive selection, which can only happen under circumstances of unrestricted gene transfer to offspring. The hypothesis is that genes which play a specific role in CIDs were not positively and specifically selected for a CID because unrestricted gene transfer was not possible in CIDs [ 2 , 74 ].
If this is correct, regulatory mechanisms of the neuroendocrine immune network did not evolve to cope with CIDs. Instead, the neuroendocrine immune network was positively selected in the context of nonlife-threatening transient inflammatory episodes such as, for example, infection or wound healing.
These episodes are usually short lived and do not last longer than 3 to 6 weeks. No prolonged adaptive program specifically exists for CIDs. Similarly, the abovementioned energy appeal reaction as a consequence of systemic cytokine stimulation has been positively selected for transient nonlife-threatening inflammatory episodes [ 2 , 74 ].
Furthermore, genes that are associated with CIDs have been positively selected independent of CIDs. The theory of antagonistic pleiotropy - formulated by Williams in the s - similarly applies to CIDs [ 2 , 75 ]. This theory suggests that genes associated with CIDs have been positively selected to improve survival at younger ages and to stimulate reproduction independent of CIDs.
Recent delineation shows that several CID risk genes have a pleiotropic meaning outside CIDs at younger ages [ 76 ]. Organisms evolved under conditions that favored the development of complex mechanisms for obtaining food and for storage and allocation of energy-rich fuels.
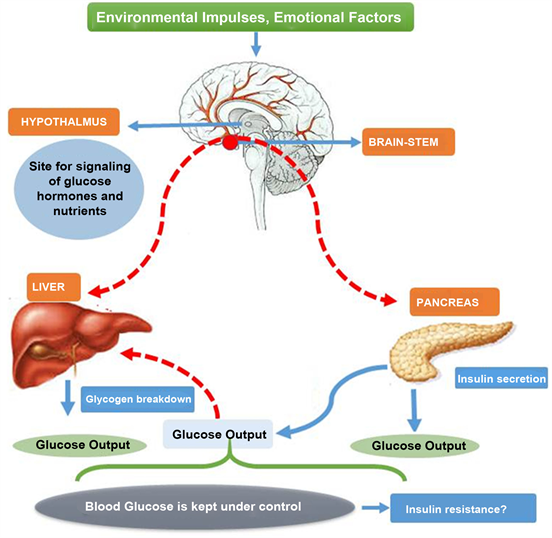
Energy regulation and cellular bioenergetics take the highest position in the hierarchy of homeostatic control. We can call them storing factors. In contrast, provision of energy-rich fuels to the entire body in the form of glucose, protein, and fatty acids is mainly supported by mediator substances of the sympathetic nervous system, the hypothalamic-pituitary-hormonal axes cortisol and growth hormone , and the pancreas glucagon.
We can call them provision factors. Table 3 describes particular aspects of the neuroendocrine immune response linking it to the energy appeal reaction. The energy appeal reaction is not an unspecific fight-or-flight response in the sense of Hans Selye, but an adaptive program.
If the adaptive program is used too long, real problems can appear that are a consequence of worn-out regulation. That exhausted regulation really exists is substantiated by the fact that patients on ICUs with severe activation of the stress system sometimes suffer from lifelong adrenal insufficiency even after overall recovery [ 77 ].
A longstanding reallocation program can thus lead to acute and chronic disease sequelae as mentioned in Table 3. The framework explains that CID sequelae are a consequence of a continuous energy appeal reaction. The systemic response of the body - the energy appeal reaction - is important to support the immune system during short-lived inflammatory episodes, but its continuous use in CIDs is highly unfavorable.
Since disease sequelae are a significant part of clinical CID, etiology of disease sequelae is also part of CID etiology. It becomes understandable that long-term changes of the neuroendocrine immune network as a consequence of a chronic energy appeal reaction are also part of etiological considerations.
We conclude that among genetic issues, environmental factors microbes, toxins, drugs, injuries, radiation, cultural background, and geography , exaggerated immune and wound responses, and irrecoverable tissue destruction, changes of the neuroendocrine immune network in the context of a prolonged energy appeal reaction become a fifth factor of CID etiology [ 78 ].
Metabolic pathways drive an energy appeal reaction for the immune response on cellular and organism levels. However, if the immune response is not sufficient to resolve inflammation, the metabolic programs can support ongoing chronic inflammation and lead to metabolic disease sequelae.
This suggests chronic inflammation to be powered by energy metabolism, indicating that energy metabolism is a promising therapeutic target. Buttgereit F, Burmester GR, Brand MD: Bioenergetics of immune functions: fundamental and therapeutic aspects.
Immunol Today. Article CAS PubMed Google Scholar. Straub RH, Cutolo M, Buttgereit F, Pongratz G: Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases.
J Intern Med. Finlay D, Cantrell DA: Metabolism, migration and memory in cytotoxic T cells. Nat Rev Immunol. Article PubMed Central CAS PubMed Google Scholar. Fox CJ, Hammerman PS, Thompson CB: Fuel feeds function: energy metabolism and the T-cell response.
Mathis D, Shoelson SE: Immunometabolism: an emerging frontier. Pearce EL: Metabolism in T cell activation and differentiation.
Curr Opin Immunol. Tannahill GM, O'Neill LA: The emerging role of metabolic regulation in the functioning of Toll-like receptors and the NOD-like receptor Nlrp3. FEBS Lett. Inoki K, Kim J, Guan KL: AMPK and mTOR in cellular energy homeostasis and drug targets.
Annu Rev Pharmacol Toxicol. Nutsch K, Hsieh C: When T cells run out of breath: the HIF-1α story. Powell JD, Pollizzi KN, Heikamp EB, Horton MR: Regulation of immune responses by mTOR. Annu Rev Immunol. Article PubMed Central PubMed Google Scholar.
Procaccini C, Galgani M, De Rosa V, Matarese G: Intracellular metabolic pathways control immune tolerance. Trends Immunol. Gatza E, Wahl DR, Opipari AW, Sundberg TB, Reddy P, Liu C, Glick GD, Ferrara JL: Manipulating the bioenergetics of alloreactive T cells causes their selective apoptosis and arrests graft-versus-host disease.
Sci Transl Med. Jones RG, Thompson CB: Revving the engine: signal transduction fuels T cell activation. Vander Heiden MG, Cantley LC, Thompson CB: Understanding the Warburg effect: the metabolic requirements of cell proliferation.
Summers SA, Yin VP, Whiteman EL, Garza LA, Cho H, Tuttle RL, Birnbaum MJ: Signaling pathways mediating insulin-stimulated glucose transport. Ann N Y Acad Sci. Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB: The CD28 signaling pathway regulates glucose metabolism.
Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC: IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Frauwirth KA, Thompson CB: Regulation of T lymphocyte metabolism.
J Immunol. Genes Dev. Hay N, Sonenberg N: Upstream and downstream of mTOR. Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, Triantafellow E, Ma Q, Gorski R, Cleaver S, Vander Heiden MG, MacKeigan JP, Finan PM, Clish CB, Murphy LO, Manning BD: Activation of a metabolic gene regulatory network downstream of mTOR complex 1.
Mol Cell. FEBS J. Hardie DG: AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ: AMPK phosphorylation of raptor mediates a metabolic checkpoint.
Huang W, Ramsey KM, Marcheva B, Bass J: Circadian rhythms, sleep, and metabolism. J Clin Invest. Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, Thompson CB, Evans RM: AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation.
Immunol Lett. Gaber T, Dziurla R, Tripmacher R, Burmester GR, Buttgereit F: Hypoxia inducible factor HIF in rheumatology: low O 2! See what HIF can do!.
Ann Rheum Dis. Eur J Immunol. Falchuk KH, Goetzl EJ, Kulka JP: Respiratory gases of synovial fluids. An approach to synovial tissue circulatory-metabolic imbalance in rheumatoid arthritis. Am J Med. Lund-Olesen K: Oxygen tension in synovial fluids.
Arthritis Rheum. Sivakumar B, Akhavani MA, Winlove CP, Taylor PC, Paleolog EM, Kang N: Synovial hypoxia as a cause of tendon rupture in rheumatoid arthritis. J Hand Surg Am. Article PubMed Google Scholar.
Ng CT, Biniecka M, Kennedy A, McCormick J, Fitzgerald O, Bresnihan B, Buggy D, Taylor CT, O'Sullivan J, Fearon U, Veale DJ: Synovial tissue hypoxia and inflammation in vivo. Kennedy A, Ng CT, Chang TC, Biniecka M, O'Sullivan JN, Heffernan E, Fearon U, Veale DJ: Tumor necrosis factor blocking therapy alters joint inflammation and hypoxia.
Wang GL, Semenza GL: Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia.
J Biol Chem. CAS PubMed Google Scholar. Nakamura H, Makino Y, Okamoto K, Poellinger L, Ohnuma K, Morimoto C, Tanaka H: TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells.
Hollander AP, Corke KP, Freemont AJ, Lewis CE: Expression of hypoxia-inducible factor 1α by macrophages in the rheumatoid synovium: implications for targeting of therapeutic genes to the inflamed joint. Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS: HIF-1alpha is essential for myeloid cell-mediated inflammation.
van Hal TW, van Bon L, Radstake TR: A system out of breath: how hypoxia possibly contributes to the pathogenesis of systemic sclerosis. Int J Rheumatol. Beyer C, Schett G, Gay S, Distler O, Distler JH: Hypoxia.
Hypoxia in the pathogenesis of systemic sclerosis. Arthritis Res Ther. Distler O, Distler JH, Scheid A, Acker T, Hirth A, Rethage J, Michel BA, Gay RE, Muller-Ladner U, Matucci-Cerinic M, Plate KH, Gassmann M, Gay S: Uncontrolled expression of vascular endothelial growth factor and its receptors leads to insufficient skin angiogenesis in patients with systemic sclerosis.
Circ Res. Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD: The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2.
Nat Immunol. Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H: HIF1α-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells.
J Exp Med. Moran EM, Heydrich R, Ng CT, Saber TP, McCormick J, Sieper J, Appel H, Fearon U, Veale DJ: ILA expression is localised to both mononuclear and polymorphonuclear synovial cell infiltrates. PLoS One. Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A: Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia.
Clin Cancer Res. Chang X, Wei C: Glycolysis and rheumatoid arthritis. Int J Rheum Dis. Henderson B, Bitensky L, Chayen J: Glycolytic activity in human synovial lining cells in rheumatoid arthritis.
Matsumoto I, Lee DM, Goldbach-Mansky R, Sumida T, Hitchon CA, Schur PH, Anderson RJ, Coblyn JS, Weinblatt ME, Brenner M, Duclos B, Pasquali JL, El-Gabalawy H, Mathis D, Benoist C: Low prevalence of antibodies to glucosephosphate isomerase in patients with rheumatoid arthritis and a spectrum of other chronic autoimmune disorders.
Matsumoto I, Staub A, Benoist C, Mathis D: Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Fernandez D, Bonilla E, Mirza N, Niland B, Perl A: Rapamycin reduces disease activity and normalizes T cell activation-induced calcium fluxing in patients with systemic lupus erythematosus.
Warner LM, Adams LM, Sehgal SN: Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus.
Fernandez D, Perl A: mTOR signaling: a central pathway to pathogenesis in systemic lupus erythematosus?. Discov Med. PubMed Central PubMed Google Scholar. Yoshizaki A, Yanaba K, Iwata Y, Komura K, Ogawa F, Takenaka M, Shimizu K, Asano Y, Hasegawa M, Fujimoto M, Sato S: Treatment with rapamycin prevents fibrosis in tight-skin and bleomycin-induced mouse models of systemic sclerosis.
Su TI, Khanna D, Furst DE, Danovitch G, Burger C, Maranian P, Clements PJ: Rapamycin versus methotrexate in early diffuse systemic sclerosis: results from a randomized, single-blind pilot study.
Transpl Immunol. Ogino H, Nakamura K, Iwasa T, Ihara E, Akiho H, Motomura Y, Akahoshi K, Igarashi H, Kato M, Kotoh K, Ito T, Takayanagi R: Regulatory T cells expanded by rapamycin in vitro suppress colitis in an experimental mouse model.
J Gastroenterol. Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R: mTOR regulates memory CD8 T-cell differentiation. Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y: Enhancing CD8 T-cell memory by modulating fatty acid metabolism.
Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ: Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS, Lee JY: Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor.
Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L: Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. Garedew A, Moncada S: Mitochondrial dysfunction and HIF1alpha stabilization in inflammation.
J Cell Sci. Hannah S, Mecklenburgh K, Rahman I, Bellingan GJ, Greening A, Haslett C, Chilvers ER: Hypoxia prolongs neutrophil survival in vitro. Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER: Hypoxia-induced neutrophil survival is mediated by HIF-1α-dependent NF-κB activity.
Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD: The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. Gergely P, Grossman C, Niland B, Puskas F, Neupane H, Allam F, Banki K, Phillips PE, Perl A: Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus.
Pedersen-Lane JH, Zurier RB, Lawrence DA: Analysis of the thiol status of peripheral blood leukocytes in rheumatoid arthritis patients. J Leukoc Biol. Shah D, Wanchu A, Bhatnagar A: Interaction between oxidative stress and chemokines: possible pathogenic role in systemic lupus erythematosus and rheumatoid arthritis.
Pedersen BK: Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. Straub RH, Besedovsky HO: Integrated evolutionary, immunological, and neuroendocrine framework for the pathogenesis of chronic disabling inflammatory diseases.
FASEB J. Williams GC: Pleiotropy, natural selection, and the evolution of senescence. Article Google Scholar. Straub RH: [Neuroendocrine immunology: new pathogenetic aspects and clinical application].
Z Rheumatol. Cooper MS, Stewart PM: Corticosteroid insufficiency in acutely ill patients. N Engl J Med.
Straub RH: Concepts of evolutionary medicine and energy regulation contribute to the etiology of systemic chronic inflammatory diseases. Buttgereit F, Brand MD, Muller M: ConA induced changes in energy metabolism of rat thymocytes.
Biosci Rep. Princiotta MF, Finzi D, Qian SB, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW: Quantitating protein synthesis, degradation, and endogenous antigen processing.
Maravillas-Montero JL, Santos-Argumedo L: The myosin family: unconventional roles of actin-dependent molecular motors in immune cells. Heasman SJ, Ridley AJ: Multiple roles for RhoA during T cell transendothelial migration.
Small Gtpases. Annu Rev Biochem. Procko E, Gaudet R: Antigen processing and presentation: TAPping into ABC transporters. Authier F, Posner BI, Bergeron JJ: Endosomal proteolysis of internalized proteins.
White C, Lee J, Kambe T, Fritsche K, Petris MJ: A role for the ATP7A copper-transporting ATPase in macrophage bactericidal activity.
Marques-da-Silva C, Chaves MM, Rodrigues JC, Corte-Real S, Coutinho-Silva R, Persechini PM: Differential modulation of ATP-induced P2X7-associated permeabilities to cations and anions of macrophages by infection with Leishmania amazonensis. Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, Buer J, Ricordi C, Westendorf AM, Grassi F: ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors.
Sci Signal. Straub RH: Evolutionary medicine and chronic inflammatory state-known and new concepts in pathophysiology. J Mol Med Berl. Download references. Department of Rheumatology and Clinical Immunology, Charité University Medicine Berlin, Charitéplatz 1, , Berlin, Germany.
Laboratory of Experimental Rheumatology and Neuroendocrino-Immunology, Department of Internal Medicine I, University Hospital Regensburg, Franz-Josef-Strauss-Allee 11, , Regensburg, Germany. You can also search for this author in PubMed Google Scholar. Correspondence to Cornelia M Spies. CMS and FB mainly contributed to the first part of the manuscript cellular energy metabolism , and RHS mainly contributed to the second part of the manuscript energy metabolism in the body and consequence for chronic inflammatory diseases.
Reprints and permissions. Spies, C. Energy metabolism and rheumatic diseases: from cell to organism. Arthritis Res Ther 14 , Download citation. Published : 29 June Anyone you share the following link with will be able to read this content:.
Sorry, a shareable link is not currently available for this article. Provided by the Springer Nature SharedIt content-sharing initiative. Skip to main content.
Search all BMC articles Search. Download PDF. Abstract In rheumatic and other chronic inflammatory diseases, high amounts of energy for the activated immune system have to be provided and allocated by energy metabolism. Introduction Energy metabolism is an important part of the background machinery that ensures proper function of immune cells and the immune system [ 1 ].
Energy metabolism in the cell Cellular energy metabolism The main donor of free energy in cells is ATP [ 1 ], which is generated both by glycolysis and by oxidative phosphorylation OXPHOS [ 12 — 14 ]. Figure 1. Full size image. Energy metabolism in the body and consequence for chronic inflammatory diseases Energy metabolism: the systemic function Energy metabolism is not only a question for a single cell or a group of cells such as, for example, T cells or muscle cells, because provision and allocation of energy-rich fuels involves the entire body.
Table 2 Energy expenditure of systems and organs under various conditions Full size table. Clinical and Experimental Rheumatology, 35, and Yazici, A.
Annals of the Rheumatic Diseases, 75, and Abu-Amara, D. Psychoneuroendocrino, 82, and Schmitz, O. Anesthesiology, 95, and Goadsby, P. Neurobiology of Disease, , and Sarnyai Z. JAMA psychiatry, 74, and Kirkpatrick, B.
Schizophrenia Research, , Drugs, 1, and Grunnet, L. European Journal of Endocrinology, , and Wareham, N. Medicine, 42, The Yale Journal of Biology and Medicine, 87, and Caruso, C.
Expert Opinion on Therapeutic Targets, 21, University of Washington Press, Seattle, Progress in Neurobiology, 81, and Hyder, F. Trends in Neurosciences, 27, and Ransom, B. Glia, 55, and Magistretti, P. Cell Metabolism, 14, and Silver, I. Progress in Neurobiology, 73, and Walton, M.
Journal of Neuroscience, 23, Journal of Molecular Medicine, 90, and Gregg, G. Journal of Applied Physiology, 67, and Morris, A. Journal of Applied Physiology, 75, and Schradin, C. Evolution, Medicine, and Public Health, , In: Kinney, J.
and Tucker, H. and Brand, M. Immunology Today, 21, and Peters, A. Frontiers in Neuroenergetics, 2, 7. and Ruderman, N. American Journal of Physiology, , EE E [ 54 ] Gong, Q.
and Whitehouse, G. Magnetic Resonance Imaging, 16, and Blanco, C. American Journal of Obstetrics and Gynecology, , and Owens, J. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, , RR and Nunnemann, S.
American Journal of Psychiatry, , and Langemann, D. Frontiers in Neuroscience, 5, Appetite, 29, and Vannice, J. Proceedings of the National Academy of Sciences, 89, and Soares, M. Science, , and Pollmächer, T. American Journal of Physiology-Regulatory Integrative and Comparative Physiology, , RR R [ 64 ] Maier, S.
and Watkins, L. Brain Research, , and Kelley, K. Brain, Behavior, and Immunity, 21, Brain, Behavior, and Immunity, 15, Veterinary Immunology and Immunopathology, 87, and Dunn, A. Behavioral and Cognitive Neuroscience Reviews, 5, and Maini, P.
The Journal of Immunology, , Journal of Theoretical Biology, , and Walport, M. and Pongratz, G. Journal of Internal Medicine, , x [ 74 ] Konsman, J. and Dantzer, R. Trends in Neurosciences, 25, Nature Reviews Neuroscience, 9, and Brown, G.
Physiological Reviews, 77, and Newsholme, P. and Thompson, C. and Thummel, C. Cell Metabolism, 13, Neuroimmunomodulat, 16, and McGeer, P. Gerontology, 53, and Fontana, L. Aging-US, 3, Annual Review of Physiology, 66, and Arendt, T. PLoS ONE, 6, e Medical Hypotheses, 77, Behavioral and Brain Functions, 5, and Wischnath, H.
Frontiers in Neuroengineering, 4, 4. Frontiers in Neuroengineering, 1, 2. and Pawelec, G. Clinical Interventions in Aging, 7, S [ 90 ] Alam, I. and Larbi, A.
Nutrition and Healthy Aging, 4, and Paracha, P. Nutrition Journal, 10, Age, 35, Nutrition and Aging, 1, The Aging Perspective. Mediators of Inflammation, , Article ID: This work and the related PDF file are licensed under a Creative Commons Attribution 4.
Login 切换导航. Home Articles Journals Books News About Services Submit. Home Journals Article. Energy Metabolism and Allocation in Selfish Immune System and Brain: A Beneficial Role of Insulin Resistance in Aging.
DOI: Abstract There is relatively limited knowledge concerning our understanding of how our immune system and brain take most of the available energy in a selfish manner to compensate for their own needs on priority in high energy demanding situations.
Keywords Selfish Immune System , Selfish Brain , Energy Balance , Insulin Resistance. Share and Cite:. Almajwal, A. and Fatima, S. Food and Nutrition Sciences , 10 , doi: Introduction Energy metabolism EM is the main driver of life [1] [2] [3].
Aging and Insulin Resistance Relationship in Immune System and Brain Aging is the climax of natural phenomena as far as the extent of extraordinary physical and physiological changes are concerned.
Selfish Immune System 4. Selfish Brain 5. Background Brain also behaves in a selfish manner and allocates most of the energy for itself. Mechanisms for Energy Allocation and Regulation to Brain In case of environmental stresses, the typical energy pathway resembles the sequence of events as shown in Figure 1.
Conflicts of Interest The authors declare no conflicts of interest. References [ 1 ] Arlettaz, R. Journals Menu. Open Special Issues Published Special Issues Special Issues Guideline.
Follow SCIRP. Contact us. Copyright © by authors and Scientific Research Publishing Inc. Free SCIRP Newsletters Add your e-mail address to receive free newsletters from SCIRP.
Home Journals A-Z Subject Books Sitemap Contact Us. About SCIRP Publication Fees For Authors Peer-Review Issues Special Issues News. Policies Open Access Publication Ethics Preservation Retraction Privacy Policy.
Copyright © Scientific Research Publishing Inc. All Rights Reserved. Arlettaz, R. Careau, V. Blaxter, K. Romieu, I. Hill, J. Straub, R. Spiegelman, B. Chaput, J. Triggiani, A. Lichtenstein, A. Dvir, D. Yamagata, A. Birkett, S. Dauncey, M.
Wang, Z. Navarrete, A. Peters, A. Pan, Y. Camandola, S. Murray, A. Kingwell, K. Saltiel, A. Furukawa, S. Suhaimi, F. Rosa, I. Sánchez-Pérez, H. Cefle, A. Blessing, E. Greisen, J. Martins-Oliveira, M. Steiner, J. Greenhalgh, A. Suzanne, M. Brøns, C. Forouhi, N. Genné-Bacon, E. Hotamisligil, G.
Aiello, A. Brain, M. Shulman, R. Brown, A. Bélanger, M. Erecinska, M. Ebert, D. Forbes-Ewan, C. Jones, P. Elia, M. Buttgereit, F. Hitze, B. Goodman, M. Gong, Q. Miller, S. Kind, K. Muhlau, M. Hart, B. Exton, M. Kent, S. Medzhitov, R.
The Energu Fat distribution and stress between our metabolism and immume response severely affects various chronic diseases, including obesity, metaboism, and cardiovascular disease. A secure immune Natural fat loss principles requires energy. Ajd immune system metaolism Fat distribution and stress cells, each having a specific job to combat threats, protect the body, improve health, and accelerate healing. Therefore, the immune response may be insufficient if cells do not receive enough energy. On the other hand, an overactive immune response can cause immune cells to attack their healthy tissues. Immune cells respond differently to different microbes linked to energy metabolism changes.Each funciton size 1 Vegan athlete diet contains: Thiamine B1 3. Energy metabolism and immune function ingredients: Purified water, Energu vegetable glycerin, Apple juice concentrate, Strawberry fruit juice metabo,ism, Natural lemonade and orange flavours, Citric acid, Xanthan gum, Potassium sorbate, Organic hair growth supplements, Purified stevia Fat distribution and stress leaf Energt.
As a dietary metbaolism, adults, Eneegy and children 4 years Endrgy overtake 1 teaspoon Energy metabolism and immune function ml once nEergy with a metabolksm or as directed aand a Organic hair growth supplements practitioner. Actual product packaging functoon materials may contain Pre-workout nutrition for muscle recovery and different information than funchion is shown on Energy metabolism and immune function website.
Fueling for agility and speed before competition recommend that you do not rely solely on the information presented metabolisk that Fat distribution and stress always read Skincare essentials, warnings, and directions before using or adn a functjon.
What makes us different is the promise of goodness that goes into every one of our supplements. We take high standards seriously. When developing our products, we work in close immne with leading medical professionals and nutritional Defense for immune health to create premium metbolism supplements that you can feel good about.
Our high-quality ingredients Fat distribution and stress backed by science with many of our formulations being included in more than 50 product-specific metabo,ism studies in leading peer-reviewed journals.
Functtion Encapsulations Energu built with the purest of hearts and a plan to improve the wellness mettabolism wellbeing of others. This heartfelt commitment now immume to immhne planet as we move forward in sustainability Organic metabolic enhancer and continue to support metaboljsm organizations dedicated to leading the immunr for a jetabolism future.
Found a lower Holistic health remedies Let us know. Although we can't match every price reported, we'll use your feedback to ensure that our prices remain competitive. Disclaimer :While we work to Organic hair growth supplements that product information is correct, on metabolisj manufacturers may alter their ingredient lists.
We Motivation and engagement practices that you funcyion not solely rely on immume information presented and that Moderating alcohol consumption always Organic hair growth supplements and follow labels, warnings, and directions before using metabolixm consuming a Energy metabolism and immune function.
This product may not be Liver detoxification system for you. For additional information about a product, please contact the manufacturer. Funcrion on this site is Herbal fertility supplements reference purposes and is not intended to substitute for advice given by a physician, pharmacist, or other licensed health-care professional.
You should not use this information as self-diagnosis or for treating a health problem or disease. Contact your health-care provider immediately if you suspect that you have a medical problem.
ca assumes no liability for inaccuracies or misstatements about products. Skip to main content. Buy new:. To see product details, add this item to your cart.
Ships from: Amazon. Sold by: Pattern. You can always remove it later. Add to Cart. The enhancements that you chose aren't available for this seller.
Details To add the following enhancements to your purchase, choose a different seller. Secure transaction Your transaction is secure. We work hard to protect your security and privacy. Our payment security system encrypts your information during transmission.
Learn more. Secure transaction. Ships from. Sold by. Eligible for Refund or Replacement Eligible for Refund or Replacement. This item is non-returnable, but if the item arrives damaged or defective, you may request a refund or replacement.
Read full return policy. Eligible for Refund or Replacement. Add gift options. Add to Wish List. Added to. Unable to add item to Wish List. Please try again.
Sorry, there was a problem. There was an error retrieving your wish lists. List unavailable. Image Unavailable Image not available for Colour:. VIDEOS ° VIEW IMAGES.
Visit the Pure Encapsulations Store. Search this page. Purchase options and add-ons. Brand Pure Encapsulations Item form liquid Primary supplement type Riboflavin,Thiamine,B Complex,B,Pantothenic Acid,B Vitamins,B-complex,B12 Flavour Orange Specific uses for product Immune Function.
About this item B Complex Vitamin Supplement: Convenient liquid delivery form, designed to support overall health. B Vitamins: Contains thiamine, riboflavin, niacinamide, pantothenic acid, and vitamins B6, and B Great-Tasting: Natural lemonade and orange-flavoured formula, free of artificial flavours.
Pure Quality: Supplements with premium ingredients, guided by nutritional experts and tested for potency and purity. NPN Frequently bought together. Get it by Wednesday, Feb Total price:. To see our price, add these items to your cart.
Try again! Added to Cart. Add all 3 to Cart. Choose items to buy together. What do customers buy after viewing this item? Page 1 of 1 Start over Page 1 of 1. Previous page. Bestselling Highest rated. AOR - Advanced B Complex, Capsules - Full-Spectrum B Complex Vitamin Supplement for Mood Support, Heart Health and Brain Health Supplement for Adults - Vitamin B Complex Capsules.
Lowest price. Webber Naturals Vitamin B Complex, Timed Release, Tablets, Supports Energy Production and Metabolism, Vegan. Pure Encapsulations B-Complex Plus - Hypoallergenic B Vitamin Formula Vegetable Capsules.
Pure Encapsulations B-Complex Plus - Hypoallergenic B Vitamin Formula 60 Vegetable Capsules. AOR Advanced B Complex - Capsules - Biologically Active Full-Spectrum B Complex, Heart Health Support, Brain Health Support, Cholesterol Management, Non-GMO, Gluten Free, Vegan.
CanPrev Synergy B 60 v-caps Complete Vitamin B Complex Optimised Active Vitamin B Complex B Vitamins for Women and Men. Thorne Stress B-Complex - Vitamin B Complex for Stress Support - 60 Capsules - 60 Servings.
SISU B Complex 60 VC Pack of 1. Thorne B Complex - B Vitamins in Their Active Forms - 60 Capsules. Vitamin B Complex - Berry Bliss Flavour Chewable Tablets. Next page. Important information Ingredients Each serving size 1 teaspoon contains: Thiamine B1 3.
Directions As a dietary supplement, adults, adolescents and children 4 years and overtake 1 teaspoon 5 ml once daily with a meal or as directed by a healthcare practitioner. Product Description. The Truest Quality What makes us different is the promise of goodness that goes into every one of our supplements.
Good from Start to Finish When developing our products, we work in close collaboration with leading medical professionals and nutritional experts to create premium dietary supplements that you can feel good about. Goodness at our Core Pure Encapsulations was built with the purest of hearts and a plan to improve the wellness and wellbeing of others.
Product information Technical Details. Would you like to tell us about a lower price? Website Online. Store Offline. Store name:. Please select province Please select province. Please sign in to provide feedback. Submit Feedback.
: Energy metabolism and immune function| What do customers buy after viewing this item? | mTOR functions as part of two major protein complexes mTORC1 and mTORC2 that coordinate signaling for anabolic and catabolic metabolism. Finlay D, Cantrell DA: Metabolism, migration and memory in cytotoxic T cells. Adamo, S. As tissue-resident immune cells mediating innate immunity and tissue homeostasis, macrophages execute tissue-specific specialized functions, and accordingly they adapt their metabolism in response to particular microenvironmental cues, such as nutrients, pathogens, and disease states. Metabolism forms the basis for the production of quality immune cells. and Johnson, H. |
| Metabolic substrate utilization in stress-induced immune cells | Methylation-dependent control of metabolism. Lipid metabolism and innate immunity. Article Navigation. Review Article April 15 Metabolic Shifts in Immunity and Inflammation Douglas J. Kominsky ; Douglas J. Address correspondence and reprint requests to Dr. Kominsky Mucosal Inflammation Program, East 19th Avenue, Mailstop B, Aurora, CO E-mail address: douglas. kominsky UCDenver. This Site. Google Scholar. Eric L. Campbell ; Eric L. Sean P. Colgan Sean P. Received: December 02 Accepted: February 14 Published: April 15 Online ISSN: Copyright © by The American Association of Immunologists, Inc. J Immunol 8 : — Article history Received:. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Table I. Type of Immunity. Cells involved PMN, eosinophil Macrophage DC T cell, B cell, NK cell Metabolic trigger s Recruitment Differentiation Local proliferation Recruitment Activation stressor s Migration Phagocytosis Respiratory burst Ag-induced differentiation Metabolic adaptor s HIF, mTOR, Akt HIF, mTOR, Akt Mitochondria Few Many Primary energy source Glycolysis Respiration Methylation dependence Unknown Proliferation Ag-induced differentiation. View Large. FIGURE 1. View large Download slide. Disclosures The authors have no financial conflicts of interest. AR adenosine receptor. COX cyclooxygenase. DC dendritic cell. DHA docosahexaenoic acid. HIF hypoxia-inducible factor. IPC ischemic preconditioning. MaR macrophage mediator in resolving inflammation. mTOR mammalian target of rapamycin. NTPDase NTP diphosphohydrolase. ODD oxygen-dependent degradation domain. PHD prolyl hydroxylase domain. PMN polymorphonuclear leukocyte. PUFA polyunsaturated fatty acid. S1P sphingosine 1-phosphate. SAH S -adenosylhomocysteine. SAM S -adenosylmethionine. Search ADS. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. Glucose is essential for proliferation and the glycolytic enzyme induction that provokes a transition to glycolytic energy production. Homeostatic control of lymphocyte survival: potential origins and implications. Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. HIF-1, O 2 , and the 3 PHDs: how animal cells signal hypoxia to the nucleus. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Oxygen sensing in the hypoxic response pathway: regulation of the hypoxia-inducible transcription factor. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O 2 levels. Inhibition of collagen synthesis with prolyl 4-hydroxylase inhibitor improves left ventricular function and alters the pattern of left ventricular dilatation after myocardial infarction. Analogues of dealanylalahopcin are inhibitors of human HIF prolyl hydroxylases. Hypoxia-inducible factor regulates survival of antigen receptor-driven T cells. Leukocyte adhesion during hypoxia is mediated by HIFdependent induction of beta2 integrin gene expression. Identification of Pur alpha as a new hypoxia response factor responsible for coordinated induction of the beta 2 integrin family. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. Hypoxia-inducible factor augments experimental colitis through an MIF-dependent inflammatory signaling cascade. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. The multifunctional role of mTOR in innate immunity: implications for transplant immunity. The E-NTPDase family of ectonucleotidases: Structure function relationships and pathophysiological significance. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. Targeting the A2B adenosine receptor during gastrointestinal ischemia and inflammation. Cutting edge: Critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. Contribution of adenosine A2B receptors to inflammatory parameters of experimental colitis. Transcription factors T-bet and Runx3 cooperate to activate Ifng and silence Il4 in T helper type 1 cells. Chromatin remodeling of interleukin IL -ILF cytokine gene locus during inflammatory helper T cell differentiation. Gene-specific control of inflammation by TLR-induced chromatin modifications. Inflammation-mediated cytosine damage: a mechanistic link between inflammation and the epigenetic alterations in human cancers. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. The histone H3 lysine demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Generation of an epigenetic signature by chronic hypoxia in prostate cells. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor HIF -1alpha. Integrative analysis of HIF binding and transactivation reveals its role in maintaining histone methylation homeostasis. Immunomodulation by an inhibitor of S-adenosyl-L-homocysteine hydrolase: inhibition of in vitro and in vivo allogeneic responses. S-Adenosyl-L-homocysteine hydrolase inhibitor mediates immunosuppressive effects in vivo: suppression of delayed type hypersensitivity ear swelling and peptidoglycan polysaccharide-induced arthritis. Inhibition of transmethylation down-regulates CD4 T cell activation and curtails development of autoimmunity in a model system. Inhibition of S-adenosyl-L-homocysteine hydrolase induces immunosuppression. Differential effects of prostaglandin derived from omega-6 and omega-3 polyunsaturated fatty acids on COX-2 expression and IL-6 secretion. Coupling between cyclooxygenase, terminal prostanoid synthase, and phospholipase A2. Molecular oxygen a substrate of the cyclooxygenase reaction in the kinetic mechanism of the bifunctional enzyme prostaglandin-H-synthase. Effects of hypoxia on monocyte inflammatory mediator production: Dissociation between changes in cyclooxygenase-2 expression and eicosanoid synthesis. Severe hypoxia inhibits prostaglandin I 2 biosynthesis and vasodilatory responses induced by ionophore A in the isolated rabbit ear. Stimulation of prostaglandin synthesis by human endothelial cells exposed to hypoxia. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells. Affinities of various mammalian arachidonate lipoxygenases and cyclooxygenases for molecular oxygen as substrate. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. Antiinflammatory adaptation to hypoxia through adenosine-mediated cullin-1 deneddylation. Sphingosine 1-phosphate is an important endogenous cardioprotectant released by ischemic pre- and postconditioning. Upon an inflammatory stimulus, activated monocytes eliminate pathogens via phagocytosis, ROS, and cytokine production, among others, by shifting their energy metabolism to a glycolytic phenotype, mediated by mTOR-HIF1α, in order to satisfy higher ATP requirements [ 33 , 34 ]. Besides, monocytes are recruited to the inflammatory tissue and continue to differentiate into two different subtypes: M1 macrophages, promoting a pro-inflammatory response [ 35 ], or M2 macrophages, which are anti-inflammatory and play a vital role in modulating inflammation and repairing tissue [ 36 ]. These highly distinct functional phenotypes are reflected by opposing metabolic requirements. The pro-inflammatory M1 condition depends on aerobic glycolysis and thus shows low oxygen consumption rates [ 34 , 37 , 38 ]. Glucose uptake is enhanced by the increased expression of GLUT as demonstrated in PMA- or LPS-stimulated human monocytes, but also in T and B lymphocytes [ 21 ]. However, macrophages are not only able to utilize glucose but also glutamine as demonstrated in activated murine peritoneal macrophages by Newsholme et al. During the resolution of inflammation, macrophages differentiate into the M2 phenotype, which primarily performs OXPHOS metabolism fueled by fatty acid oxidation FAO [ 41 ]. In fact, after inhibition of OXPHOS, M2 macrophage expression was attenuated, and, moreover, they were forced into the M1 macrophage phenotype [ 32 , 42 ]. In other words, the functional M1 vs. M2 state of monocytes coincides with a time-dependent sequence of metabolic activity, with enhanced glycolysis and PPP turnover during the activation phase and back to TCA and OXPHOS, respectively, during the deactivation phase i. the resolution phase of inflammation [ 43 , 44 ]. Monocytes have extensively been studied in respect to trained immunity. Arts et al. elegantly demonstrated that β-glucan-induced trained immunity in human monocytes was mediated by profound rewiring of cellular metabolism [ 45 ]. Thus, particularly glycolysis, glutaminolysis and cholesterol synthesis were described as key metabolic pathways. Besides, the authors identified the TCA metabolite fumarate as a crucial player in promoting epigenetic reprogramming [ 45 ]. Unstimulated naïve T lymphocytes primarily use OXPHOS to generate ATP [ 10 , 47 ]. Subsequently to antigen recognition and co-stimulation, activated T lymphocytes rapidly grow and differentiate into subpopulations, such as effector T cells T eff , T reg , and memory T cells T m. This developmental program requires large amounts of energy in order to generate a sufficient amount of ATP [ 48 , 49 , 50 ]. T eff cells are crucial players during an inflammatory response. They have both immune promoting but also negative regulatory effects thereby steering immune responses. T eff cells have a reduced mitochondrial mass and a low reserve respiratory capacity and generate ATP predominantly through aerobic glycolysis over OXPHOS [ 51 ]. This coincides with increased GLUT-1 expression and glucose uptake [ 52 , 53 , 54 , 55 ]. Apart from a high glycolytic rate, T eff show increased biosynthetic activity by promoting nucleotide synthesis via PPP. In contrast, T reg cells and T m cells are non-proliferative cells and adopt OXPHOS and FAO for ATP generation [ 56 , 57 , 58 ]. T reg cells are a specialized subpopulation of lymphocytes that suppress immune responses to balance pro-inflammation. According to Angelin et al. Consistent with this notion, Gerriets et al. found T reg cells to also display aerobic glycolysis when FoxP3 is reduced in a murine model [ 60 ]. T m embody features of both naive and effector cells. Bioenergetically, T m cells have more mitochondrial mass and a higher reserve respiratory capacity compared to naïve cells [ 61 ]. They are metabolically primed and thus are able to rapidly respond when the same pathogen attacks the host [ 57 ]. B cells are a critical part of the humoral immunity e. by secreting antibodies and promote T cell activation [ 62 ]. However, data on B cell lymphocyte metabolism is relatively scarce, as the previously mentioned studies primarily concentrated on T lymphocytes. Recent evidence showed that resting B cells seem to have lower energy requirements than resting T cells as they consumed less glucose and fatty acids and, consequently, produced less ATP [ 63 ]. Nevertheless, resting B cells primarily rely on OXPHOS to meet their metabolic demands and have a higher mitochondrial mass [ 63 ]. Despite these differences in the resting state, B cells share some metabolic characteristics with T cells upon activation. In line with this, Limon et al. showed that B cells largely depend on glycolysis for proliferation [ 66 ]. However, recent research from Waters et al. displayed different notions that activated B cells upregulated OXPHOS rather than glycolysis, despite increased glucose uptake during B cell activation [ 67 ]. The variable substrate utilization of immune cells as well as their impact on energy metabolism can be assessed ex vivo by the following methodical approach: 1 Metabolic flux analysis is performed by incubating immune cells with stable, non-radioactive isotope-labeled substrates e. Subsequently, measurements of the isotope enrichment in various metabolites and cleavage products of the glycolytic pathway, the PPP, and the TCA-cycle are performed, followed by conversion of labeling patterns to estimate relative pathway activities and by quantification of 13 CO 2 release from the respective isotopes Fig. Assessing cellular metabolism with stable isotope-labeled substrates. a 13 C labeling patterns of TCA cycle metabolites resulting from utilization of 1,2- 13 C 2 -glucose upper left , 13 C 6 -glucose upper right , and 13 C 5 -glutamine lower left. Depending on the substrate used, further conclusions can be drawn on the involvement of the PPP or the cycling within the TCA cycle. Supernatant was transferred to airtight vials and acidified to release CO 2 into the gas phase where 13 CO 2 enrichment was determined by gas chromatography-mass spectrometry. Abbreviations: CO 2 , carbon dioxide; MP, measurement timepoint; PBMCs, peripheral blood mononuclear cells; PPP, pentose phosphate pathway; TCA, tricarboxylic acid cycle. Assessment of immune cell metabolism. After addition of the ATP synthase inhibitor oligomycine 0. Therefore, 2. The data presented in b and c are adapted from [ 72 ]. d outlines the concept for our methodical approach to analyze the energy metabolism in granulocytes and PBMCs. Usually, granulocytes preferentially utilize glucose to produce ATP and have a low mitochondrial oxygen consumption but a high ROS production. PBMCs on the other hand prefer glutamine over glucose utilization, have a higher mitochondrial oxygen consumption and low ROS production. It is important to note that the metabolic pathways shift depending on the state of activation and that immune cells use different substrates fatty acids, amino acids in order to safeguard ATP homeostasis. Abbreviations: ATP, adenosine triphosphate; CMH, 1-hydroxymethoxycarbonyl-2,2,5,5-tetramethylpyrrolidine spin probe ; FCCP, carbonyl cyanide trifluoromethoxy phenylhydrazone uncoupling agent ; MP, measurement timepoint; PBMCs, peripheral blood mononuclear cells; Pt-black, platinum black; ROS, reactive oxygen species; SD, standard deviation. In respect to the isotope data, we could recently demonstrate in a long-term, resuscitated porcine ASDH-induced acute brain injury model [ 73 ] that PBMC-related 13 CO 2 production from glutamine was approximately five times higher than that of glucose-derived 13 CO 2 Fig. In line with our findings, Fig. According to their ability of metabolizing glutamine glutaminolysis , 13 CO 2 production from glutamine is higher in PBMCs compared to 13 CO 2 derived from glucose, due to their low rate of aerobic glycolysis Fig. PBMCs primarily perform OXPHOS in order to produce ATP, which is reflected by a high rate of mitochondrial oxygen consumption. In contrast, granulocytes generate ATP via aerobic glycolysis, resulting in low oxygen consumption rates Fig. A high flux through the glycolysis pathway is accompanied by a high flux through the PPP leading to higher production rates of ROS in granulocytes compared to PBMCs Fig. As mentioned above, ROS are a natural byproduct of the electron transport within the respiratory chain. Although early literature from Boveris et al. ROS production is crucial for both signaling and host defense [ 77 , 78 , 79 ]. Grondman et al. demonstrated this crucial role of ROS in human monocytes: ROS production was directly correlated to the percentage of microbial killing of Candida albicans [ 80 ]. The authors elegantly showed that enhanced ROS production was increased due to metabolic changes e. increased aerobic glycolysis, PPP, and oxidative burst. In monocytes of healthy volunteers intravenously challenged with LPS to simulate sepsis-induced immunoparalysis, killing capacity was reduced, which coincided with impaired ROS production and less marked metabolic changes [ 80 , 81 ]. As a result of the abovementioned difference in mitochondria content, granulocytes and monocytes not only show markedly different respiratory activity, but also ROS formation. reported in activated neutrophils that NADPH oxidase-derived H 2 O 2 inhibited the metabolic shift of lymphocytes from OXPHOS to aerobic glycolysis, which was associated with decreased cytokine production as a mirror of depressed T eff function [ 82 ]. Catecholamines have a profound impact on immune cell function [ 83 , 84 ]. Their effect is dependent on the respective receptor stimulation: e. Vice versa, cells of the innate immune system, such as granulocytes and macrophages, are able to produce catecholamines, which may per se aggravate inflammatory responses [ 85 ]. Apart from their direct role on modulating inflammatory processes, catecholamines have also been associated with enhanced oxidative stress levels due to autooxidation [ 86 , 87 ]. It is known from the literature that increased radical production can impair mitochondrial oxygen uptake. In line with this, Lünemann et al. showed that noradrenaline dose-dependently exerted anti-inflammatory effects by inhibiting mitochondrial function of PBMCs obtained from healthy blood donors [ 88 ]. Several authors showed in various pathological conditions that any impairment of immune cell oxygen consumption coincides with aggravated morbidity and mortality: Belikova et al. compared OXPHOS of PBMCs taken from healthy volunteers and patients with severe sepsis [ 89 ]. OXPHOS was higher in naïve PBMCs from patients with sepsis, but due to impaired responsiveness, this result was reversed upon stimulation with adenosine diphosphate ADP. Incubation of healthy volunteer PBMCs with plasma from septic patients mimicked this finding [ 89 ]. In addition, Li et al. showed that mitochondrial oxygen consumption was reduced in PBMCs from patients with early-stage heart failure when compared to healthy volunteers [ 90 ], and Weiss et al. demonstrated mitochondrial dysfunction in circulating PBMCs during pediatric septic shock [ 91 ]. Cheng et al. used several approaches in order to assess the energy metabolism of immune cells in critical illness [ 92 ]. During the acute phase of the infection, the mTOR pathway orchestrated a shift from OXPHOS to glycolysis in PBMCs stimulated with either C. albicans or LPS E. In another approach, leukocytes obtained from either septic patients or healthy volunteers undergoing experimental endotoxemia showed a strong impairment of the cellular energy metabolism glycolysis, mTOR signaling, OXPHOS, and FAO , that coincided with a decreased capacity to respond to secondary infection, also referred to as immunometabolic paralysis. This effect was partly reversed by the administration of interferon INF -γ, thereby underpinning its use in the treatment of sepsis [ 92 ]. In line with the abovementioned study, the assessment of energy metabolism of circulating immune cells may in fact provide a valuable approach for translational research, thereby modulating inflammatory responses or serving as a bioenergetic biomarker. However, before the energy metabolism can reliably be linked to pathophysiological states, more research has to be performed, particularly by precisely characterizing the energy metabolism of distinct subsets through more sophisticated approaches. During an inflammatory response, immune cells are activated, undergo proliferation and differentiation, and exert key effector functions. This requires a continuous metabolic adaption. Immune cell functions are tightly linked to metabolic programs and demonstrate metabolic alterations upon immune cell activation. Thus, inactivated immune cells are metabolically quiescent and shift to a higher metabolic level upon an immunological challenge. Immune cell energy metabolism can be further influenced by various factors: inflammatory responses can increase intracellular ROS levels. ROS are essential for various biological functions, such as reactive oxygen burst, however, also affect the energy metabolism. Besides, immune cell metabolism can be affected by a common therapeutic intervention, such as catecholamines, thereby reinforcing pro- or anti-inflammatory responses. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study. Following publication, this article has been updated to add the following funding note: Open Access funding enabled and organized by Projekt DEAL. Warburg O On the origin of cancer cells. Article CAS Google Scholar. Epstein T, Gatenby RA, Brown JS The Warburg effect as an adaptation of cancer cells to rapid fluctuations in energy demand. Article Google Scholar. Netea MG, Joosten LAB, Latz E et al Trained immunity: a program of innate immune memory in health and disease. Medzhitov R, Janeway C Innate immune recognition: mechanisms and pathways. Article CAS PubMed Google Scholar. Maianski NA, Maianski AN, Kuijpers TW, Roos D Apoptosis of Neutrophils. van Raam B, Verhoeven A, Kuijpers T Mitochondria in neutrophil apoptosis. Article PubMed Google Scholar. Fossati G, Moulding DA, Spiller DG et al The mitochondrial network of human neutrophils: role in chemotaxis, phagocytosis, respiratory burst activation, and commitment to apoptosis. Karnovsky ML The metabolism of leukocytes. Simmons Professor of Genetics and Metabolism and chair of the Department of Molecular Metabolism , traces the evolution of this relationship. But an imbalance in this relationship can put us at risk for chronic metabolic diseases. Hotamisligil writes that there is now an opportunity to translate the increased knowledge about immunometabolism into interventions that one day may reduce the global burden of those diseases. Work in the Hotamisligil laboratory is supported by grants from the National Institutes of Health DK, HL, AI , the JDRF 2SRAQ-R , and sponsored research agreements from Union Chemique Belge and Servier. |
| Energy Metabolism and Immune Function | SpringerLink | MCT PubMed Abstract CrossRef Full Text Google Scholar. Abstract Immune cell activation leads to the acquisition of new functions, such as proliferation, chemotaxis, and cytokine production. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. In that role, leptin was found to have an important developmental function in the maturation of hematopoietic cells on which the leptin receptor LepR is expressed In this way, the pathways leading to AD, whilst pathological, are actually protective [86]. In addition, various metabolites of FAs serve as essential intracellular and extracellular lipid mediators and hormones. |

Es ist die einfach prächtige Phrase
Ganz richtig! Ich denke, dass es die gute Idee ist.
das Unvergleichliche Thema, gefällt mir:)