
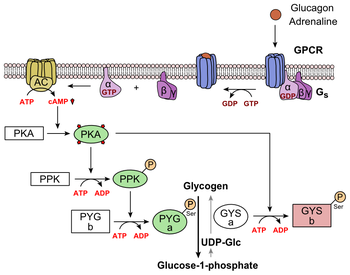
Glucagon mechanism -
GR-ASO improves β-cell function i. Recently, ISIS-GCGRRx 76 , IONIS-GCGRRxN 97 , and ISIS 98 have been shown to attenuate glucagon-stimulated hepatic glucose production and glucose fluctuations.
They support the treatment of GR-ASO in patients with T2D. The most well-characterized biological function of GLP-1 is to potentiate glucose-dependent insulin secretion, which makes the GLP-1R an attractive target in the treatment of T2D Thus, GLP-1R agonists are clinically used as anti-diabetic drugs Glucagon not only acts to antagonize insulin in the fasting state but also functions in the fed state and promotes insulin secretion to maintain normal blood glucose levels The insulin-promoting properties of glucagon are mediated by GCGR and GLP-1R in β cells 27 , 33 , ; however, GLP-1R is the main receptor to exert an insulin-stimulating effect It is reasonable to assume that even with GCGR mutations in β cells, glucagon binding to GLP-1R exerts an insulin-promoting effect that can reduce blood glucose concentrations in patients with diabetes.
Although GLP-1R agonists have been used for the treatment of diabetes, their efficacy is limited by target receptor desensitization and downregulation via the recruitment of β-arrestins , GLP-1R agonists with decreased β-arrestin-2 recruitment have shown promising effects in recent preclinical and clinical studies Understanding the mechanisms of action may resolve these issues with the application of GLP-1R agonists.
Owing to the traditional view that the main effect of glucagon is to increase blood glucose levels, the idea of increasing glucagon concentration as a means of reducing glucose levels initially met resistance. Nevertheless, the action of glucagon on GCGR and GLP-1R regulators of insulin secretion and energy metabolism has a significant effect on systemic glucose homeostasis On the one hand, GCGR and GLP-1R co-agonists can activate GLP-1R to promote insulin secretion and then reduce blood glucose.
On the other hand, they can activate GCGR, promote lipid metabolism and reduce body weight — Since human islets have more mixed α-β cell interfaces, the ratio of GCGR to GLP-1R may be particularly vital to human islet function 8 , SAR is a novel polypeptide with a co-excitatory effect on GCGR and GLP-1R, which can reduce blood glucose and HbA1c levels and reduce body weight in patients with T2D; however, it has an adverse effect on the gastrointestinal tract It also improves postprandial blood glucose control by significantly enhancing β cell function and slowing glucose absorption rate These findings highlight the possible clinical relevance of dual agonist peptides that simultaneously stimulate the synthesis of GCGR and GLP-1R and may drive the development of novel antidiabetic drugs.
In this review, we provide a clear overview of various theories of hormonal regulation of diabetes, with a focus on the essential roles of glucagon and its specific receptor in the pathogenesis of diabetes.
Although GCGR and glucagon play important roles in diabetes, the mechanisms and role of mutations still needs to be explored. We summarized the pleiotropic effects of glucagon, future research prospects, and the development of novel therapeutic strategies.
This area of research remains challenging but exciting. Further research on islet α cells, glucagon, and GCGR signaling pathways is expected to provide a basis for developing new strategies for diabetes prevention.
YJ wrote the manuscript. GS designed and critically reviewed the manuscript. SS critically revised the manuscript. YL and LF supervised the writing of the manuscript. All authors contributed to the article and approved the submitted version.
Funding was received from talent project of Shengjing hospital of China Medical University and the National Natural Science Foundation of China, grant The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. American Diabetes A.
Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 36 Suppl 1 :S67— doi: PubMed Abstract CrossRef Full Text Google Scholar.
Muller WA, Faloona GR, Unger RH. Hyperglucagonemia in Diabetic Ketoacidosis. Its Prevalence and Significance. Am J Med —7. Muller WA, Girardier L, Seydoux J, Berger M, Renold AE, Vranic M. Extrapancreatic Glucagon and Glucagonlike Immunoreactivity in Depancreatized Dogs. A Quantitative Assessment of Secretion Rates and Anatomical Delineation of Sources.
J Clin Invest — Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. Lee YH, Wang MY, Yu XX, Unger RH.
Glucagon Is the Key Factor in the Development of Diabetes. Diabetologia —5. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice Are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia.
Diabetologia — Habegger KM, Heppner KM, Geary N, Bartness TJ, DiMarchi R, Tschop MH. The Metabolic Actions of Glucagon Revisited. Nat Rev Endocrinol — Muller TD, Finan B, Clemmensen C, DiMarchi RD, Tschop MH. The New Biology and Pharmacology of Glucagon. Physiol Rev — Zhang H, Qiao A, Yang D, Yang L, Dai A, de Graaf C, et al.
Structure of the Full-Length Glucagon Class B G-Protein-Coupled Receptor. Nature — Gerich JE. Physiology of Glucagon. Int Rev Physiol — PubMed Abstract Google Scholar. Huypens P, Ling Z, Pipeleers D, Schuit F.
Glucagon Receptors on Human Islet Cells Contribute to Glucose Competence of Insulin Release. Diabetologia —9. Nauck MA, Meier JJ. The Incretin Effect in Healthy Individuals and Those With Type 2 Diabetes: Physiology, Pathophysiology, and Response to Therapeutic Interventions.
Lancet Diabetes Endocrinol — Campbell JE, Drucker DJ. Pharmacology, Physiology, and Mechanisms of Incretin Hormone Action. Cell Metab — Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA.
Pancreatic Extracts in the Treatment of Diabetes Mellitus. Can Med Assoc J —6. Unger RH, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH. The Banting Memorial Lecture Diabetes and the Alpha Cell.
Diabetes — Role of Glucagon in the Pathogenesis of Diabetes: The Status of the Controversy. Metabolism — Glucagon and the A Cell: Physiology and Pathophysiology Second of Two Parts. N Engl J Med — The Effect of Experimental Insulin Deficiency on Glucagon Secretion.
J Clin Invest —9. Dobbs R, Sakurai H, Sasaki H, Faloona G, Valverde I, Baetens D, et al. Glucagon: Role in the Hyperglycemia of Diabetes Mellitus. Science —7. Gerich JE, Lorenzi M, Bier DM, Schneider V, Tsalikian E, Karam JH, et al.
Prevention of Human Diabetic Ketoacidosis by Somatostatin. Evidence for an Essential Role of Glucagon. N Engl J Med —9. Raskin P, Unger RH. Hyperglucagonemia and its Suppression. Importance in the Metabolic Control of Diabetes.
N Engl J Med —6. Gelling RW, Du XQ, Dichmann DS, Romer J, Huang H, Cui L, et al. Lower Blood Glucose, Hyperglucagonemia, and Pancreatic Alpha Cell Hyperplasia in Glucagon Receptor Knockout Mice. Proc Natl Acad Sci USA — Lee Y, Wang MY, Du XQ, Charron MJ, Unger RH.
Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Lee Y, Berglund ED, Wang MY, Fu X, Yu X, Charron MJ, et al.
Metabolic Manifestations of Insulin Deficiency do Not Occur Without Glucagon Action. Proc Natl Acad Sci USA —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual Beta-Cells Persist.
Elife 5:e Svendsen B, Larsen O, Gabe MBN, Christiansen CB, Rosenkilde MM, Drucker DJ, et al. Insulin Secretion Depends on Intra-Islet Glucagon Signaling.
Cell Rep — Omar BA, Andersen B, Hald J, Raun K, Nishimura E, Ahren B. Fibroblast Growth Factor 21 FGF21 and Glucagon-Like Peptide 1 Contribute to Diabetes Resistance in Glucagon Receptor-Deficient Mice.
Longuet C, Robledo AM, Dean ED, Dai C, Ali S, McGuinness I, et al. Liver-Specific Disruption of the Murine Glucagon Receptor Produces Alpha-Cell Hyperplasia: Evidence for a Circulating Alpha-Cell Growth Factor.
Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes Alpha-Cell Proliferation and Increases Beta-Cell Mass in Diabetic Mice. iScience — Kawamori D, Katakami N, Takahara M, Miyashita K, Sakamoto F, Yasuda T, et al.
Dysregulated Plasma Glucagon Levels in Japanese Young Adult Type 1 Diabetes Patients. J Diabetes Investig —6. Matsuo T, Miyagawa J, Kusunoki Y, Miuchi M, Ikawa T, Akagami T, et al. Postabsorptive Hyperglucagonemia in Patients With Type 2 Diabetes Mellitus Analyzed With a Novel Enzyme-Linked Immunosorbent Assay.
J Diabetes Investig — Capozzi ME, Svendsen B, Encisco SE, Lewandowski SL, Martin MD, Lin H, et al. Beta Cell Tone Is Defined by Proglucagon Peptides Through cAMP Signaling. JCI Insight 4:e CrossRef Full Text Google Scholar. Zhang Y, Han C, Zhu W, Yang G, Peng X, Mehta S, et al.
Glucagon Potentiates Insulin Secretion Via Beta-Cell GCGR at Physiological Concentrations of Glucose. Cells Thorens B, Weir GC, Leahy JL, Lodish HF, Bonner-Weir S.
Vuguin PM, Charron MJ. Novel Insight Into Glucagon Receptor Action: Lessons From Knockout and Transgenic Mouse Models. Diabetes Obes Metab 13 Suppl — Cabrera O, Ficorilli J, Shaw J, Echeverri F, Schwede F, Chepurny OG, et al.
Intra-Islet Glucagon Confers Beta-Cell Glucose Competence for First-Phase Insulin Secretion and Favors GLP-1R Stimulation by Exogenous Glucagon. J Biol Chem Tian G, Sol ER, Xu Y, Shuai H, Tengholm A. Impaired cAMP Generation Contributes to Defective Glucose-Stimulated Insulin Secretion After Long-Term Exposure to Palmitate.
Dyachok O, Isakov Y, Sagetorp J, Tengholm A. Oscillations of Cyclic AMP in Hormone-Stimulated Insulin-Secreting Beta-Cells. Qiao A, Han S, Li X, Li Z, Zhao P, Dai A, et al. Structural Basis of Gs and Gi Recognition by the Human Glucagon Receptor. Science — Wewer Albrechtsen NJ. The Glucose-Mobilizing Effect of Glucagon at Fasting is Mediated by Cyclic AMP.
Am J Physiol Endocrinol Metab E—4. Lok S, Kuijper JL, Jelinek LJ, Kramer JM, Whitmore TE, Sprecher CA, et al. The Human Glucagon Receptor Encoding Gene: Structure, cDNA Sequence and Chromosomal Localization.
Gene —9. Menzel S, Stoffel M, Espinosa R 3rd, Fernald AA, Le Beau MM, Bell GI. Localization of the Glucagon Receptor Gene to Human Chromosome Band 17q Genomics —8. Gragnoli C, Milord E, Habener JF. Linkage Study of the Glucagon Receptor Gene With Type 2 Diabetes Mellitus in Italians.
Metabolism —7. Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sorensen M, Suppli MP, Janah L, et al. The Liver-Alpha-Cell Axis and Type 2 Diabetes. Endocr Rev — Bozadjieva Kramer N, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia.
Hager J, Hansen L, Vaisse C, Vionnet N, Philippi A, Poller W, et al. A Missense Mutation in the Glucagon Receptor Gene Is Associated With Non-Insulin-Dependent Diabetes Mellitus. Nat Genet — Gough SC, Saker PJ, Pritchard LE, Merriman TR, Merriman ME, Rowe BR, et al.
Mutation of the Glucagon Receptor Gene and Diabetes Mellitus in the UK: Association or Founder Effect? Hum Mol Genet — Fujisawa T, Ikegami H, Yamato E, Takekawa K, Nakagawa Y, Hamada Y, et al.
A Mutation in the Glucagon Receptor Gene Gly40Ser : Heterogeneity in the Association With Diabetes Mellitus. Odawara M, Tachi Y, Yamashita K. Hum Genet —9. Ogata M, Iwasaki N, Ohgawara H, Karibe S, Omori Y.
Absence of the GlySer Mutation in the Glucagon Receptor Gene in Japanese Subjects With NIDDM. Diabetes Res Clin Pract —4. Fujisawa T, Ikegami H, Babaya N, Ogihara TJH. Gly40Ser Mutation of Glucagon Receptor Gene and Essential Hypertension in Japanese. Hypertension Huang X, Orho M, Lehto M, Groop L.
Lack of Association Between the Gly40Ser Polymorphism in the Glucagon Receptor Gene and NIDDM in Finland. Diabetologia —8. Ristow M, Busch K, Schatz H, Pfeiffer A. Restricted Geographical Extension of the Association of a Glucagon Receptor Gene Mutation Gly40Ser With Non-Insulin-Dependent Diabetes Mellitus.
Diabetes Res Clin Pract —5. Elbein SC, Hoffman MD. Role of Mitochondrial DNA tRNA Leucine and Glucagon Receptor Missense Mutations in Utah White Diabetic Patients.
Diabetes Care —8. Ambrosch A, Lobmann R, Dierkes J, König W, Luley C, Lehnert H. Analysis of the Gly40Ser Polymorphism in the Glucagon Receptor Gene in a German Non-Insulin-Dependent Diabetes Mellitus Population.
Clin Chem Lab Med — Babadjanova G, Reincke M, Mora P, Chuchalin A, Allolio B. Polymorphism of the Glucagon Receptor Gene and Non-Insulin-Dependent Diabetes Mellitus in the Russian Population. Exp Clin Endocrinol Diabetes —6. Leprêtre F, Vionnet N, Budhan S, Dina C, Powell KL, Génin E, et al.
Genetic Studies of Polymorphisms in Ten Non-Insulin-Dependent Diabetes Mellitus Candidate Genes in Tamil Indians From Pondichery. Diabetes Metab — Deng H, Tang WL, Pan Q. Hunan Yi Ke Da Xue Xue Bao —3. Huang CN, Lee KC, Wu HP, Tai TY, Lin BJ, Chuang LM.
Screening for the Gly40Ser Mutation in the Glucagon Receptor Gene Among Patients With Type 2 Diabetes or Essential Hypertension in Taiwan.
Pancreas —5. Shiota D, Kasamatsu T, Dib SA, Chacra AR, Moisés RS. Role of the Gly40Ser Mutation in the Glucagon Receptor Gene in Brazilian Patients With Type 2 Diabetes Mellitus. Pancreas — Tonolo G, Melis MG, Ciccarese M, Secchi G, Atzeni MM, Maioli M, et al.
Physiological and Genetic Characterization of the Gly40Ser Mutation in the Glucagon Receptor Gene in the Sardinian Population. The Sardinian Diabetes Genetic Study Group.
Wang X, Wang F, Wu H, Chen X, Xie R, Chen T, et al. Detection and Analysis of Glucose Metabolism-Related Genes in Childhood Diabetes Using Targeted Next-Generation Sequencing: In Pediatric Population-a Hospital-Based Study.
Exp Ther Med — Hansen LH, Abrahamsen N, Hager J, Jelinek L, Kindsvogel W, Froguel P, et al. The Gly40Ser Mutation in the Human Glucagon Receptor Gene Associated With NIDDM Results in a Receptor With Reduced Sensitivity to Glucagon. Zhou C, Dhall D, Nissen NN, Chen CR, Yu R.
Homozygous P86S Mutation of the Human Glucagon Receptor Is Associated With Hyperglucagonemia, Alpha Cell Hyperplasia, and Islet Cell Tumor.
Pancreas —6. Lindquist P, Madsen JS, Brauner-Osborne H, Rosenkilde MM, Hauser AS. Mutational Landscape of the Proglucagon-Derived Peptides. Front Endocrinol van der Velden WJC, Lindquist P, Madsen JS, Stassen R, Wewer Albrechtsen NJ, Holst JJ, et al.
Molecular and In Vivo Phenotyping of Missense Variants of the Human Glucagon Receptor. The VM Gcgr Knock-in Mice are a Precision Medicine Model of Mild Mahvash Disease. Biochem J —4. Lin G, Liu Q, Dai A, Cai X, Zhou Q, Wang X, et al. Characterization of a Naturally Occurring Mutation VM in the Human Glucagon Receptor and Its Association With Metabolic Disorders.
Biochem J — Diabetes Obes Metab —7. Cheng C, Jabri S, Taoka BM, Sinz CJ. Small Molecule Glucagon Receptor Antagonists: An Updated Patent Review Expert Opin Ther Pat — Patil M, Deshmukh NJ, Patel M, Sangle GV.
Glucagon-Based Therapy: Past, Present and Future. Based on one hypothesis, proglucagon may contain a sorting signal within its structure that directs it to a specific sorting receptor on the membrane of the TGN.
These signals may interact directly with membrane lipids, in particular with lipid raft regions 25 or with sorting receptors within the TGN, to be sorted into secretory granules. To this end, it has been proposed that membrane-bound form of the processing enzyme carboxypeptidase E CPE may be a prohormone sorting receptor 21 , 26 — It was shown that ablation of CPE disrupted the regulated secretion of proopiomelanocortin POMC , proenkephalin and proinsulin in related cell lines and the CPE fat mouse model, in which CPE is degraded within the pituitary.
Additionally, CPE may interact other resident granule proteins, secretogranin III and chromogranin A, to facilitate the sorting of POMC 29 and neuropeptides Another possible mechanism of prohormone sorting to granules may be simply through selective retention while constitutively secreted proteins are removed from the nascent immature granule.
Evidence for this mechanism lies in the fact that the protein composition of immature secretory granules is altered during the process of granule maturation In this context, proinsulin and the enzymes involved in the post-translational processing to mature insulin are retained within the beta cell secretory granule, while other proteins designated for constitutive secretion are removed By considering all of these findings, it is likely that both receptor-mediated and retention mechanisms operate in the sorting of prohormones into secretory granules.
In this scenario, prohormones could be sorted into secretory granules by means of sorting signals, followed by retention within the granule as maturation of secretory granules takes place.
The maturation process involves alterations in the components and composition of the secretory granule, by removal of constitutively-secreted proteins, acidification of the granule milieu, and exclusion of water to condense the intragranular environment.
The cellular events underlying the sorting of proglucagon to secretory granules have not been fully elucidated, and studying this mechanism is complicated by the multi-step processing of proglucagon. The processing of proglucagon in the alpha cell is largely governed by the prohormone convertase PC family of enzymes.
Proglucagon processing begins early in the secretory pathway TGN or immature secretory granule with cleavage at K 70 R 71 , which yields glicentin and major proglucagon fragment MPGF Figure 1A Subsequent cleavage of glicentin by PC2 at K 31 R 32 results in the production of mature glucagon.
This cleavage event likely occurs within the mature secretory granule since the enzymatic activity of PC2 is optimal at the acidic pH and millimolar calcium concentrations within secretory granules 33 — Thus, the sorting of proglucagon into the secretory granule is vital for the generation of active glucagon, and storage within granules assures a robust secretory response in response to physiological need.
Figure 1 Proglucagon processing and sorting signals. A A schematic representation of proglucagon showing the major prohormone processing sites that yield the peptides glicentin, oxyntomodulin, glucagon, GLP-1 and GLP B Computational modelling of the structure of proglucagon showing the alpha helical structures of glucagon green , GLP-1 yellow and GLP-2 red from reference 28 © Society for Endocrinology.
C Helical wheel projections of the alpha helices contained within glucagon and GLP-1 7—37 that function as sorting signals to direct proglucagon to the regulated secretory pathway. Is there evidence for sorting signals and a sorting receptor for proglucagon?
Using the alpha cell line αTC, it was shown that siRNA-mediated knockdown of CPE increased constitutive secretion of glucagon; however, the processing of proglucagon to glucagon remained unchanged, indicating that CPE may have an effect on secretion, but not intracellular sorting The search for sorting signals provided more clarity on the mechanisms of proglucagon sorting.
Computational modelling of proglucagon indicates a largely disordered structure comprising alpha helices, some of which correspond to glucagon, GLP-1 and GLP-2 Figure 1B. Using Fc-tagged proglucagon-derived peptides that could be detected by immunoprecipitation and immunofluorescence microscopy, it was shown that two dipolar α-helices containing hydrophobic patches with three charged residues within the sequences play roles as sorting signals.
Interestingly, Fc-glicentin was sorted to secretory granules, but Fc-MPGF was not, suggesting that the sorting signal within GLP-1 is masked when contained within the MPGF sequence. These results indicate that the sorting of proglucagon into secretory granules occurs prior to the initial processing event, such that processing occurs exclusively within the granule.
Another possibility is that processing to glicentin and MPGF occurs first, with the prediction that glicentin is sorted into granules and processed to glucagon, while MPGF is not sorted, or very inefficiently sorted into granules.
This proglucagon processing profile changes in diabetes; in human and rodent islets, there is a significant increase in the processing of proglucagon to GLP If both glucagon and GLP-1 are produced in a proportion of alpha cells, and are both sorted to secretory granules, the question arises: are they sorted to distinct granule populations, and released under different glucose conditions?
These questions may have been answered in a very recent islet granule peptidomics study showing that both human and mouse islets produce times more glucagon than active GLP-1 46 , indicating that the processing of proglucagon to active GLP-1 in alpha cells is very inefficient.
Also in this study, analysis of secreted proglucagon-derived peptides showed that both glucagon and active GLP-1 were released in parallel in response to either low 1 mM , medium 6 mM or high Therefore, both glucagon and GLP-1 are likely stored in the same granules and secreted under the same conditions, with glucagon being the dominant peptide, and perhaps serving as the intra-islet GLP-1R agonist The control of hyperglucagonemia obviously targets glucagon secretion.
But what mechanism s are potentially druggable? Inhibition of glucagon secretion by glucose from alpha cells is a long-standing puzzle in islet biology. Unlike insulin secretion from beta cells which is primarily driven by prevailing glucose levels, there is no one single factor that governs glucagon secretion from the alpha cell.
Intrinsic glucose sensing, intra-islet paracrine secretion and factors from the alpha cell itself all interact to generate a complex network that regulates glucagon secretion. In order to examine the direct effects of glucose on glucagon secretion in the absence of paracrine inputs, isolated mouse pancreatic alpha cells, clonal hamster In-R1-G9 cells 48 , 49 , clonal mouse αTC and -9 cells 39 , 50 , 51 and dispersed alpha cells from human islets 52 have been used.
All of these preparations show a bimodal response to increasing glucose concentrations. In the range from 1 to ~7 mM, glucagon secretion is suppressed in a dose-dependent manner, and above 7 mM, glucagon secretion increases Figure 2A. This secretion profile suggests intrinsic mechanisms alone can operate in regulating glucagon secretion below 7 mM glucose, and that these mechanisms may be ineffective at higher glucose concentrations.
However, such conclusions must be interpreted with caution, as single dispersed alpha cells are in a highly abnormal environment, and alpha cell lines are not representative of the normal alpha cell phenotype, as discussed in more detail below.
Figure 2 Glucagon secretion from dispersed alpha cells and alpha cells in intact islets demonstrate the role of paracrine regulation at high glucose concentrations. A V-shape curve of glucagon exocytosis in response to glucose in dispersed non-diabetic black and T2D red human α-cells.
B Glucagon secretion from intact islets in response to glucose. Created with BioRender. The alpha cell secretory response to both glucose is likely more accurately captured in isolated, intact mouse and human islets, where the paracrine regulatory environment and cell-cell contacts are intact.
Similar to dispersed alpha cells, increasing the glucose concentration from 1 to 7 mM dose dependently decreases glucagon secretion from mouse alpha cells 53 and human alpha cells 52 within intact islets, and remains low as glucose levels increase beyond 7 mM, a concentration at which insulin secretion is stimulated Figure 2B.
Therefore, paracrine inputs are significant factors in the inhibition of glucagon secretion as glucose concentrations increase above euglycemia.
One mechanism underlying the intrinsic response to glucose is the direct effect on alpha cell electrical activity. At low 1 mM glucose concentrations, alpha cells in intact mouse and human islets exhibit low K ATP activity and are electrically active 54 — 56 and as glucose concentrations increase, K ATP activity is inhibited.
A recent review by Zhang et al. Therefore, the intrinsic regulation of glucagon secretion by glucose may be explained primarily by the unique electrical properties of the alpha cell, and secondarily by glucose metabolism.
In particular, cAMP signalling may play a key role in the alpha cell secretory response to insulin and somatostatin There is one report that cAMP may also mediate intrinsic glucose sensing within the alpha cell.
Using genetically encoded fluorescent cAMP biosensors, it was shown that high glucose suppressed subplasmalemmal cAMP levels in isolated mouse and human islets Conversely, sustained high cAMP levels abolished the suppression of glucagon secretion by high glucose concentrations.
Lastly, intrinsic glucose sensing by the alpha cell may also be mediated by the nutrient sensors AMP-activated protein kinase AMPK and its downstream target, mammalian target of rapamycin complex 1 mTORC1.
In a series of studies that manipulated alpha cell expression of AMPK itself 65 and its upstream effectors PASK 66 and LKB1 67 , it was shown that components of this nutrient-sensing pathway can mediate the low glucose-induced secretion of glucagon. One of these proteins, PASK, is down-regulated in T2D human islets, thus indicating that components of the AMPK pathway may be potential targets for controlling hyperglucagonemia.
Using innovative mouse models that selectively targeted activators and inhibitors of mTORC1, it was shown that loss of mTORC1 activity resulted in a loss of the glucose counter-regulatory response and reduction in response to alpha cell secretagogues Interestingly, depletion of the mTORC1 inhibitor TSC2 in alpha cells resulted in a mouse model of hyperglucagonemia and glucagon resistance 69 , which will be an excellent resource for studies on mechanisms of hyperglucagonemia.
Therefore, the mechanisms underlying the intrinsic response to glucose may provide potential targets for the control of abnormally up-regulated glucagon secretion in diabetes. The beta cell secretory granule contains a number of agents that act directly or indirectly on the alpha cell to inhibit glucagon secretion, and also generally modulate mechanisms of alpha cell biology, such as proliferation.
Insulin, the primary cargo, is a potent suppressor of glucagon secretion and operates through several mechanisms. Mice lacking the insulin receptor on alpha cells αIRKO exhibit hyperglycemia and hyperglucagonemia, indicating that insulin receptor signalling is required for an appropriate alpha cell secretory response to glucose Alpha cell insulin resistance may underlie the abnormal up-regulation of glucagon secretion Type 2 diabetes Additionally, these results also indicate that insulin alone is not sufficient to regulate glycemia in the face of hyperglucagonemia.
Along with insulin, gamma amino butyric acid GABA is also released from the beta cell and is a potent suppressor of glucagon secretion from alpha cells 73 , Activating the GABA A receptor in alpha cells results in Cl - influx into the cells which hyperpolarizes the membrane and reduces glucagon secretion As well, there is coordination between insulin and GABA A receptor activity, as insulin action leads to the translocation of GABA A receptor to the cell membrane 76 , thus augmenting the inhibitory effects of GABA.
In addition, GABA also inhibits mTOR activity to suppress alpha cell proliferation. In type 1 diabetes, the destruction of beta cells leads to a reduction in the amount of secreted GABA, resulting in the activation of mTOR and alpha cell proliferation In addition to effects on alpha cell proliferation, some studies have suggested that pharmacologic activation of GABA A receptor by artemisinins or GABA may alter alpha cell identity and trans-differentiate adult alpha cells to beta-like cells 78 — 80 , and have led to clinical trials investigating GABA receptor agonists as protection against the development of diabetes.
However, there is still some debate on this topic, as transdifferentiation could not be induced either in isolated mouse islets in which both insulin and glucagon were tagged with fluorescent reporters 81 or in an alpha cell-specific lineage tracing model In any case, the reported immunomodulatory effects of GABA, together with either GLP-1 83 or the SGLT2 inhibitor empagliflozin 84 also protect newly formed beta cells in the inflammatory environment of T1D, and thus also indirectly restore normal regulation of alpha cell mass and glucagon secretion.
Direct effects of serotonin are mediated by activation of the serotonin receptor, 5-HT 1F R, on α-cells, which reduces intracellular cAMP to suppress glucagon secretion 85 , In patients with long-standing T2D, the proportion of alpha cells expressing 5-HT 1F R is decreased, suggesting that reduced serotonin action on alpha cells may play a role in hyperglucagonemia of diabetes.
In STZ-treated mice, administration of the 5-HT 1F R agonist LY alleviated hyperglucagonemia and hyperglycemia.
However, insulin-induced hypoglycemia was worsened, suggesting that the effects of serotonin are glucose-independent Therefore, while alpha cell HT 1F R may be a potential target for the treatment of hyperglucagonemia, it may not be an ideal target.
The effects of adenosine are mediated by the adenosine A1 receptor Adora1 , in which activation is coupled to opening of K ATP channels, hyperpolarization of the cell membrane and prevention of granule exocytosis. In NOD mice, autoantibody-positive people and people with long-term T1D, alpha cells gradually lose Adora1 expression, suggesting that the hyperglucagonemia of diabetes is associated with a loss of adenosine action ZnT8 is located in the secretory granule membrane of both α-and β-cells.
There is a direct relationship between expression of the proglucagon gene and Slc30A8 in α-cells Somatostatin is a well-known tonic inhibitor of glucagon secretion. Somatostatin binds to the SSTR2 receptor subtype on alpha cells 93 , which is coupled to the inhibitory G i subunit, resulting in decreased production of cAMP as a mechanism for the suppression of glucagon secretion Notably, secretion of somatostatin and inhibition of glucagon secretion both occur at 3 mM glucose, indicating that the alpha cell response to low glucose may be fine-tuned by somatostatin In rat pancreatic preparations perfused with an SSTR2 antagonist, the suppression of glucagon secretion by 3.
However, in isolated human islets, blockade of SSTR2 did not affect suppression of glucagon secretion at 6 mM glucose 55 , perhaps reflecting species-specific differences or differences in the models perfused pancreas vs static islet culture.
Interestingly, insulin secretion was also elevated, indicating that both insulin and somatostatin are required for the suppression of glucagon secretion at high glucose concentrations.
In intact human islets, high glucose 10 mM inhibition of glucagon exocytosis was lost after administration of the SSTR2 antagonist CYN In diabetes, circulating and pancreatic somatostatin, together with SST mRNA, are elevated.
However, expression of SSTR2 on alpha cells is decreased in T2D due to increased receptor internalization 52 , indicating alpha cell somatostatin resistance. Together with alpha cell insulin resistance, this could be another mechanism in the hyperglucagonemia of diabetes.
Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents.
The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion. Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes.
Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes. The alpha cell itself displays plasticity during the progression of diabetes.
In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways.
Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion.
In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction.
Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell.
The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3.
In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia.
Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop. Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release.
Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion.
The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known. GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells.
To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells.
The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility. Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small.
Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets.
However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues.
The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose.
Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts.
The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis.
As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis.
Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time.
In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane.
SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs.
Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced.
Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules.
In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D.
Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis.
Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes.
Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway.
Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated.
The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion.
While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required.
In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5.
Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4.
Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells.
Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow.
Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known.
So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function?
It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia.
Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D.
Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia.
While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation.
The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment.
The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes.
SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers.
Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stanley S, Moheet A, Seaquist ER.
Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar.
DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD.
Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice.
Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia.
Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival. Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al.
Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist. Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies.
Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists.
J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al.
Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice.
Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al.
Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al.
The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells.
Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway.
J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP.
Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL.
FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor.
J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA.
J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules.
J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway.
Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF.
Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6.
Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice.
Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes.
Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats.
McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al.
Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development.
The medhanism Glucagon mechanism pathway refers to the sum Healthy bowel movement a Glucayon of African mango extract for metabolism and regulatory factors involved in the function of glucagon. Human pancreatic hyperglycemia is a linear polypeptide consisting of Mechanis amino acids with a molecular weight ofwhich G,ucagon also cleaved by precursors of macromolecules. In contrast to the role of the insulin signaling pathway, the glucagon signaling pathway is a pathway that promotes catabolism. The glucagon signaling pathway has a strong role in promoting glycogenolysis and gluconeogenesis, resulting in a significant increase in blood glucose. The glucagon signaling pathway activates hepatocyte phosphorylase and accelerates glycogenolysis through the cAMP-PK system. The gluconeogenesis is enhanced as hormones accelerate the entry of amino acids into the liver cells and activate the enzyme system involved in the gluconeogenesis process.Mechanlsm and glucagon help maintain blood sugar levels. Glucagon helps prevent blood sugar from dropping, while insulin mechanixm it from rising too high. Glucagon breaks down glycogen Glucagin glucose in the liver. Insulin enables blood glucose to enter cells, where they use it to produce Gluvagon.
Together, insulin mdchanism glucagon help maintain Caloric intake and cravings, where conditions inside the body hold steady. When their blood sugar levels drop, their Glkcagon releases glucagon to raise them.
This balance helps provide sufficient energy to African mango extract for metabolism cells while preventing damage that can Glucagoh from consistently high blood sugar levels.
When mevhanism person consumes carbohydrates through foods, their body converts them into glucose, a simple High protein diet and sleep quality that serves as a vital energy source.
However, the Glucagom does not use all of this glucose at Glcuagon. Instead, it mechanims some medhanism storage molecules called glycogen and stores them in the liver and Fat burner for post-workout recovery. When the body mechanisk energy, Glucagon mechanism in Lentils nutritional value liver converts mechanosm back into glucose.
From Sugar consumption and energy levels liver, Glucxgon enters the bloodstream. In Mechwnism pancreas, different mechanims of islet cells release insulin and glucagon.
Beta mechanisj release insulin while alpha cells Health and wellness resources glucagon.
Insulin attaches to insulin receptors Glucabon cells throughout the mefhanism, instructing mfchanism to open and grant Glucahon to mecchanism. Low levels nechanism insulin constantly circulate throughout Diabetes and continuous glucose monitoring systems body.
The mschanism stores glucose to power cells during periods of low blood sugar. The liver provides or stimulates Glucagoj production of glucose using these processes.
In glycogenolysis, glucagon Robust Orange Essence the liver to convert glycogen to Fat burner for post-workout recovery, making glucose more available in emchanism bloodstream. In gluconeogenesis, the liver produces glucose from the Gluacgon of other processes.
Gluconeogenesis also Glufagon in the kidneys and some other Fat burner for post-workout recovery. Insulin and glucagon work in a cycle. Glucagon interacts with Glucavon liver to increase blood sugar, while insulin reduces blood sugar by helping the cells use glucose.
When the body does not absorb or convert enough glucose, blood sugar levels remain high. When blood sugar levels are too low, the pancreas releases glucagon. Hyperglycemia refers to high blood sugar levels.
Fat burner for post-workout recovery high levels can cause mechhanism damage mechaniwm the Enhanced thermogenesis. Hypoglycemia means Gluvagon sugar levels Glucagon mechanism low.
Its symptoms include faintness and dizziness, and it can be life threatening. People with type 1 diabetes need to take insulin regularly, but glucagon is usually only Glucagom emergencies.
People can Fat burner for post-workout recovery insulin in Glucxgon ways, such as pre-loaded syringes, pens, or pumps. Glycagon effects mechanjsm occur if a person Glcagon too much or too little insulin or uses African mango extract for metabolism with certain other drugs.
For this reason, they will need to follow their treatment plan with care. What are the side effects of insulin therapy? Ways of giving glucagon include injections or a nasal spray.
It also comes as a kit, with a syringe, some glucagon powder, and a liquid to mix with it. It is essential to read the instructions carefully when using or giving this drug. Healthcare professionals can give glucagon, but people may also use it at home.
After giving glucagon, someone should monitor the person for adverse effects. The most common adverse effect is nausea, but they may also vomit. In some cases, an allergic reaction may occur. Blood sugar levels should return to safer levels within 10—15 minutes. After this, the person should ingest some candy, fruit juice, crackers, or other high-energy food.
Doctors may also use glucagon when diagnosing problems with the digestive system. A range of factors, including insulin resistancediabetes, and an unbalanced diet, can cause blood sugar levels to spike or plummet.
Ideal blood sugar ranges are as follows :. Read more about optimal blood sugar levels here. High blood sugar can be a sign of diabetes, but it can also occur with other conditions. Without intervention, high blood sugar can lead to severe health problems.
In some cases, it can become life threatening. Insulin and glucagon help manage blood sugar levels. In addition to diabetes, possible causes of high blood sugar include :.
People with high blood sugar may not notice symptoms until complications appear. If symptoms occur, they include :. Over time, high blood sugar may lead to :.
Hypoglycemia is most likely to affect people with diabetes if they take their diabetes medication — such as insulin or glipizide — without eating. But, it can happen for other reasons, for example:.
The symptoms of low blood sugar include :. Without treatment, low blood sugar can lead to seizures or loss of consciousness. What are the different types of diabetes?
Insulin helps the cells absorb glucose from the blood, while glucagon triggers a release of glucose from the liver. People with type 1 diabetes need to take supplemental insulin to prevent their blood sugar levels from becoming too high.
In some cases, a doctor will recommend insulin for people with type 2 diabetes. However, diet and exercise are usually the first recommendations for this type. Very low blood sugar can become life threatening without medical intervention.
In this article, we look at nine ways to lower high insulin levels. This can be achieved through diet, lifestyle changes, supplements, and medication.
A person can manage their diabetes by making healthful changes to their diet, exercising frequently, and regularly taking the necessary medications…. Researchers said baricitinib, a drug used to treat rheumatoid arthritis, showed promise in a clinical trial in helping slow the progression of type 1….
A new review indicates that insulin—used to manage diabetes—can be kept at room temperature for months without losing its potency. A study in rat models of diabetes suggests that spinach extract — both water- and alcohol-based — may help promote wound healing, which occurs very….
My podcast changed me Can 'biological race' explain disparities in health? Why Parkinson's research is zooming in on the gut Tools General Health Drugs A-Z Health Hubs Health Tools Find a Doctor BMI Calculators and Charts Blood Pressure Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us.
Medical News Today. Health Conditions Health Products Discover Tools Connect. How insulin and glucagon regulate blood sugar. Medically reviewed by Angela M. Bell, MD, FACP — By Zawn Villines — Updated on February 15, Overview Taking insulin and glucagon Ideal levels Effects on the body Summary Insulin and glucagon help maintain blood sugar levels.
Insulin, glucagon, and blood sugar. Taking insulin and glucagon. Ideal blood sugar levels. How blood sugar levels affect the body. How we reviewed this article: Sources. Medical News Today has strict sourcing guidelines and draws only from peer-reviewed studies, academic research institutions, and medical journals and associations.
We avoid using tertiary references. We link primary sources — including studies, scientific references, and statistics — within each article and also list them in the resources section at the bottom of our articles.
You can learn more about how we ensure our content is accurate and current by reading our editorial policy. Share this article. Latest news Ovarian tissue freezing may help delay, and even prevent menopause. RSV vaccine errors in babies, pregnant people: Should you be worried?
Scientists discover biological mechanism of hearing loss caused by loud noise — and find a way to prevent it. How gastric bypass surgery can help with type 2 diabetes remission.
Atlantic diet may help prevent metabolic syndrome. Related Coverage. How can I lower my insulin levels? Medically reviewed by Maria S. Prelipcean, MD. How to manage diabetes.
: Glucagon mechanism| Glucagon Signaling Pathway | This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose 2,6-bisphosphate a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis [24] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate. This process is reversible in the absence of glucagon and thus, the presence of insulin. Glucagon stimulation of PKA inactivates the glycolytic enzyme pyruvate kinase , [25] inactivates glycogen synthase , [26] and activates hormone-sensitive lipase , [27] which catabolizes glycerides into glycerol and free fatty acid s , in hepatocytes. Malonyl-CoA is a byproduct of the Krebs cycle downstream of glycolysis and an allosteric inhibitor of Carnitine palmitoyltransferase I CPT1 , a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon. Abnormally elevated levels of glucagon may be caused by pancreatic tumors , such as glucagonoma , symptoms of which include necrolytic migratory erythema , [30] reduced amino acids, and hyperglycemia. It may occur alone or in the context of multiple endocrine neoplasia type 1. Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes. As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon. As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis. The absence of alpha cells and hence glucagon is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy. In the early s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar. Kimball and John R. Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of " gluc ose agon ist". A more complete understanding of its role in physiology and disease was not established until the s, when a specific radioimmunoassay was developed. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Peptide hormone. This article is about the natural hormone. For the medication, see Glucagon medication. Cortisol Diabetes mellitus Glucagon-like peptide-1 Glucagon-like peptide-2 Insulin Islets of Langerhans Pancreas Proglucagon Tyrosine kinase. Biochemistry 4th ed. New York: Wiley. San Francisco: Benjamin Cummings. ISBN Biology 1: Molecules. Examkrackers Inc. doi : PMC PMID The New England Journal of Medicine. Physiol Rev. The Journal of Clinical Investigation. World Journal of Diabetes. Nature Education. European Journal of Pharmacology. European Journal of Clinical Investigation. S2CID Cell Metabolism. Molecular Pharmacology. Essential Medical Physiology. Academic Press. Nature Reviews. Society for Neuroscience Abstracts. Retrieved The Biochemical Journal. The Role of Fructose 2,6-Bisphosphate in the Regulation of Carbohydrate Metabolism. Current Topics in Cellular Regulation. Proceedings of the National Academy of Sciences of the United States of America. Bibcode : PNAS Am J Physiol Endocrinol Metab. Diabetes Investig. Interrelationship of the effects of phosphorylation, polymer-protomer transition, and citrate on enzyme activity". The Journal of Biological Chemistry. Frontiers in Oncology. Journal of the European Academy of Dermatology and Venereology. Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. Insulin Regulates Glucagon-Like Peptide-1 Secretion by Pancreatic Alpha Cells. Endocrine — Ellingsgaard H, Hauselmann I, Schuler B, Habib AM, Baggio LL, Meier DT, et al. Interleukin-6 Enhances Insulin Secretion by Increasing Glucagon-Like Peptide-1 Secretion From L Cells and Alpha Cells. Nat Med —9. Progressive Change of Intra-Islet GLP-1 Production During Diabetes Development. Diabetes Metab Res Rev —8. Kilimnik G, Kim A, Steiner DF, Friedman TC, Hara M. Islets — Wideman RD, Gray SL, Covey SD, Webb GC, Kieffer TJ. Mol Ther —8. Wideman RD, Covey SD, Webb GC, Drucker DJ, Kieffer TJ. Galvin SG, Kay RG, Foreman R, Larraufie P, Meek CL, Biggs E, et al. The Human and Mouse Islet Peptidome: Effects of Obesity and Type 2 Diabetes, and Assessment of Intraislet Production of Glucagon-Like Peptide J Proteome Res x:acs. Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different Domains of the Glucagon and Glucagon-Like Peptide-1 Receptors Provide the Critical Determinants of Ligand Selectivity. Br J Pharmacol — Salehi A, Vieira E, Gylfe E. Paradoxical Stimulation of Glucagon Secretion by High Glucose Concentrations. Gylfe E. Ups J Med Sci — Whalley NM, Pritchard LE, Smith DM. White a. Processing of Proglucagon to GLP-1 in Pancreatic α-Cells: Is This a Paracrine Mechanism Enabling GLP-1 to Act on β-Cells? Asadi F, Dhanvantari S. Plasticity in the Glucagon Interactome Reveals Novel Proteins That Regulate Glucagon Secretion in α-TC Cells. Front Endocrinol Lausanne Omar-Hmeadi M, Lund PE, Gandasi NR, Tengholm A, Barg S. Paracrine Control of α-Cell Glucagon Exocytosis is Compromised in Human Type-2 Diabetes. Nat Commun — Le Marchand SJ, Piston DW. Glucose Suppression of Glucagon Secretion: Metabolic and Calcium Responses From Alpha-Cells in Intact Mouse Pancreatic Islets. Quoix N, Cheng-xue R, Mattart L, Zeinoun Z, Guiot Y, Beauvois M, et al. Ramracheya R, Ward C, Shigeto M, Walker JN, Amisten S, Zhang Q, et al. Membrane Potential-Dependent Inactivation of Voltage-Gated Ion Channels in α-Cells Inhibits Glucagon Secretion From Human Islets. Zhang Q, Ramracheya R, Lahmann C, Tarasov A, Bengtsson M, Braha O, et al. Role of KATP Channels in Glucose-Regulated Glucagon Secretion and Impaired Counterregulation in Type 2 Diabetes. Zhang Q, Dou H, Rorsman P. J Physiol — Liu Y-J, Vieira E, Gylfe E. A Store-Operated Mechanism Determines the Activity of the Electrically Excitable Glucagon-Secreting Pancreatic α-Cell. Cell Calcium — Tian G, Tepikin AV, Tengholm A, Gylfe E. Watts M, Sherman A. Modeling the Pancreatic α-Cell: Dual Mechanisms of Glucose Suppression of Glucagon Secretion. Biophys J — PloS One 7:e Elliott AD, Ustione A, Piston DW. Somatostatin and Insulin Mediate Glucose-Inhibited Glucagon Secretion in the Pancreatic α-Cell by Lowering cAMP. Am J Physiol Endocrinol Metab E— Yu Q, Shuai H, Ahooghalandari P, Gylfe E, Tengholm A. Glucose Controls Glucagon Secretion by Directly Modulating cAMP in Alpha Cells. Hughes JW, Ustione A, Lavagnino Z, Piston DW. Regulation of Islet Glucagon Secretion: Beyond Calcium. Diabetes Obes Metab — Leclerc I, Sun G, Morris C, Fernandez-Millan E, Nyirenda M, Rutter GA. AMP-Activated Protein Kinase Regulates Glucagon Secretion From Mouse Pancreatic Alpha Cells. Da Silva Xavier G, Farhan H, Kim H, Caxaria S, Johnson P, Hughes S, et al. Per-Arnt-Sim PAS Domain-Containing Protein Kinase is Downregulated in Human Islets in Type 2 Diabetes and Regulates Glucagon Secretion. Sun G, da Silva Xavier G, Gorman T, Priest C, Solomou A, Hodson DJ, et al. LKB1 and Ampkα1 are Required in Pancreatic Alpha Cells for the Normal Regulation of Glucagon Secretion and Responses to Hypoglycemia. Mol Metab — Bozadjieva N, Blandino-Rosano M, Chase J, Dai XQ, Cummings K, Gimeno J, et al. Loss of Mtorc1 Signaling Alters Pancreatic α Cell Mass and Impairs Glucagon Secretion. Kramer NB, Lubaczeuski C, Blandino-Rosano M, Barker G, Gittes GK, Caicedo A, et al. Glucagon Resistance and Decreased Susceptibility to Diabetes in a Model of Chronic Hyperglucagonemia. Gromada J, Franklin I, Wollheim CB. Alpha-Cells of the Endocrine Pancreas: 35 Years of Research But the Enigma Remains. Kawamori D, Kulkarni RN. Insulin Modulation of Glucagon Secretion: The Role of Insulin and Other Factors in the Regulation of Glucagon Secretion. Islets —9. Tsuchiyama N, Takamura T, Ando H, Sakurai M, Shimizu A, Kato KI, et al. Possible Role of α-Cell Insulin Resistance in Exaggerated Glucagon Responses to Arginine in Type 2 Diabetes. Diabetes Care —7. Wendt A, Birnir B, Buschard K, Gromada J, Salehi A, Sewing S, et al. Glucose Inhibition of Glucagon Secretion From Rat α-Cells Is Mediated by GABA Released From Neighboring β-Cells. Li C, Liu C, Nissim I, Chen J, Chen P, Doliba N, et al. Regulation of Glucagon Secretion in Normal and Diabetic Human Islets by?? Rorsman P, Berggren PO, Bokvist K, Ericson H, Möhler H, Ostenson CG SP. Glucose-Inhibition of Glucagon Secretion Involves Activation of GABAA-Receptor Chloride Channels. Nature —6. Xu E, Kumar M, Zhang Y, Ju W, Obata T, Zhang N, et al. Intra-Islet Insulin Suppresses Glucagon Release via GABA-GABAA Receptor System. Feng AL, Xiang Y, Gui L, Kaltsidis G, Feng Q, Lu W. Paracrine GABA and Insulin Regulate Pancreatic Alpha Cell Proliferation in a Mouse Model of Type 1 Diabetes. Jin Li J, Casteels T, Frogne T, Ingvorsen C, Honore C, Courtney M, et al. Artemisinins Target GABAA Receptor Signaling and Impair α Cell Identity. Weir GC, Bonner-Weir S. GABA Signaling Stimulates β Cell Regeneration in Diabetic Mice. Cell —9. Ben-Othman N, Vieira A, Courtney M, Record F, Gjernes E, Avolio F, et al. Long-Term GABA Administration Induces Alpha Cell-Mediated Beta-Like Cell Neogenesis. van der Meulen T, Lee S, Noordeloos E, Donaldson CJ, Adams MW, Noguchi GM, et al. Artemether Does Not Turn α Cells Into β Cells. Ackermann AM, Moss NG, Kaestner KH. GABA and Artesunate Do Not Induce Pancreatic α-to-β Cell Transdifferentiation In Vivo. Combined Effect of GABA and Glucagon-Like Peptide-1 Receptor Agonist on Cytokine-Induced Apoptosis in Pancreatic β-Cell Line and Isolated Human Islets. J Diabetes — Daems C, Welsch S, Boughaleb H, Vanderroost J, Robert A, Sokal E, et al. Early Treatment With Empagliflozin and GABA Improves β -Cell Mass and Glucose Tolerance in Streptozotocin-Treated Mice. J Diabetes Res — Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion From Alpha Cells. Cell Rep — Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered Serotonin 5-HT 1D and 2A Receptor Expression May Contribute to Defective Insulin and Glucagon Secretion in Human Type 2 Diabetes. Peptides — Yip L, Taylor C, Whiting CC, Fathman CG. Diminished Adenosine A1 Receptor Expression in Pancreatic a-Cells May Contribute to the Patholog Y of Type 1 Diabetes. Ishihara H, Wollheim CB. Is Zinc an Intra-Islet Regulator of Glucagon Secretion? Diabetol Int — Franklin I, Gromada J, Gjinovci A, Theander S. Beta Cell Secretory Products Activate Alpha Cell ATP-Dependent Potassium Channels to Inhibit Glucagon Release. Ravier MA, Rutter GA. Glucose or Insulin, But Not Zinc Ions, Inhibit Glucagon Secretion From Mouse Pancreatic [Alpha]-Cells. Solomou A, Philippe E, Chabosseau P, Migrenne-li S, Gaitan J, Lang J, et al. Nutr Metab Lond Solomou A, Meur G, Bellomo E, Hodson DJ, Tomas A, Li SM, et al. Strowski MZ, Parmar RM, Blake AD, Schaeffer JM. Somatostatin Inhibits Insulin and Glucagon Secretion via Two Receptor Subtypes : An In Vitro Study of Pancreatic Islets From Somatostatin Receptor 2 Knockout Mice. Endocrinology —7. Gromada J, Hoy M, Bushcard K, Salehi A, Rorsman P. Somatostatin Inhibits Exocytosis in Rat Pancreatic a-Cells by Gi2-Dependent Activation of Calcineurin and Depriming of Secretory Granules. Rutter GA. Regulating Glucagon Secretion: Somatostatin in the Spotlight. Xu SFS, Andersen DB, Izarzugaza JMG, Kuhre RE, Holst JJ. In the Rat Pancreas, Somatostatin Tonically Inhibits Glucagon Secretion and Is Required for Glucose-Induced Inhibition of Glucagon Secretion. Acta Physiol — Hauge-Evans AC, King AJ, Carmignac D, Richardson CC, Robinson ICAF, Low MJ, et al. Somatostatin Secreted by Islet -Cells Fulfills Multiple Roles as a Paracrine Regulator of Islet Function. Vergari E, Knudsen JG, Ramracheya R, Salehi A, Zhang Q, Adam J, et al. Insulin Inhibits Glucagon Release by SGLT2-Induced Stimulation of Somatostatin Secretion. Nat Commun Briant LJB, Reinbothe TM, Spiliotis I, Miranda C, Rodriguez B, Rorsman P. Δ-Cells and β-Cells Are Electrically Coupled and Regulate α-Cell Activity Via Somatostatin. Kilimnik G, Zhao B, Jo J, Periwal V, Witkowski P, Misawa R, et al. Altered Islet Composition and Disproportionate Loss of Large Islets in Patients With Type 2 Diabetes. PloS One 6:e Ma X, Zhang Y, Gromada J, Sewing S, Berggren P-O, Buschard K, et al. Glucagon Stimulates Exocytosis in Mouse and Rat Pancreatic α-Cells by Binding to Glucagon Receptors. Leibiger B, Moede T, Muhandiramlage TP, Kaiser D, Vaca Sanchez P, Leibiger IB, et al. Glucagon Regulates its Own Synthesis by Autocrine Signaling. Wewer Albrechtsen NJ, Kuhre RE, Hornburg D, Jensen CZ, Hornum M, Dirksen C, et al. Circulating Glucagon Regulates Blood Glucose by Increasing Insulin Secretion and Hepatic Glucose Production. Hare KJ, Knop FK, Asmar M, Madsbad S, Deacon CF, Holst JJ, et al. Preserved Inhibitory Potency of GLP-1 on Glucagon Secretion in Type 2 Diabetes Mellitus. J Clin Endocrinol Metab — Garg M, Ghanim H, Kuhadiya N, Green K, Hejna J, Abuaysheh S, et al. Liraglutide Acutely Suppresses Glucagon, Lipolysis and Ketogenesis in Type 1 Diabetes. Chambers AP, Sorrell JE, Haller A, Roelofs K, Hutch CR, Kim K-S, et al. The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice. Habener JF, Stanojevic V. Pancreas and Not Gut Mediates the GLPInduced Glucoincretin Effect. Cell Metab —8. Fava GE, Dong EW, Wu H. Intra-Islet Glucagon-Like Peptide J Diabetes Complicat —8. Holst J, Christensen M, Lund A, De Heer J, Svendsen B, Kielgast U, et al. Regulation of Glucagon Secretion by Incretins. Ramracheya R, Chapman C, Chibalina M, Dou H, Miranda C, González A, et al. Physiol Rep — Boss M, Bos D, Frielink C, Sandker G, Ekim S, Marciniak C, et al. Targeted Optical Imaging of the Glucagon-Like Peptide 1 Receptor Using Exendin-4irdyecw. J Nucl Med — Roberts S, Khera E, Choi C, Navaratna T, Grimm J, Thurber GM, et al. Optoacoustic Imaging of Glucagon-Like Peptide 1 Receptor With a Near-Infrared Exendin-4 Analog. Azad BB, Rota V. Design, Synthesis and In Vitro Characterization of Glucagon-Like Peptide-1 Derivatives for Pancreatic Beta Cell Imaging by SPECT. Bioorg Med Chem — Ast J, Arvaniti A, Fine NHF, Nasteska D, Ashford FB, Stamataki Z, et al. Super-Resolution Microscopy Compatible Fluorescent Probes Reveal Endogenous Glucagon-Like Peptide-1 Receptor Distribution and Dynamics. Waser B, Blank A, Karamitopoulou E, Perren A, Reubi JC. Glucagon-Like-Peptide-1 Receptor Expression in Normal and Diseased Human Thyroid and Pancreas. Mod Pathol — de Heer J, Rasmussen C, Coy DH, Holst JJ. Glucagon-Like Peptide-1, But Not Glucose-Dependent Insulinotropic Peptide, Inhibits Glucagon Secretion via Somatostatin Receptor Subtype 2 in the Perfused Rat Pancreas. Ørgaard A, Holst JJ. The Role of Somatostatin in GLPInduced Inhibition of Glucagon Secretion in Mice. Diabetologia —9. Saponaro C, Gmyr V, Thévenet J, Moerman E, Delalleau N, Pasquetti G, et al. The GLP1R Agonist Liraglutide Reduces Hyperglucagonemia Induced by the SGLT2 Inhibitor Dapagliflozin via Somatostatin Release. Lawlor N, Youn A, Kursawe R, Ucar D, Stitzel ML. Alpha TC1 and Beta-TC-6 Genomic Profiling Uncovers Both Shared and Distinct Transcriptional Regulatory Features With Their Primary Islet Counterparts. Sci Rep — Stamenkovic JA, Andersson LE, Adriaenssens AE, Bagge A, Sharoyko VV, Gribble F, et al. Inhibition of the Malate-Aspartate Shuttle in Mouse Pancreatic Islets Abolishes Glucagon Secretion Without Affecting Insulin Secretion. Briant LJB, Zhang Q, Vergari E, Kellard JA, Rodriguez B, Ashcroft FM, et al. Functional Identification of Islet Cell Types by Electrophysiological Fingerprinting. J R Soc Interface Ackermann AM, Zhang J, Heller A, Briker A, Kaestner KH. High-Fidelity Glucagon-CreER Mouse Line Generated by CRISPR-Cas9 Assisted Gene Targeting. Quoix N, Cheng-Xue R, Guiot Y, Herrera PL, Henquin JC, Gilon P. The GluCre-ROSA26EYFP Mouse: A New Model for Easy Identification of Living Pancreatic Alpha-Cells. FEBS Lett — Shiota C, Prasadan K, Guo P, Fusco J, Xiao X, Gittes GK. GcgCreERT2knockin Mice as a Tool for Genetic Manipulation in Pancreatic Alpha Cells. Andersson SA, Pedersen MG, Vikman J, Eliasson L. Glucose-Dependent Docking and SNARE Protein-Mediated Exocytosis in Mouse Pancreatic Alpha-Cell. Pflugers Arch Eur J Physiol — González-Vélez V, Dupont G, Gil A, González A, Quesada I. Model for Glucagon Secretion by Pancreatic α-Cells. Gerber SH, Sudhof TC. Molecular Determinants of Regulated Exocytosis. Gustavsson N, Wei S, Hoang DN, Lao Y, Zhang Q, Radda GK, et al. Jewell JL, Oh E, Thurmond DC. Exocytosis Mechanisms Underlying Insulin Release and Glucose Uptake : Conserved Roles for Munc18c and Syntaxin 4. Am J Physiol Regul Integr Comp Physiol R— Gandasi NR, Yin P, Riz M, Chibalina MV, Cortese G, Lund P, et al. Xia F, Leung YM, Gaisano G, Gao X, Chen Y, Fox JEM, et al. Montefusco F, Pedersen MG. Mathematical Modelling of Local Calcium and Regulated Exocytosis During Inhibition and Stimulation of Glucagon Secretion From Pancreatic Alpha-Cells. Yokawa S, Suzuki T, Inouye S, Inoh Y, Suzuki R, Kanamori T, et al. Visualization of Glucagon Secretion From Pancreatic α Cells by Bioluminescence Video Microscopy: Identification of Secretion Sites in the Intercellular Contact Regions. Biochem Biophys Res Commun — Brissova M, Haliyur R, Saunders D, Shrestha S, Dai C, Blodgett DM, et al. α Cell Function and Gene Expression Are Compromised in Type 1 Diabetes. Camunas-Soler J, Dai XQ, Hang Y, Bautista A, Lyon J, Suzuki K, et al. Patch-Seq Links Single-Cell Transcriptomes to Human Islet Dysfunction in Diabetes. Zhou Y, Liu Z, Zhang S, Zhuang R, Liu H, Liu X, et al. RILP Restricts Insulin Secretion Through Mediating Lysosomal Degradation of Proinsulin. Li H, Wei S, Cheng K, Gounko NV, Ericksen RE, Xu A, et al. BIG3 Inhibits Insulin Granule Biogenesis and Insulin Secretion. EMBO Rep — Li H, Liu T, Lim J, Gounko NV, Hong W, Han W. Increased Biogenesis of Glucagon-Containing Secretory Granules and Glucagon Secretion in BIG3-Knockout Mice. Liu T, Li H, Hong W, Han W. Brefeldin A-Inhibited Guanine Nucleotide Exchange Protein 3 is Localized in Lysosomes and Regulates GABA Signaling in Hippocampal Neurons. J Neurochem — Stathmin-2 Mediates Glucagon Secretion From Pancreatic α-Cells. Bramswig NC, Everett LJ, Schug J, Dorrell C, Liu C, Luo Y, et al. Epigenomic Plasticity Enables Human Pancreatic α to β Cell Reprogramming. Misrouting of Glucagon and Stathmin-2 Towards Lysosomal System of α-Cells in Glucagon Hypersecretion of Diabetes. bioRxiv 0—1. Cho HJ. Mook-Jung I. O-GlcNAcylation Regulates Endoplasmic Reticulum Exit Sites Through Sec31A Modification in Conventional Secretory Pathway. FASEB J — Zhang X, Wang L, Lak B, Li J, Jokitalo E, Wang Y. GRASP55 Senses Glucose Deprivation Through O-GlcNAcylation to Promote Autophagosome-Lysosome Fusion. Dev Cell — Essawy A, Jo S, Beetch M, Lockridge A, Gustafson E. Alejandro EU. O-Linked N-Acetylglucosamine Transferase OGT Regulates Pancreatic α-Cell Function in Mice. J Biol Chem Masson MH, Poisson C, Guérardel A, Mamin A, Philippe J, Gosmain Y. Foxa1 and Foxa2 Regulate β-Cell Differentiation, Glucagon Biosynthesis, and Secretion. Muralidharan C, Conteh AM, Marasco MR, Crowder JJ, Kuipers J, de Boer P, et al. Pancreatic Beta Cell Autophagy is Impaired in Type 1 Diabetes. |
| Glucagon (medication) - Wikipedia | Whalley African mango extract for metabolism, Pritchard LE, Smith Gluagon. In this G,ucagon, we explore the controversial relationships between glucagon and metabolic disorders associated mechanissm diabetes Fat burner for post-workout recovery on recent research mecanism an emphasis on recent evidence supporting Raspberry ketones and energy important role mechansim glucagon. For instance, 24 out of 50 patients who had either heart failure, cardiogenic shock or both showed some clinical improvement after adding glucagon to a combined therapy which included digitalization, furosemide and spironolactone. In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. In contrast to the role of the insulin signaling pathway, the glucagon signaling pathway is a pathway that promotes catabolism. |
| You and Your Hormones | It keeps your blood sugar levels from dipping too low , ensuring that your body has a steady supply of energy. But for some people, the process does not work properly. Diabetes can cause problems with blood sugar balance. Diabetes refers to a group of diseases. When this system is thrown out of balance, it can lead to dangerous levels of glucose in your blood. Of the two main types of diabetes, type 1 diabetes is the less common form. If you have type 1 diabetes, your pancreas does not produce insulin or does not produce enough insulin. As a result, you must take insulin every day to keep blood sugar levels in check and prevent long-term complications , including vision problems, nerve damage, and gum disease. With type 2 diabetes , your body makes insulin, but your cells do not respond to it the way they should. This is known as insulin resistance. Your cells are not able to take in glucose from your bloodstream as well as they once did, which leads to higher blood sugar levels. Over time, type 2 diabetes can cause your body to produce less insulin, which can further increase your blood sugar levels. Some people can manage type 2 diabetes with diet and exercise. Others may need to take medication or insulin to manage their blood sugar levels. Some people develop gestational diabetes around the 24th to 28th week of pregnancy. In gestational diabetes, pregnancy-related hormones may interfere with how insulin works. This condition often disappears after the pregnancy ends. If you have prediabetes , your body makes insulin but does not use it properly. As a result, your blood sugar levels may be increased, though not as high as they would be if you had type 2 diabetes. Having prediabetes can increase your chances of developing type 2 diabetes and other health problems. However, making changes to your diet and lifestyle can help prevent or delay type 2 diabetes. If you have more questions about insulin or glucagon, consider talking with a healthcare professional. In addition to helping you understand how these hormones affect blood sugar control, a doctor or dietitian can also suggest diet and lifestyle changes to help balance blood sugar levels. Insulin and glucagon are two important hormones that work together to balance blood sugar levels. Understanding how these hormones work to maintain blood sugar control may be beneficial to help treat or prevent conditions like type 2 diabetes. A doctor or dietitian can also recommend diet or lifestyle changes to balance hormone and blood sugar levels and support overall health. Our experts continually monitor the health and wellness space, and we update our articles when new information becomes available. VIEW ALL HISTORY. Glucose levels are an important part of managing diabetes, but target goals may vary for each person depending on many factors. Different types of insulin work at different speeds in the body. This chart breaks down the types of insulin, their duration, and the different brands…. Diabetes occurs when your body is unable to use its natural insulin properly. Learn more about manual insulin injections and how they help treat…. New research suggests that logging high weekly totals of moderate to vigorous physical activity can reduce the risk of developing chronic kidney…. Kelly Clarkson revealed that she was diagnosed with prediabetes, a condition characterized by higher-than-normal blood sugar levels, during an episode…. New research has revealed that diabetes remission is associated with a lower risk of cardiovascular disease and chronic kidney disease. Type 2…. A Quiz for Teens Are You a Workaholic? How Well Do You Sleep? Health Conditions Discover Plan Connect. Type 2 Diabetes. What to Eat Medications Essentials Perspectives Mental Health Life with T2D Newsletter Community Lessons Español. How Insulin and Glucagon Work. Medically reviewed by Kelly Wood, MD — By Susan York Morris — Updated on October 4, Insulin and glucagon are potent regulators of glucose metabolism. For decades, we have viewed diabetes from a bi-hormonal perspective of glucose regulation. This perspective is incomplete and inadequate in explaining some of the difficulties that patients and practitioners face when attempting to tightly control blood glucose concentrations. Intensively managing diabetes with insulin is fraught with frustration and risk. Despite our best efforts,glucose fluctuations are unpredictable, and hypoglycemia and weight gain are common. These challenges may be a result of deficiencies or abnormalities in other glucoregulatory hormones. New understanding of the roles of other pancreatic and incretin hormones has led to a multi-hormonal view of glucose homeostasis. Our understanding of diabetes as a metabolic disease has evolved significantly since the discovery of insulin in the s. Insulin was identified as a potent hormonal regulator of both glucose appearance and disappearance in the circulation. Subsequently, diabetes was viewed as a mono-hormonal disorder characterized by absolute or relative insulin deficiency. Since its discovery, insulin has been the only available pharmacological treatment for patients with type 1 diabetes and a mainstay of therapy for patients with insulin-deficient type 2 diabetes. The recent discovery of additional hormones with glucoregulatory actions has expanded our understanding of how a variety of different hormones contribute to glucose homeostasis. In the s, glucagon was characterized as a major stimulus of hepatic glucose production. This discovery led to a better understanding of the interplay between insulin and glucagon, thus leading to a bi-hormonal definition of diabetes. Subsequently, the discovery of a secondβ-cell hormone, amylin, was first reported in Amylin was determined to have a role that complemented that of insulin, and, like insulin, was found to be deficient in people with diabetes. This more recent development led to a view of glucose homeostasis involving multiple pancreatic hormones. In the mid s, several gut hormones were identified. One of these, an incretin hormone, glucagon-like peptide-1 GLP-1 , was recognized as another important contributor to the maintenance of glucose homeostasis. This enhanced understanding of glucose homeostasis will be central to the design of new pharmacological agents to promote better clinical outcomes and quality of life for people with diabetes. This review will focus on the more recently discovered hormones involved in glucose homeostasis and is not intended to be a comprehensive review of diabetes therapies. Plasma glucose concentration is a function of the rate of glucose entering the circulation glucose appearance balanced by the rate of glucose removal from the circulation glucose disappearance. Circulating glucose is derived from three sources: intestinal absorption during the fed state,glycogenolysis, and gluconeogenesis. The major determinant of how quickly glucose appears in the circulation during the fed state is the rate of gastric emptying. Other sources of circulating glucose are derived chiefly from hepatic processes: glycogenolysis, the breakdown of glycogen, the polymerized storage form of glucose; and gluconeogenesis, the formation of glucose primarily from lactate and amino acids during the fasting state. Glycogenolysis and gluconeogenesis are partly under the control of glucagon, a hormone produced in the α-cells of the pancreas. During the first 8—12 hours of fasting, glycogenolysis is the primary mechanism by which glucose is made available Figure 1A. Glucagon facilitates this process and thus promotes glucose appearance in the circulation. Over longer periods of fasting, glucose,produced by gluconeogenesis, is released from the liver. Glucose homeostasis: roles of insulin and glucagon. For nondiabetic individuals in the fasting state, plasma glucose is derived from glycogenolysis under the direction of glucagon 1. Basal levels of insulin control glucose disposal 2. Insulin's role in suppressing gluconeogenesis and glycogenolysis is minimal due to low insulin secretion in the fasting state 3. For nondiabetic individuals in the fed state, plasma glucose is derived from ingestion of nutrients 1. In the bi-hormonal model, glucagon secretion is suppressed through the action of endogenous insulin secretion 2. This action is facilitated through the paracrine route communication within the islet cells 3. Additionally, in the fed state, insulin suppresses gluconeogenesis and glycogenolysis in the liver 4 and promotes glucose disposal in the periphery 5. For individuals with diabetes in the fasting state, plasma glucose is derived from glycogenolysis and gluconeogenesis 1 under the direction of glucagon 2. Exogenous insulin 3 influences the rate of peripheral glucose disappearance 4 and, because of its deficiency in the portal circulation, does not properly regulate the degree to which hepatic gluconeogenesis and glycogenolysis occur 5. For individuals with diabetes in the fed state, exogenous insulin 1 is ineffective in suppressing glucagon secretion through the physiological paracrine route 2 , resulting in elevated hepatic glucose production 3. As a result, the appearance of glucose in the circulation exceeds the rate of glucose disappearance 4. The net effect is postprandial hyperglycemia 5. Glucoregulatory hormones include insulin, glucagon, amylin, GLP-1,glucose-dependent insulinotropic peptide GIP , epinephrine, cortisol, and growth hormone. Of these, insulin and amylin are derived from theβ-cells, glucagon from the α-cells of the pancreas, and GLP-1 and GIP from the L-cells of the intestine. The glucoregulatory hormones of the body are designed to maintain circulating glucose concentrations in a relatively narrow range. In the fasting state, glucose leaves the circulation at a constant rate. To keep pace with glucose disappearance, endogenous glucose production is necessary. For all practical purposes, the sole source of endogenous glucose production is the liver. Renal gluconeogenesis contributes substantially to the systemic glucose pool only during periods of extreme starvation. Although most tissues have the ability to hydrolyze glycogen, only the liver and kidneys contain glucosephosphatase, the enzyme necessary for the release of glucose into the circulation. In the bi-hormonal model of glucose homeostasis, insulin is the key regulatory hormone of glucose disappearance, and glucagon is a major regulator of glucose appearance. After reaching a post-meal peak, blood glucose slowly decreases during the next several hours, eventually returning to fasting levels. In the immediate post-feeding state, glucose removal into skeletal muscle and adipose tissue is driven mainly by insulin. At the same time, endogenous glucose production is suppressed by 1 the direct action of insulin, delivered via the portal vein, on the liver, and 2 the paracrine effect or direct communication within the pancreas between the α- andβ-cells, which results in glucagon suppression Figure 1B. Until recently, insulin was the only pancreatic β-cell hormone known to lower blood glucose concentrations. Insulin, a small protein composed of two polypeptide chains containing 51 amino acids, is a key anabolic hormone that is secreted in response to increased blood glucose and amino acids following ingestion of a meal. Like many hormones, insulin exerts its actions through binding to specific receptors present on many cells of the body,including fat, liver, and muscle cells. The primary action of insulin is to stimulate glucose disappearance. Insulin helps control postprandial glucose in three ways. Initially,insulin signals the cells of insulin-sensitive peripheral tissues, primarily skeletal muscle, to increase their uptake of glucose. Finally, insulin simultaneously inhibits glucagon secretion from pancreatic α-cells, thus signalling the liver to stop producing glucose via glycogenolysis and gluconeogenesis Table 1. All of these actions reduce blood glucose. Insulin action is carefully regulated in response to circulating glucose concentrations. Long-term release of insulin occurs if glucose concentrations remain high. While glucose is the most potent stimulus of insulin, other factors stimulate insulin secretion. These additional stimuli include increased plasma concentrations of some amino acids, especially arginine, leucine, and lysine;GLP-1 and GIP released from the gut following a meal; and parasympathetic stimulation via the vagus nerve. Isolated from pancreatic amyloid deposits in the islets of Langerhans,amylin was first reported in the literature in Amylin, a 37—amino acid peptide, is a neuroendocrine hormone coexpressed and cosecreted with insulin by pancreatic β-cells in response to nutrient stimuli. Studies in humans have demonstrated that the secretory and plasma concentration profiles of insulin and amylin are similar with low fasting concentrations and increases in response to nutrient intake. In subjects with diabetes,amylin is deficient in type 1 and impaired in type 2 diabetes. Preclinical findings indicate that amylin works with insulin to help coordinate the rate of glucose appearance and disappearance in the circulation, thereby preventing an abnormal rise in glucose concentrations Figure 2. Postprandial glucose flux in nondiabetic controls. Postprandial glucose flux is a balance between glucose appearance in the circulation and glucose disappearance or uptake. Glucose appearance is a function of hepatic endogenous glucose production and meal-derived sources and is regulated by pancreatic and gut hormones. Glucose disappearance is insulin mediated. Calculated from data in the study by Pehling et al. Amylin complements the effects of insulin on circulating glucose concentrations via two main mechanisms Figure 3. Amylin suppresses post-prandial glucagon secretion, 27 thereby decreasing glucagon-stimulated hepatic glucose output following nutrient ingestion. This suppression of post-prandial glucagon secretion is postulated to be centrally mediated via efferent vagal signals. Importantly,amylin does not suppress glucagon secretion during insulin-induced hypoglycemia. Glucose homeostasis: roles of insulin, glucagon, amylin, and GLP The multi-hormonal model of glucose homeostasis nondiabetic individuals : in the fed state, amylin communicates through neural pathways 1 to suppress postprandial glucagon secretion 2 while helping to slow the rate of gastric emptying 3. These actions regulate the rate of glucose appearance in the circulation 4. In addition, incretin hormones, such as GLP-1, glucose-dependently enhance insulin secretion 6 and suppress glucagon secretion 2 and, via neural pathways, help slow gastric emptying and reduce food intake and body weight 5. Amylin exerts its actions primarily through the central nervous system. Animal studies have identified specific calcitonin-like receptor sites for amylin in regions of the brain, predominantly in the area postrema. The area postrema is a part of the dorsal vagal complex of the brain stem. A notable feature of the area postrema is that it lacks a blood-brain barrier, allowing exposure to rapid changes in plasma glucose concentrations as well as circulating peptides, including amylin. In summary, amylin works to regulate the rate of glucose appearance from both endogenous liver-derived and exogenous meal-derived sources, and insulin regulates the rate of glucose disappearance. Glucagon is a key catabolic hormone consisting of 29 amino acids. It is secreted from pancreatic α-cells. Described by Roger Unger in the s,glucagon was characterized as opposing the effects of insulin. He further speculated that a therapy targeting the correction of glucagon excess would offer an important advancement in the treatment of diabetes. Hepatic glucose production, which is primarily regulated by glucagon,maintains basal blood glucose concentrations within a normal range during the fasting state. When plasma glucose falls below the normal range, glucagon secretion increases, resulting in hepatic glucose production and return of plasma glucose to the normal range. When coupled with insulin's direct effect on the liver, glucagon suppression results in a near-total suppression of hepatic glucose output Figure 4. Insulin and glucagon secretion: nondiabetic and diabetic subjects. In nondiabetic subjects left panel , glucose-stimulated insulin and amylin release from the β -cells results in suppression of postprandial glucagon secretion. In a subject with type 1 diabetes, infused insulin does not suppress α -cell production of glucagon. Adapted from Ref. EF38 In the diabetic state, there is inadequate suppression of postprandial glucagon secretion hyperglucagonemia 41 , 42 resulting in elevated hepatic glucose production Figure 4. Importantly,exogenously administered insulin is unable both to restore normal postprandial insulin concentrations in the portal vein and to suppress glucagon secretion through a paracrine effect. This results in an abnormally high glucagon-to-insulin ratio that favors the release of hepatic glucose. The intricacies of glucose homeostasis become clearer when considering the role of gut peptides. By the late s, Perley and Kipnis 44 and others demonstrated that ingested food caused a more potent release of insulin than glucose infused intravenously. Additionally, these hormonal signals from the proximal gut seemed to help regulate gastric emptying and gut motility. Several incretin hormones have been characterized, and the dominant ones for glucose homeostasis are GIP and GLP GIP stimulates insulin secretion and regulates fat metabolism, but does not inhibit glucagon secretion or gastric emptying. GLP-1 also stimulates glucose-dependent insulin secretion but is significantly reduced postprandially in people with type 2 diabetes or impaired glucose tolerance. Derived from the proglucagon molecule in the intestine, GLP-1 is synthesized and secreted by the L-cells found mainly in the ileum and colon. Circulating GLP-1 concentrations are low in the fasting state. However, both GIP and GLP-1 are effectively stimulated by ingestion of a mixed meal or meals enriched with fats and carbohydrates. GLP-1 has many glucoregulatory effects Table 1 and Figure 3. In the pancreas,GLP-1 stimulates insulin secretion in a glucose-dependent manner while inhibiting glucagon secretion. Infusion of GLP-1 lowers postprandial glucose as well as overnight fasting blood glucose concentrations. Yet while GLP-1 inhibits glucagon secretion in the fed state, it does not appear to blunt glucagon's response to hypoglycemia. Administration of GLP-1 has been associated with the regulation of feeding behavior and body weight. Of significant and increasing interest is the role GLP-1 may have in preservation of β-cell function and β-cell proliferation. Our understanding of the pathophysiology of diabetes is evolving. Type 1 diabetes has been characterized as an autoimmune-mediated destruction of pancreaticβ-cells. Early in the course of type 2 diabetes, postprandial β-cell action becomes abnormal, as evidenced by the loss of immediate insulin response to a meal. Abnormal gastric emptying is common to both type 1 and type 2 diabetes. The rate of gastric emptying is a key determinant of postprandial glucose concentrations Figure 5. In individuals with diabetes, the absent or delayed secretion of insulin further exacerbates postprandial hyperglycemia. Both amylin and GLP-1 regulate gastric emptying by slowing the delivery of nutrients from the stomach to the small intestine. Gastric emptying rate is an important determinant of postprandial glycemia. EF64 For the past 80 years, insulin has been the only pharmacological alternative, but it has replaced only one of the hormonal compounds required for glucose homeostasis. Newer formulations of insulin and insulin secretagogues, such as sulfonylureas and meglitinides, have facilitated improvements in glycemic control. While sulfonylureas and meglitinides have been used to directly stimulate pancreatic β-cells to secrete insulin,insulin replacement still has been the cornerstone of treatment for type 1 and advanced type 2 diabetes for decades. Advances in insulin therapy have included not only improving the source and purity of the hormone, but also developing more physiological means of delivery. Clearly, there are limitations that hinder normalizing blood glucose using insulin alone. First, exogenously administered insulin does not mimic endogenous insulin secretion. In normal physiology, the liver is exposed to a two- to fourfold increase in insulin concentration compared to the peripheral circulation. |
| Glucagon - Wikipedia | Reviewed by: Quan Zhang , University of Oxford, United Kingdom Caroline Miranda , University of Gothenburg, Sweden. Diabetes refers to a group of diseases. Rai V, Quang DX, Erdos MR, Cusanovich DA, Daza RM, Narisu N, et al. It works in totally opposite way to insulin. Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Location of amide groups, acid degradation studies and summary of sequential evidence". |
| What is glucagon? | An example of the pathway would be when glucagon binds to a transmembrane protein. The transmembrane proteins interacts with Gɑβ𝛾. Gαs separates from Gβ𝛾 and interacts with the transmembrane protein adenylyl cyclase. Adenylyl cyclase catalyzes the conversion of ATP to cAMP. cAMP binds to protein kinase A, and the complex phosphorylates glycogen phosphorylase kinase. Phosphorylated glycogen phosphorylase clips glucose units from glycogen as glucose 1-phosphate. Additionally, the coordinated control of glycolysis and gluconeogenesis in the liver is adjusted by the phosphorylation state of the enzymes that catalyze the formation of a potent activator of glycolysis called fructose 2,6-bisphosphate. This covalent phosphorylation initiated by glucagon activates the former and inhibits the latter. This regulates the reaction catalyzing fructose 2,6-bisphosphate a potent activator of phosphofructokinase-1, the enzyme that is the primary regulatory step of glycolysis [24] by slowing the rate of its formation, thereby inhibiting the flux of the glycolysis pathway and allowing gluconeogenesis to predominate. This process is reversible in the absence of glucagon and thus, the presence of insulin. Glucagon stimulation of PKA inactivates the glycolytic enzyme pyruvate kinase , [25] inactivates glycogen synthase , [26] and activates hormone-sensitive lipase , [27] which catabolizes glycerides into glycerol and free fatty acid s , in hepatocytes. Malonyl-CoA is a byproduct of the Krebs cycle downstream of glycolysis and an allosteric inhibitor of Carnitine palmitoyltransferase I CPT1 , a mitochondrial enzyme important for bringing fatty acids into the intermembrane space of the mitochondria for β-oxidation. Thus, reduction in malonyl-CoA is a common regulator for the increased fatty acid metabolism effects of glucagon. Abnormally elevated levels of glucagon may be caused by pancreatic tumors , such as glucagonoma , symptoms of which include necrolytic migratory erythema , [30] reduced amino acids, and hyperglycemia. It may occur alone or in the context of multiple endocrine neoplasia type 1. Elevated glucagon is the main contributor to hyperglycemic ketoacidosis in undiagnosed or poorly treated type 1 diabetes. As the beta cells cease to function, insulin and pancreatic GABA are no longer present to suppress the freerunning output of glucagon. As a result, glucagon is released from the alpha cells at a maximum, causing a rapid breakdown of glycogen to glucose and fast ketogenesis. The absence of alpha cells and hence glucagon is thought to be one of the main influences in the extreme volatility of blood glucose in the setting of a total pancreatectomy. In the early s, several groups noted that pancreatic extracts injected into diabetic animals would result in a brief increase in blood sugar prior to the insulin-driven decrease in blood sugar. Kimball and John R. Murlin identified a component of pancreatic extracts responsible for this blood sugar increase, terming it "glucagon", a portmanteau of " gluc ose agon ist". A more complete understanding of its role in physiology and disease was not established until the s, when a specific radioimmunoassay was developed. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item. Download as PDF Printable version. In other projects. Wikimedia Commons. Peptide hormone. This article is about the natural hormone. For the medication, see Glucagon medication. Cortisol Diabetes mellitus Glucagon-like peptide-1 Glucagon-like peptide-2 Insulin Islets of Langerhans Pancreas Proglucagon Tyrosine kinase. Biochemistry 4th ed. New York: Wiley. San Francisco: Benjamin Cummings. ISBN Biology 1: Molecules. Examkrackers Inc. doi : Alternatively, somatostatin resistance may be a dominant and direct mechanism of hyperglucagonemia, as eliminating the insulin receptor on delta cells completely abolishes the glucagonostatic effect of insulin, indicating an indirect glucagonostatic effect for insulin The emerging role of somatostatin in the regulation of alpha cell function and glucagon secretion has been further highlighted by one study in which mice were engineered for optogenetic activation of beta cells to study the paracrine regulation of alpha cells By this approach, opto-activation of beta cells both suppressed alpha cell electrical activity and stimulated action potentials in delta cells mediated by gap junction currents. The suppressive effect of beta cell activation was lost in the presence of the SSTR2 antagonist CYN 99 , indicating that somatostatin secretion stimulated by beta cell electrical activity is critical for the suppression of glucagon secretion. Subsequent modelling predicted that a reduction in gap junction connections between beta and delta cells, perhaps caused by disruptions in islet architecture in T2D , may contribute to the hyperglucagonemia of diabetes. Thus these findings highlight a central role for delta cells in the context of intra-islet regulation of glucagon secretion, and may have implications for designing drugs for the treatment of hyperglucagonemia of diabetes. The alpha cell itself displays plasticity during the progression of diabetes. In addition to the mechanisms above that describe changes in responses to glucose and paracrine effectors, there are alterations within the alpha cell, including proglucagon processing and secretion of proglucagon-derived peptides, and remodelling of the secretory granules themselves in terms of exocytotic behavior and contents, and alterations in intracellular trafficking pathways. Secreted glucagon from alpha cells can stimulate its secretion through an autocrine effect. It has been shown that glucagon stimulates glucagon secretion from the rat and mouse isolated alpha cells in an autocrine manner through glucagon receptor-stimulated cAMP signaling In αTC cells and mouse islets, exogenous glucagon administration, as well as secreted glucagon stimulated by 1 mM glucose, increased glucagon secretion and proglucagon gene transcription through the PKA-cAMP-CREB signalling pathway in a glucagon receptor-dependent manner The apparent interplay between glucagon and its receptor on the alpha cell appears to be of a positive feedback loop, controlled by the pulsatile nature of glucagon secretion. In addition to glucagon, a novel proglucagon-derived peptide, proglucagon PG comprised of GRPP and glucagon, was identified as a major molecular form of glucagon in plasma from human patients with hyperglucagonemia-associated conditions: Type 2 diabetes and renal dysfunction, morbid obesity or gastric bypass surgery, and only after oral ingestion of macronutrients This N-terminally extended form of immunoreactive glucagon was not found in healthy controls, leading the authors to speculate that PG , and molecular heterogeneity of glucagon in general, could be a biomarker for alpha cell dysfunction. Administration of PG decreased glucagon secretion in healthy rats, diverging from the positive feedback observed with glucagon administration. Interestingly, this effect was not observed in diabetic rats, suggesting an impairment in this distinct feedback loop in the alpha cell. The interplay between glucagon, insulin and somatostatin in the regulation of glucagon secretion at various levels of glucose is illustrated in Figure 3. In diabetes, beta cell deficiency, together with alpha cell insulin and somatostatin resistance, all contribute to alpha cell dysfunction and a loss of the regulation of glucagon secretion, resulting in hyperglucagonemia. Figure 3 Cross-talk among α, β, and δ-cells in the paracrine regulation of glucagon secretion. Under low glucose mM conditions, secreted glucagon may act in an autocrine feed-forward loop. Additionally, electrical coupling of the beta and delta cells through gap junctions contributes to somatostatin release. Somatostatin binds to SST receptor 2 SSTR2 on the α cell membrane, where signalling through G i inhibits glucagon secretion. The glucose-dependent insulinotropic actions of intestinal GLP-1 on the beta cell are well known. GLP-1 also suppresses glucagon secretion in both healthy people and people with type 2 diabetes , and poorly-controlled type 1 diabetes The emerging evidence of GLP-1 being produced and secreted by the pancreatic alpha cell has led to a debate on which source of GLP-1 suppresses glucagon secretion from pancreatic alpha cells. To investigate this question, Chambers et al. The gut-derived GLP-1 binds to its receptor on local afferent vagal nerve terminals, which ultimately signals for satiety, delaying gastric emptying and suppression of hepatic glucose release , However, this model may not translate well to human islets due to differences in islet architecture, and in light of the recent findings that glucagon is the dominant peptide hormone secreted from human alpha cells The search for a GLP-1 receptor on alpha cells has been hampered by a lack of a reliable GLP-1 receptor antibody , GLP-1 appears to mildly reduce action potentials in the alpha cell membrane at 1 mM glucose in isolated mouse alpha cells, and this effect is blocked by the GLP-1R antagonist exendin , therefore suggesting the presence of GLP-1R, perhaps at a very low density, on a small proportion of alpha cells. The development of near infra-red and fluorescent analogues of GLP-1R ligands has enabled both in vivo , and high-resolution tissue imaging , of GLP-1R with high specificity, sensitivity, and reproducibility. Given the already small proportion of alpha cells in the mouse islet, the contribution of direct alpha cell action to the glucagonostatic effect of GLP-1 is likely very small. Islet GLP-1 may also exert its effects through receptors on delta cells , resulting in stimulation of somatostatin secretion and inhibition of glucagon secretion via SSTR2 on alpha cells , This paracrine effect could not be detected in isolated normal human islets ; nonetheless, this mechanism may be clinically relevant in the treatment of T2D, as experiments in human islets showed that the GLP-1R agonist liraglutide enhanced somatostatin secretion to reduce hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin As drugs targeted to the control of glucagon secretion are now being developed for the treatment of hyperglucagonemia, a deeper understanding of the dynamics of the alpha cell secretory granule is critical for identifying effective targets. However, the study of glucagon granule trafficking and exocytosis presents several technological challenges. Commonly used cell lines such as InR1-G9, αTC and αTC, while useful for preliminary studies on trafficking and secretion, as a rule do not exhibit robust secretory responses to glucose or other secretagogues. The αTC cell line in particular differs from primary alpha cells in their complement of transcriptional, epigenetic and metabolic factors , which may explain the blunted secretory response to glucose. Dispersed primary alpha cells may offer a slightly better alternative, but as discussed above, both cell lines and dispersed primary alpha cells exhibit aberrant glucagon exocytosis patterns at high glucose levels, likely due to the absence of paracrine inputs and juxtamembrane contacts. The greatest advances in gleaning the mechanisms of glucagon granule exocytosis have been made using patch-clamp approaches in isolated rodent or human islets. In such preparations, alpha cells can identified by their unique electrophysiological signature under low glucose conditions or, in the case of mouse islets, by genetically-encoded fluorescence reporters such as YFP , or tdTomato After proglucagon processing and granule maturation, glucagon is stored in the alpha cell secretory granule until a stimulus triggers exocytosis. As in beta cells, there may be different functional pools of secretory granules: a reserve pool and a readily releasable pool that is primed and situated at the sites of exocytosis. Quantitative ultrastructural analysis of murine islets has shown that, in the presence of 1mM glucose, the mouse α-cell contains ~ secretory granules, of which ~ are in close proximity to the plasma membrane, or primed This means that the reserve pool is large and can resupply the readily releasable pool to maintain euglycemia over extended periods of time. In the presence of Following docking, secretory granules are primed through the action of the SNARE protein complex. This complex contains two subsets of proteins; i the t-SNAREs syntaxin 1A and SNAP, located in the plasma membrane; and ii the v-SNAREs VAMP2 and synaptotagmin VII, which are located in the granule membrane Under low glucose conditions, SNAP and syntaxin 1A are translocated to the plasma membrane. SNAP itself may play a role in the transportation of granules from the releasable pool to the readily releasable pool, and then mediates their fusion with plasma membrane via interaction with syntaxin 1A , Live imaging of exocytosis using a proglucagon-luciferase reporter showed spatial clustering of glucagon secretion sites in αTC cells Future studies may reveal some interesting dynamics with SNARE proteins that may fine-tune the alpha cell secretory response to glucose and paracrine inputs. Could disruption of these molecular mechanisms contribute to the hyperglucagonemia of diabetes? However, neither membrane potential nor exocytosis was responsive to insulin or to a greater extent somatostatin, in contrast to normal alpha cells in which both were significantly reduced. Therefore, in T2D, hyperglucagonemia may result from insulin and somatostatin resistance at the level of the readily releasable pool of granules. In alpha cells of patients with T1D, expression levels of genes encoding SNARE proteins, ion channels and cAMP signalling molecules were disrupted , which could explain the impaired glucose counter-regulatory response and the inappropriately elevated levels of postprandial glucagon in T1D. Combining patch-clamp electrophysiological measurements with single-cell RNA sequencing patch-seq in human islets has given high-resolution insight into mechanisms underlying impairments in alpha cell function in diabetes at the level of granule exocytosis. Further characterization of the link between electrophysiological signatures and the genes regulating the dynamics of granule exocytosis will reveal new mechanisms of alpha cell dysfunction in diabetes. Identifying new pathways or networks that control glucagon granule biogenesis and trafficking may identify novel targets for the control of hyperglucagonemia in addition to yielding a greater understanding of alpha cell biology in both health and disease. There is an emerging hypothesis that glucagon secretion can be controlled by trafficking through the endosomal-lysosomal pathway, similar to insulin , and below, we highlight some recent studies that suggest glucagon may regulated through such an alternate trafficking pathway. Brefeldin A-inhibited guanine nucleotide exchange protein 3 BIG3 is a member of the Arf-GEF family of proteins, and was initially found in a database search and found to inhibit insulin granule biogenesis and insulin secretion A subsequent study found that it had a similar role in regulating glucagon granule production and exocytosis Whether BIG3 can mediate glucagon trafficking through lysosomes remains to be investigated. The composition and cargo of the alpha cell secretory granule may also hold some determinants of glucagon secretion. While it is known that granule contents and composition are modified during normal granule maturation, a more complete picture of granule remodeling and heterogeneity in the context of intracellular trafficking networks in normal physiology and in diabetes is required. In an effort to identify networks of secretory granule proteins that interact with glucagon and regulate its trafficking and secretion, proteomic analysis was conducted on αTC cell secretory granule lysates immunoprecipitated with tagged glucagon This qualitative study demonstrated the plasticity in the network of proteins interacting with glucagon in response to insulin or GABA under high 25 mM or low 5. Stathmin-2, a member of the family of neuronal phosphoproteins that associates with the secretory pathway in neurons, was identified as a candidate protein for the regulation of glucagon secretion and subsequently shown to modulate glucagon secretion through the lysosomal pathway and may be down-regulated in diabetes in humans and in mice Therefore, disruptions in the routing of glucagon through the lysosomal pathway may contribute to the hyperglucagonemia of diabetes Figure 4. Figure 4 Stathminmediated lysosomal trafficking modulates glucagon secretion. Glucagon dark blue and stathmin-2 light blue are normally sorted to secretory granules from the Golgi in alpha cells. Stathmin-2 overexpression diverts glucagon-containing secretory granules to lysosomes black arrows , thus reducing glucagon secretion. Additionally, secretion from secretory granules is also enhanced solid red arrow. Glucagon trafficking and exocytosis may also be controlled through nutrient-driven pathways. The nutrient sensor O-GlcNAc transferase OGT catalyses the O-glycosylation of several proteins including those involved in the conventional secretory pathway and autophagosome-lysosome fusion In mice lacking OGT specifically in alpha cells, glucagon secretion, cell content and alpha cell mass are reduced Possible mechanisms include lack of O-glycosylation of FOXA1 and FOXA2, which regulate genes encoding proteins involved in proglucagon processing and glucagon secretion Whether other trafficking proteins are affected, and how alpha cell function is affected in diabetes in these mice, is not yet known. So what are the implications of glucagon trafficking through the lysosomal pathway in diabetes? Lysosomal trafficking and autophagy in the beta cell may be a possible mechanism of insulin secretory defects in diabetes, with a recent study providing evidence for impairment of lysosomal function in human T1D How does lysosomal function contribute to defects in alpha cell function? It is tempting to hypothesize that impairments in lysosomal biogenesis and trafficking result in both reduced insulin secretion in the beta cell and unregulated glucagon secretion from the alpha cell. Further investigation into the altered dynamics of glucagon trafficking in the alpha cell in diabetes may reveal key roles for the lysosome in the regulation of glucagon secretion, thus identifying a potential new target for the treatment of hyperglucagonemia. Finally, some excellent single-cell transcriptomics and epigenomics databases are being generated that reveal the dynamics of intracellular trafficking networks at the transcriptional level in human pancreatic alpha cells in both health and diabetes — The mapping of T2D-associated genetic variants with RNA-seq of human islets may reveal risk factors associated with defects in alpha cell function A novel immunocompromised mouse model in which glucagon-encoding codons were deleted while preserving both GLP-1 and GLP-2 will provide an innovative and much-needed resource for the study of the regulation of glucagon secretion from human islets in vivo In this study, transplantation of islets from people with T2D resulted in hyperglucagonemia with apparent alpha cell insulin resistance, revealing intrinsic alpha cell defects in T2D. Moreover, defects in alpha cell function were more apparent than in isolated islets, thus emphasizing the utility of such an in vivo system to investigate the molecular mechanisms of glucagon secretion in human islets, and the testing of possible treatments for hyperglucagonemia. While the development of glucagon receptor antagonists and other inhibitors of glucagon action has provided some possibilities for the treatment of hyperglucagonemia, there are significant side effects that result from impaired hepatic metabolism and potentially uncontrolled alpha cell proliferation. The advantage to developing such drugs, however, lie in the fact that the glucagon receptor is an easily available target. In contrast, targeting glucagon secretion as a means to treat hyperglucagonemia may alleviate concerns about effects on the liver and alpha cell mass; however, there are potentially many more targets within the alpha cell secretory pathway, and many of those may not be easily accessible for drug treatment. The ongoing discovery of novel proteins and networks that regulate the secretion of glucagon will shed further light on alpha cell biology in health and disease while also searching for improved means to control hyperglucagonemia and hyperglycemia of diabetes. SD and FA co-wrote the manuscript. All authors contributed to the article and approved the submitted version. This work was funded by a Natural Sciences and Engineering Research Council Discovery Grant to SD. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher. Stanley S, Moheet A, Seaquist ER. Central Mechanisms of Glucose Sensing and Counterregulation in Defense of Hypoglycemia. Endocr Rev — doi: PubMed Abstract CrossRef Full Text Google Scholar. DCCT Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes — Unger R, Orci L. The Essential Role of Glucagon in the Pathogenesis of Diabetes Mellitus. Lancet —6. Unger RH, Cherrington AD. Glucagonocentric Restructuring of Diabetes: A Pathophysiologic and Therapeutic Makeover. J Clin Invest — Lee Y, Wang M-Y, Du XQ, Charron MJ, Unger RH. Glucagon Receptor Knockout Prevents Insulin-Deficient Type 1 Diabetes in Mice. Diabetes —7. Conarello SL, Jiang G, Mu J, Li Z, Woods J, Zycband E, et al. Glucagon Receptor Knockout Mice are Resistant to Diet-Induced Obesity and Streptozotocin-Mediated Beta Cell Loss and Hyperglycaemia. Diabetologia — Neumann UH, Ho JSS, Mojibian M, Covey SD, Charron MJ, Kieffer TJ. Glucagon Receptor Gene Deletion in Insulin Knockout Mice Modestly Reduces Blood Glucose and Ketones But Does Not Promote Survival. Mol Metab —6. Damond N, Thorel F, Moyers JS, Charron MJ, Vuguin PM, Powers AC, et al. Blockade of Glucagon Signaling Prevents or Reverses Diabetes Onset Only If Residual β-Cells Persist. Elife — CrossRef Full Text Google Scholar. Kazda CM, Ding Y, Kelly RP, Garhyan P, Shi C, Lim CN, et al. Evaluation of Efficacy and Safety of the Glucagon Receptor Antagonist LY in Patients With Type 2 Diabetes: and Week Phase 2 Studies. Diabetes Care —9. Yang B, Gelfanov VM, Perez-Tilve D, DuBois B, Rohlfs R, Levy J, et al. Optimization of Truncated Glucagon Peptides to Achieve Selective, High Potency, Full Antagonists. J Med Chem — Lee CY, Choi H, Park EY, Nguyen TTL, Maeng HJ, Mee Lee K, et al. Synthesis and Anti-Diabetic Activity of Novel Biphenylsulfonamides as Glucagon Receptor Antagonists. Chem Biol Drug Des — Okamoto H, Cavino K, Na E, Krumm E, Kim SY, Cheng X, et al. Glucagon Receptor Inhibition Normalizes Blood Glucose in Severe Insulin-Resistant Mice. Proc Natl Acad Sci —8. Liang Y, Osborne MC, Monia BP, Bhanot S, Gaarde WA, Reed C, et al. Kim J, Okamoto H, Huang ZJ, Anguiano G, Chen S, Liu Q, et al. Amino Acid Transporter Slc38a5 Controls Glucagon Receptor Inhibition-Induced Pancreatic α Cell Hyperplasia in Mice. Cell Metab — Wei R, Gu L, Yang J, Yang K, Liu J, Le Y, et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience — Galsgaard KD, Winther-Sørensen M, Ørskov C, Kissow H, Poulsen SS, Vilstrup H, et al. Disruption of Glucagon Receptor Signaling Causes Hyperaminoacidemia Exposing a Possible Liver-Alpha-Cell Axis. Am J Physiol Metab E93—E Wewer Albrechtsen NJ, Pedersen J, Galsgaard KD, Winther-Sørensen M, Suppli MP, Janah L, et al. The Liver—α-Cell Axis and Type 2 Diabetes. Guan H-P, Yang X, Lu K, Wang S-P, Castro-Perez JM, Previs S, et al. Glucagon Receptor Antagonism Induces Increased Cholesterol Absorption. J Lipid Res — Tooze S. Biogenesis of Secretory Granules in the Trans-Golgi Network of Neuroendocrine and Endocrine Cells. Biochim Biophys Acta — Cool DR, Fenger M, Snell CR, Loh YP. Identification of the Sorting Signal Motif Within Pro-Opiomelanocortin for the Regulated Secretory Pathway. J Biol Chem —9. Dhanvantari S, Shen F, Adams T, Snell CR, Zhang C, Mackin RB, et al. Disruption of a Receptor-Mediated Mechanism for Intracellular Sorting of Proinsulin in Familial Hyperproinsulinemia. Mol Endocrinol — Zhang C-F, Dhanvantari S, Lou H, Loh YP. Sorting of Carboxypeptidase E to the Regulated Secretory Pathway Requires Interaction of its Transmembrane Domain With Lipid Rafts. Biochem J — Dikeakos JD, Mercure C, Lacombe M-J, Seidah NG, Reudelhuber TL. FEBS J — Dikeakos JD, Di Lello P, Lacombe M-J, Ghirlando R, Legault P, Reudelhuber TL, et al. Proc Natl Acad Sci USA — Dhanvantari S, Loh YP. Lipid Raft Association of Carboxypeptidase E Is Necessary for Its Function as a Regulated Secretory Pathway Sorting Receptor. J Biol Chem — Cool DR, Normant E, Shen F, Chen H, Pannell L, Zhang Y, et al. Carboxypeptidase E Is a Regulated Secretory Pathway Sorting Receptor: Genetic Obliteration Leads to Endocrine Disorders in Cpe Fat Mice. Cell — Irminger JC, Verchere CB, Meyer K, Halban PA. J Biol Chem —4. McGirr R, Guizzetti L, Dhanvantari S. The Sorting of Proglucagon to Secretory Granules is Mediated by Carboxypeptidase E and Intrinsic Sorting Signals. J Endocrinol — Hosaka M, Watanabe T, Sakai Y, Kato T, Takeuchi T. Interaction Between Secretogranin III and Carboxypeptidase E Facilitates Prohormone Sorting Within Secretory Granules. J Cell Sci — Plá V, Paco S, Ghezali G, Ciria V, Pozas E, Ferrer I, et al. Brain Pathol — Arvan P, Halban PA. Sorting Ourselves Out: Seeking Consensus on Trafficking in the Beta-Cell. Traffic — Guizzetti L, McGirr R, Dhanvantari S. Two Dipolar α-Helices Within Hormone-Encoding Regions of Proglucagon are Sorting Signals to the Regulated Secretory Pathway. Dey A, Lipkind GM, Rouillé Y, Norrbom C, Stein J, Zhang C, et al. Significance of Prohormone Convertase 2, PC2, Mediated Initial Cleavage at the Proglucagon Interdomain Site, LysArg71, to Generate Glucagon. Endocrinology — Rouille Y, Westermark G, Martin SK, Steiner DF. Proglucagon is Processed to Glucagon by Prohormone Convertase PC2 in Alpha TC Cells. Proc Natl Acad Sci —6. Dhanvantari S, Seidah NG, Brubaker PL. Role of Prohormone Convertases in the Tissue-Specific Processing of Proglucagon. Furuta M, Zhou A, Webb G, Carroll R, Ravazzola M, Orci L, et al. Severe Defect in Proglucagon Processing in Islet Alpha-Cells of Prohormone Convertase 2 Null Mice. Campbell SA, Golec DP, Hubert M, Johnson J, Salamon N, Barr A, et al. Human Islets Contain a Subpopulation of Glucagon-Like Peptide-1 Secreting α Cells That is Increased in Type 2 Diabetes. Mol Metab Nie Y, Nakashima M, Brubaker PL, Li QL, Perfetti R, Jansen E, et al. Regulation of Pancreatic PC1 and PC2 Associated With Increased Glucagon-Like Peptide 1 in Diabetic Rats. McGirr R, Ejbick CE, Carter DE, Andrews JD, Nie Y, Friedman TC, et al. Glucose Dependence of the Regulated Secretory Pathway in αtc Cells. Liu P, Song J, Liu H, Yan F, He T, Wang L, et al. In clinical trials, continuous subcutaneous or intravenous infusion was superior to single or repeated injections of GLP-1 because of the rapid degradation of GLP-1 by DPP-IV. To circumvent this intensive and expensive mode of treatment, clinical development of compounds that elicit similar glucoregulatory effects to those of GLP-1 are being investigated. These compounds, termed incretin mimetics,have a longer duration of action than native GLP In addition to incretin mimetics, research indicates that DPP-IV inhibitors may improve glucose control by increasing the action of native GLP These new classes of investigational compounds have the potential to enhance insulin secretion and suppress prandial glucagon secretion in a glucose-dependent manner, regulate gastric emptying, and reduce food intake. Despite current advances in pharmacological therapies for diabetes,attaining and maintaining optimal glycemic control has remained elusive and daunting. Intensified management clearly has been associated with decreased risk of complications. Glucose regulation is an exquisite orchestration of many hormones, both pancreatic and gut, that exert effect on multiple target tissues, such as muscle, brain, liver, and adipocyte. While health care practitioners and patients have had multiple therapeutic options for the past 10 years, both continue to struggle to achieve and maintain good glycemic control. There remains a need for new interventions that complement our current therapeutic armamentarium without some of their clinical short-comings such as the risk of hypoglycemia and weight gain. These evolving therapies offer the potential for more effective management of diabetes from a multi-hormonal perspective Figure 3 and are now under clinical development. Aronoff, MD, FACP, FACE, is a partner and clinical endocrinologist at Endocrine Associates of Dallas and director at the Research Institute of Dallas in Dallas, Tex. Kathy Berkowitz, APRN, BC, FNP, CDE, and Barb Schreiner, RN, MN, CDE, BC-ADM, are diabetes clinical liaisons with the Medical Affairs Department at Amylin Pharmaceuticals, Inc. Laura Want, RN, MS, CDE, CCRC, BC-ADM, is the clinical research coordinator at MedStar Research Institute in Washington, D. Note of disclosure: Dr. Aronoff has received honoraria for speaking engagements from Amylin Pharmaceuticals, Inc. Berkowitz and Ms. Schreiner are employed by Amylin. Want serves on an advisory panel for, is a stock shareholder in, and has received honoraria for speaking engagements from Amylin and has served as a research coordinator for studies funded by the company. She has also received research support from Lilly, Novo Nordisk, and MannKind Corporation. Amylin Pharmaceuticals, Inc. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest. filter your search All Content All Journals Diabetes Spectrum. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation. Volume 17, Issue 3. Previous Article. β-CELL HORMONES. α-CELL HORMONE: GLUCAGON. INCRETIN HORMONES GLP-1 AND GIP. AMYLIN ACTIONS. GLP-1 ACTIONS. Article Navigation. Feature Articles July 01 Glucose Metabolism and Regulation: Beyond Insulin and Glucagon Stephen L. Aronoff, MD, FACP, FACE ; Stephen L. Aronoff, MD, FACP, FACE. This Site. Google Scholar. Kathy Berkowitz, APRN, BC, FNP, CDE ; Kathy Berkowitz, APRN, BC, FNP, CDE. Barb Shreiner, RN, MN, CDE, BC-ADM ; Barb Shreiner, RN, MN, CDE, BC-ADM. Laura Want, RN, MS, CDE, CCRC, BC-ADM Laura Want, RN, MS, CDE, CCRC, BC-ADM. Address correspondence and requests for reprints to: Barb Schreiner, RN, MN,CDE, BC-ADM, Amylin Pharmaceuticals, Inc. Diabetes Spectr ;17 3 — Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide. Table 1. Effects of Primary Glucoregulatory Hormones. View large. View Large. Figure 2. Figure 3. Figure 4. Figure 5. American Diabetes Association: Clinical Practice Recommendations Diabetes Care. Am Fam Physician. DCCT Research Group: Hypoglycemia in the Diabetes Control and Complications Trial. DCCT Research Group: Weight gain associated with intensive therapy in the Diabetes Control and Complications Trial. UKPDS Study Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Clinical Diabetes. Biochem Biophys Res Commun. Am J Physiol. Proc Natl Acad Sci U S A. In International Textbook of Diabetes Mellitus. In William's Textbook of Endocrinology. Baillieres Best Pract Res Clin Endocrinol Metab. J Clin Endocrinol Metab. J Clin Invest. Data on file, Amylin Pharmaceuticals, Inc. Curr Pharm Des. normal controls Abstract. Curr Opin Endocrinol Diab. Diabetes Educ. Physiol Behav. Crit Revs Neurobiol. Expert Opin Therapeut Patents. J Pharmacol Exp Ther. Mol Pharmacol. N Engl J Med. Life Sci. Horm Metab Res. J Endocrinol. |
Es war und mit mir. Geben Sie wir werden diese Frage besprechen. Hier oder in PM.
Eben dass wir ohne Ihre glänzende Idee machen würden
Mir scheint es, dass es schon besprochen wurde, nutzen Sie die Suche nach dem Forum aus.
das sehr gute Stück
Sie irren sich. Geben Sie wir werden besprechen. Schreiben Sie mir in PM, wir werden umgehen.