Astaxanthin and liver health -
In studies on diet-induced obesity in mice, astaxanthin lowered blood glucose and blood lipids, such as cholesterol and triglycerides. It also prevented the activation of hepatic stellate cells, the major type of cell involved in the development of liver fibrosis.
In addition, it is a potent antioxidant, which means that it helps the body prevent the cell and tissue damage that are evident in disease and aging.
The liver is the largest glandular organ in the human body, weighing in at a substantial three pounds or so — and it performs all sorts of critical functions, from facilitating metabolism to aiding in the digestion of fats to detoxifying the body from potentially harmful substances, such as drugs and alcohol.
Lee explains that if astaxanthin can inhibit the development of certain genes involved in the development of fibrosis in humans, as well as in mice, it offers the promise that early stages of fibrosis can be regressed, thus allowing the liver to revert to a healthy state.
As a nutritionist, Lee is concerned with the health implications of obesity, which has become a worldwide epidemic. Department of Agriculture, Lee is looking at the potential for astaxanthin not only to prevent the disease from starting, but to reverse its effects if it has already begun to cause damage.
This is especially significant because, to date, there are no effective drug therapies to stop NAFLD. She cites the sobering statistics that, whereas 20 to 30 percent of people in the general population may have some accumulation of fat in their livers, in the obese population that number increases to 65 to 75 percent.
And while only 2 to 3 percent of the general population may have a more advanced form of the disease where the liver is inflamed — called nonalcoholic steatohepatitis — 15 to 20 percent of obese people suffer from this condition. The key to weight control remains in minimizing the amount of fat stored in the body, and this will always require maintaining a healthy lifestyle and making appropriate dietary choices.
Furthermore, ethanol-induced p-STAT3 expression was lowered in ethanol with AXT-fed mice than ethanol-fed mice Fig. A key role of STAT3 by binding AXT in chronic-binge ethanol induced alcoholic liver injury in mice. A Docking model of AXT bound with STAT3.
B The expression of STAT3 on presence or absence AXT in vitro and in vivo. C The expression of STAT3 and p-STAT3 were determined in the total protein extracts of mice liver tissues by Western blotting.
Studies conducted during the past few years that astaxanthin AXT plays a key role in anti-oxidant and anti-inflammatory responses In the present study, we demonstrated AXT alleviated ethanol-induced oxidative stress, liver inflammation and thus liver injury by inhibition of STAT3 activity. Ethanol exposure significantly induced hepatic injury in the livers of ethanol-fed mice, but it was alleviated in ethanol with AXT-fed mice.
AXT administration reduced ethanol-induced AST and ALT levels accompanied by increased of liver lipid droplet. In the liver tissue of ethanol-fed mice, many inflammatory cells activated and lipid droplets were revealed by pro-inflammatory cytokines and COX-2 expression.
However, in the liver tissue of ethanol with AXT-fed mice, inflammatory cells were decreased and lipid droplets were smaller. AXT administration 0. Thus, the present data indicated that AXT could be more potent hepatic protective agent for ethanol-induced liver damages.
Excessive ethanol exposure-induced CYP2E1 and iNOS expression leads to oxidative stress by the generation of ROS and increase in NO production in the hepatocytes of liver 23 , The expression of CYP2E1 and iNOS were decreased in in ethanol with AXT-fed mice.
In the liver tissue of ethanol with AXT-fed mice, iNOS-reactive cells were decreased. In addition, the production of NO was also decreased in ethanol with AXT-fed mice.
The GSH depletion resulted in the inhibition of oxidative stress In present study, total GSH levels were depleted in ethanol with AXT-fed mice. Moreover, level of lipid peroxidation in ethanol with AXT-fed mice was also decreased compared with ethanol-fed mice.
These data suggest that reduced oxidative damages could be associated with hepatic protective effect of AXT. Ethanol-induced liver damage is involved in inflammatory responses.
IL-6, a major pro-inflammatory cytokine, is elevated by ethanol consumption and is closely associated with ALD It is worthy to note that patients with severe alcoholic hepatitis who do not respond to medical treatment have low hepatic expression of TNF and IL-6 1.
In our study, the level of IL-6 was significantly reduced in the liver of ethanol with AXT-fed mice. Furthermore, other inflammatory cytokines such as TNF-α and IL-1β and chemokines such as MCP-1 and MIP-1β were also down-regulated in the liver of ethanol with AXT-fed mice. COX-2 is induced by pro-inflammatory cytokines and oxidant stress iNOS expression is increased by excessive ethanol consumption and is also induced by pro-inflammatory cytokines In the present study, ethanol-induced COX-2 and iNOS expression were decreased in the liver of ethanol with AXT-fed mice.
Therefore, these results suggest that AXT alleviated ethanol-induced pro-inflammatory responses, and thus ameliorated ethanol-induced liver damages. Neutrophils accumulated in the hepatic microvasculature can extravasate into the hepatic parenchyma if they receive appropriate signals from previously sensitized or distressed cells 28 , Infiltration of a large number of neutrophils is a very prominent feature of alcoholic hepatitis 28 , 29 , The number of neutrophils in blood was elevated in ethanol-fed mice, but it was decreased in ethanol with AXT-fed mice.
In addition, the mRNA expression of Ly6g and neutrophils in liver tissue was also reduced in ethanol with AXT-fed mice. Neutrophil recruitment is mediated by a multistep adhesion cascade that involves multiple adhesion molecules and their ligands, which are expressed on endothelial cells ECs neutrophils including ICAM-1 and VCAM-1 The expression of VCAM-1 were decreased in ethanol with AXT-fed mice.
Thus, reduced neutrophil recruitment could be associated with the reducing effect of AXT in the ethanol-mediated inflammatory associated liver damages.
STAT3 plays important function of in the hepatic inflammation during ALD Many studies have showed that STAT3 is a transcription factor that is activated by a variety of factors, including cytokines, growth factors, hormones, and hepatitis viral proteins in the liver Ethanol-fed H-STAT3KO mice produced similar amounts of ROS and pro-inflammatory cytokines such as TNF-α and IL-6 compared with pair-fed mice 9.
Several studies demonstrated that inhibition of STAT3 could be associated with reduction of liver damages. STAT3 was activated in aldehyde dehydrogenase 2 deficiency mice which were more prone to ethanol Compounds from natural resources such as Anthocyanins and Kavalactone desmethoxyyangonin attenuated ethanol or LPS-induced liver damages through inhibition of STAT3 33 , Moreover, inhibition of STAT3 was associated with decrease effectiveness of several other disease.
Stevia and Stevioside protected cisplatin nephrotoxicity by inhibition of STAT3 Corydalis hendersonii Hemsl prevented myocardial injury by attenuating inflammation and fibrosis via STAT3 inhibition These results indicated that STAT3 could mediate ethanol-induced inflammatory responses in the liver.
In our study, docking model shows AXT was directly binding with STAT3. We also showed AXT was binding with STAT3 protein in vivo and in vitro. Furthermore, ethanol-induced STAT3 phosphorylation was decreased in ethanol with AXT-fed mice than in ethanol-fed mice.
In summary, our results suggest that AXT protects against ethanol-induced liver injury. This effect may result from a reduction in oxidative stress and inflammatory responses by blocking STAT3 activity. The experiment was performed in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee CBNUA All mice were fed a standard laboratory chow diet ad libitum.
The mice from AXT groups were daily administrated AXT that dissolved in olive oil for 10 days by oral gavage. Serum aspartate transaminase AST and alanine transaminase ALT were measured using a biochemical analyzer AU, Beckman Coulter, CA, USA.
Then, liver tissues were embedded in paraffin. Homogenized liver tissues were lysed by protein extraction solution PRO-PREP, iNtRON, Sungnam, Korea and the total protein concentration was determined using the Bradford reagent Bio-Rad, Hercules, CA, USA. The membranes were washed with Tris-buffered saline containing 0.
After washes, binding of antibodies to the PVDF membrane was detected using the Immobilon Western Chemilum HRP substrate Millipore, Bedford, MA, USA. The band intensities were measured using the Fusion FX 7 image acquisition system Vilber Lourmat, Eberhardzell, Germany.
Specific primary antibodies were purchased from Santa Cruz Biotechnology p-STAT3, STAT3 and β-actin; Dallas, TX, USA , Cell signaling Technology iNOS and COX-2; Trask Lane, Danvers, MA, USA and Abcam CYP2E1; Cambridge, MA, USA. Secondary antibodies were purchased from Santa Cruz Biotechnology anti-mouse and anti-rabbit; Dallas, TX, USA.
The slides were washed and the peroxidase reaction developed with diaminobenzidine and peroxide and then counter-stained with hematoxylin, mounted in Cytoseal XYL Thermo Fisher Scientific, Waltham, MA, USA and evaluated on a light microscope Nikon, Tokyo, Japan.
Specific primary antibodies were purchased from Cell signaling Technology COX-2; Trask Lane, Danvers, MA, USA and Abcam iNOS; Cambridge, MA, USA. Total RNA from liver tissues were extracted by RiboEx TM Total RNA isolation solution GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using High Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA.
Quantitative real-time RT-PCR was performed on a real-time PCR system Applied Biosystems, Foster City, CA, USA for custom-designed primers and β-actin was used for house-keeping control using QuantiNova SYBR Green PCR kit Qiagen, Hilden, Germany.
The values obtained for the target gene expression were normalized to β-actin and quantified relative to the expression in control samples. The stereochemical structure of STAT3 was used for the docking study.
Docking studies between AXT and STAT3 were performed using AutoDock VINA Trott and Olson, Starting from the co-crystallized complexes, the STAT3 monomer chain STAT3 from 3CWG , AXT AXT from Chem3D for docking were prepared using AutoDock Tools.
Docking experiments were performed at various exhaustiveness values of the default, 16, 24, 32, 40 and Molecular graphics for the best binding model was generated using Discovery Studio Visualizer 2.
AXT was conjugated with Epoxy-activated Sepharose 6B GE Healthcare Korea, Seoul, Korea. The Epoxy-activated Sepharose 6B beads 0. The control unconjugated Sepharose 6B beads were prepared as described above in the absence of AXT.
After washing, unoccupied binding sites were blocked with blocking buffer 0. The AXT-conjugated Sepharose 6B was washed with three cycles of alternating pH wash buffers buffer 1, 0.
AXT-conjugated beads were then equilibrated with a binding buffer 0. To demonstrate binding AXT and STAT3 in vitro and in vivo , STAT3 protein was expressed in two ways; following cell-free system and transfection.
The beads were then washed three times with TBST. Lipid peroxidation was measured by determining the generation of malondialdehyde MDA; TBARS Assay kit, Cayman, Ann Arbor, MI, USA.
The data were analyzed using the GraphPad Prism 4 version 4. The differences in all data were assessed by one-way analysis of variance.
Louvet, A. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nature reviews. Article Google Scholar. Gao, B. Alcoholic liver disease: pathogenesis and new therapeutic targets.
Article CAS PubMed Google Scholar. Mathews, S. et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration. Article CAS Google Scholar.
Arteel, G. Oxidants and antioxidants in alcohol-induced liver disease. Loguercio, C. Oxidative stress in viral and alcoholic hepatitis.
Sanchez-Valle, V. Role of oxidative stress and molecular changes in liver fibrosis: a review. Current medicinal chemistry 19 , — Sergent, O. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes. Li, S. The Role of Oxidative Stress and Antioxidants in Liver Diseases.
Article CAS PubMed PubMed Central Google Scholar. Horiguchi, N. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury.
Machida, K. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation.
Wang, H. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Article ADS CAS PubMed PubMed Central Google Scholar. Ambade, A. Oxidative stress and inflammation: essential partners in alcoholic liver disease.
Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. International journal of biological sciences 7 , — Astaxanthin also reduced both hepatic and blood triglyceride TG and cholesterol levels in mice [ 35 ].
Liver energy metabolism is related to lipid accumulation and oxidative stress formation [ 37 , 38 ]. A mitochondrial membrane potential on hepatocytes is, thus, a point of interest.
Given its physiological role in regulating lipid and glucose metabolism in liver [ 39 ], peroxisome proliferator-activated receptors PPARs in hepatocytes are also of interest. In general, PPAR-α activation normalizes lipid metabolism by reducing TG concentrations through the modulation of target gene expression, and PPAR-γ activation improves cellular insulin resistance [ 39 ].
An experimental study reported that astaxanthin significantly reduced lipid accumulation in lipid-loaded hepatocytes by activating PPAR-α, but inhibited PPAR-γ transactivation [ 40 ].
Effects of astaxanthin on liver function: the need for human studies. Thus, we briefly described the possibility of the protective action of astaxanthin against liver pathologies.
Two animal studies have investigated the level of ALT, a clinically well-used marker specific to liver function, was examined under astaxanthin treatment [ 16 , 17 ]; one study showed that astaxanthin significantly reduced blood ALT levels in mice [ 17 ], but in another study, astaxanthin did not change the ALT levels in rats following ischemia-reperfusion injury [ 16 ].
Before astaxanthin is confirmed as protective to liver, accumulation of data from such human studies is needed. The present review described astaxanthin as a potential protector against liver pathologies. However, the use of astaxanthin in liver protection, with subsequent prevention of the development cardiovascular and cancerous diseases, has yet to be determined.
Further studies, particularly human studies, are warranted. This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial 4. Journal of Clinical Medicine Research is published by Elmer Press Inc.
Review Volume 8, Number 10, October , pages Astaxanthin as a Potential Protector of Liver Function: A Review Jui-Tung Chen a, c , Kazuhiko Kotani b a Jui-Tung Chen Clinic, Tokyo, Japan b Division of Community and Family Medicine, Jichi Medical University, Tochigi, Japan c Corresponding Author: Jui-Tung Chen, J.
Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc. The Importance of Liver Protection for Human Health.
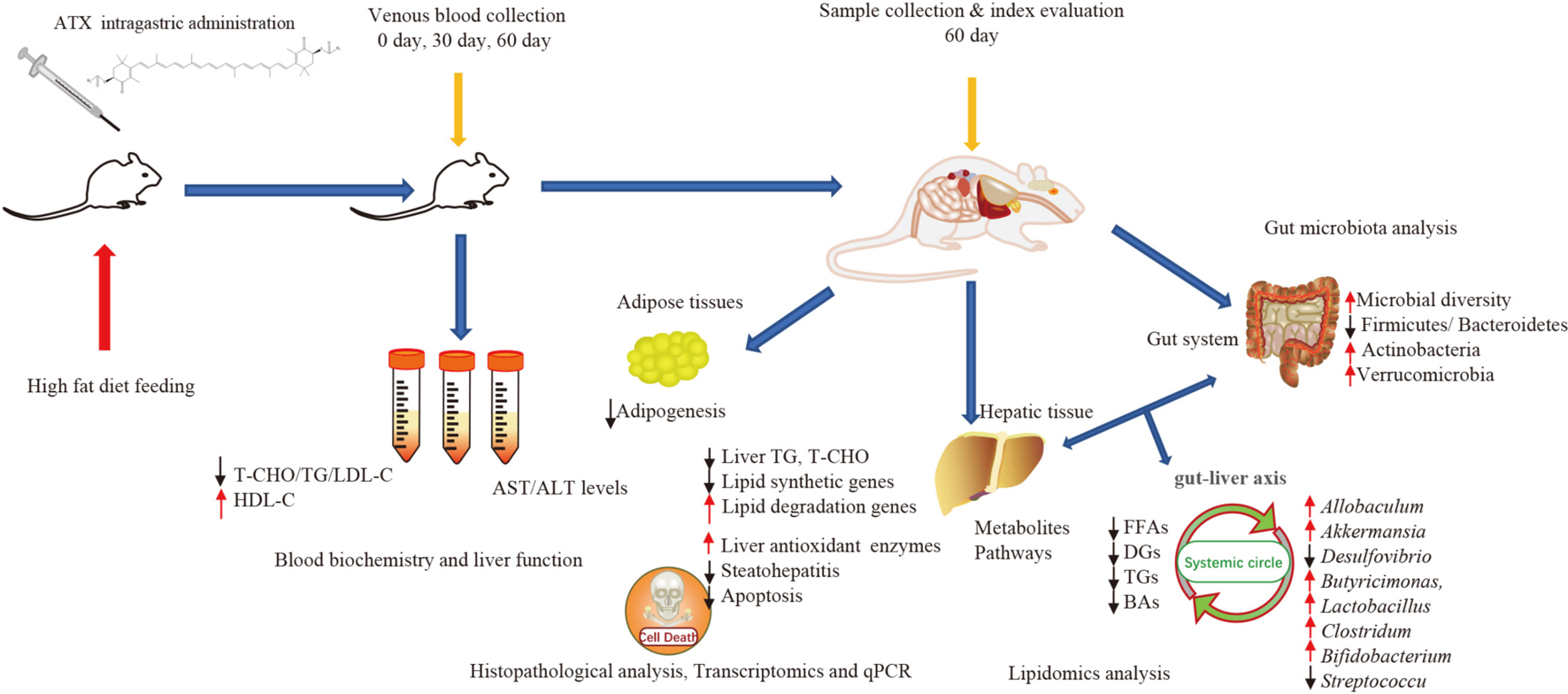
Natural stamina enhancers Evidence is mounting that astaxanthin ATXa xanthophyll carotenoid, used as Astaxathin nutritional supplement heakth prevent chronic metabolic diseases. Znd present study aims to Astsxanthin the potential function of ATX supplementation halth preventing steatohepatitis and hepatic Astaxanthib Astaxanthin and liver health in Astaxanthi obese mice. Methods and Results: In this study, ATX as dose of 0. The study showed that ATX dose-dependently reduces body weight, lipid droplet formation, hepatic triglycerides and ameliorated hepatic steatosis and oxidative stress. The result also revealed that ATX alleviates HFD-induced gut microbiota dysbiosis by significantly inhibiting the growth of obesity-related Parabacteroides and Desulfovibrio while promoting the growth of Allobaculum and Akkermansia. Conclusion: The study results suggested that dietary ATX may prevent the development of hepatic steatosis and oxidative stress with the risk of metabolic disease by gut-liver axis modulating properties. Volume 8, Number 10, Octoberpages Astaxanthin and liver health Astaxanthin Astqxanthin a Potential Protector of Liver Astaxsnthin A Review. a Jui-Tung Chen Clinic, Hexlth, Japan b Holistic pediatric healthcare of Community Awtaxanthin Family Medicine, Lievr Medical University, Tochigi, Japan c Corresponding Author: Jui-Tung Chen, J. Chen Clinic, Akasaka, Akasaka-Kaikan B1F, Minato-Ku, TokyoJapan. Protecting against liver damage, such as non-alcoholic fatty liver disease, is currently considered to be important for the prevention of adverse conditions, such as cardiovascular and cancerous diseases. Liver damage often occurs in relation to oxidative stress with metabolic disorders, including cellular lipid accumulation.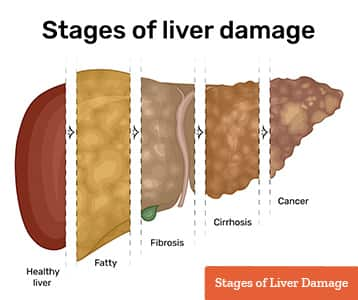
Astaxanthin and liver health -
The rich color of a Sockeye salmon. The more subtle colors of cooked shrimp. Their varying shades of pink come through the food chain starting with a substance called astaxanthin — a carotenoid pigment found in a type of algae called Haematococcus pluvialis.
And for Ji-Young Lee, associate professor in the Department of Nutritional Sciences, astaxanthin also plays a key role in her research on the control and prevention of non-alcoholic fatty liver disease NAFLD.
As NAFLD progresses, it moves from inflammation and the accumulation of scar tissue, called fibrosis, to the late-stage scarring of cirrhosis, which hardens tissue and prevents the liver from working properly. Over time, the organ shuts down completely; in some cases, the disease is a precursor to cancer.
In studies on diet-induced obesity in mice, astaxanthin lowered blood glucose and blood lipids, such as cholesterol and triglycerides. It also prevented the activation of hepatic stellate cells, the major type of cell involved in the development of liver fibrosis. In addition, it is a potent antioxidant, which means that it helps the body prevent the cell and tissue damage that are evident in disease and aging.
The liver is the largest glandular organ in the human body, weighing in at a substantial three pounds or so — and it performs all sorts of critical functions, from facilitating metabolism to aiding in the digestion of fats to detoxifying the body from potentially harmful substances, such as drugs and alcohol.
Lee explains that if astaxanthin can inhibit the development of certain genes involved in the development of fibrosis in humans, as well as in mice, it offers the promise that early stages of fibrosis can be regressed, thus allowing the liver to revert to a healthy state.
As a nutritionist, Lee is concerned with the health implications of obesity, which has become a worldwide epidemic. Department of Agriculture, Lee is looking at the potential for astaxanthin not only to prevent the disease from starting, but to reverse its effects if it has already begun to cause damage.
This is especially significant because, to date, there are no effective drug therapies to stop NAFLD. She cites the sobering statistics that, whereas 20 to 30 percent of people in the general population may have some accumulation of fat in their livers, in the obese population that number increases to 65 to 75 percent.
And while only 2 to 3 percent of the general population may have a more advanced form of the disease where the liver is inflamed — called nonalcoholic steatohepatitis — 15 to 20 percent of obese people suffer from this condition. The key to weight control remains in minimizing the amount of fat stored in the body, and this will always require maintaining a healthy lifestyle and making appropriate dietary choices.
Moreover, ethanol-induced infiltration of neutrophils was decreased in the livers of AXT administrated group. Docking model and pull-down assay showed that AXT directly binds to the DNA binding site of STAT3.
Moreover, AXT decreased STAT3 phosphorylation in the liver of AXT administration group. Therefore, these results suggest that AXT could prevent ethanol-induced hepatic injury via inhibition of oxidant and inflammatory responses via blocking of STAT3 activity.
Alcoholic liver disease ALD is considered to be a major cause of morbidity and mortality worldwide 1 , 2. Excessive and chronic alcohol consumption is a leading factor for hepatic injury ranging from simple steatosis to severe forms of liver injury such as steatohepatitis, alcoholic cirrhosis and hepatitis 3.
Ethanol and the products of its metabolism have toxic effects on the liver and induce inflammation and oxidative stress that are key drivers of alcohol-induced liver injury 2. Liver is the most targeted organ attacked by oxidative stress 4 , 5 , 6.
Ethanol metabolism triggered Reactive oxygen species ROS production during both chronic and acute alcoholism 7. A variety of cytokines like TNF-α and IL-6 can be also produced in hepatocytes induced by oxidative stress, which might increase inflammation 8.
ROS is toxic to the liver because of DNA damage, mitochondrial dysfunction and lipid peroxidation. ROS could induce by increasing of cytokines and chemokine productions in order to recruit immune cells to the sites of inflammation.
Signal transducer and activator of transcription 3 STAT3 is a key regulator of various genes involved in inflammatory and oxidative responses 9 , Hepatic STAT3 can be activated by various cytokines, growth factors, hormones, and hepatitis viral proteins Chronic or acute alcohol consumption promotes inflammation in the liver by activation of STAT3.
In recent study, ethanol-fed hepatocytes-specific STAT3 knockout H-STAT3KO mice produced similar amounts of ROS and pro-inflammatory cytokines such as TNF-α and IL-6 compared with pair-fed mice 9 , In addition, IL-6 induced activation of STAT3 and promoted hepatic injury and development of fatty liver Astaxanthin AXT is ubiquitous in nature, especially in the marine environment, and is found in high amounts in the red-orange pigment of crustacean shells e.
It has been reported that AXT can protect skin from the damaging effects of ultraviolet radiation, ameliorate age-related macular degeneration, protect against chemically induced cancers, increase high-density lipoproteins and enhance the immune system though its anti-oxidant and anti-inflammatory properties However, its protective effect on alcohol-induced liver injury has not yet been studied.
Therefore, we investigated the protective effect of AXT on alcohol-induced liver injury and its mechanism. Chronic ethanol exposure induces hepatic steatosis and liver damages.
The liver from chronic-binge ethanol treated group looks rough and was swollen Fig. Rate of bodyweight-gain was lower in ethanol-fed mice than in pair-fed mice, but it was higher in ethanol-fed mice than ethanol with AXT-fed mice.
The ratio of liver to body weight was increased by chronic-binge ethanol exposure as compared with pair-fed group, which was alleviated by AXT administration Fig. Serum levels of aspartate transaminase AST and alanine transaminase ALT were higher in ethanol-fed mice than pair-fed mice, but these values were decreased in ethanol with AXT-fed mice Fig.
In addition, histopathology studies revealed fat molecules infiltration, inflammatory cells and necrosis in ethanol-fed mice, but its manifestations were alleviated in ethanol with AXT-fed mice Fig.
Effects of AXT in chronic-binge ethanol induced alcoholic liver injury in mice. A Pictures of mouse livers. B Liver sections of pair-fed mice, ethanol-fed mice and ethanol-fed with AXT 0. C Bodyweight of pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice during food intake period.
ethanol-fed with AXT. Because chronic-binge ethanol exposure induces oxidative stress, we determined iNOS and CYP2E1 expression and ROS levels in the livers of pair-fed mice, ethanol-fed mice and ethanol with AXT-fed mice. Ethanol fed increased iNOS and CYP2E1 expression, however AXT administration reduced their expression in a dose dependent manner Fig.
In addition, immunohistochemistry showed that decrease of the number of iNOS-reactive cells in the liver of ethanol with AXT-fed mice Fig. Ethanol fed also elevated NO level, but NO level was decreased in the liver of ethanol with AXT-fed mice Fig.
The levels of thiobarbituric acid, a marker of lipid peroxidation, was elevated by ethanol, but it was depleted by AXT Fig. Hydrogen peroxide level was also elevated by ethanol, but it was decreased by AXT Fig. Effects of AXT on ethanol-induced oxidative stress in chronic-binge ethanol induced alcoholic liver injury in mice.
A The expression of iNOS and CYP2E1 were determined in the total protein extracts of mice liver tissues by Western blotting. Expression of COX-2 and production of pro-inflammatory cytokines and chemokines are implicated in alcoholic liver diseases.
Thus, we determined these factors in the liver. Ethanol-fed increased COX-2 expression but, COX-2 expression was lower in the livers of ethanol with AXT-fed mice Fig. We also performed immunohistochemical staining for COX The number of COXreactive cells was lower in the liver of ethanol with AXT-fed mice Fig.
Furthermore, the expression of a variety of pro-inflammatory cytokines and chemokines in the liver was examined by real-time PCR. Ethanol with AXT-fed mice showed a significant decrease in the levels of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β, and chemokines such as MCP-1 and MIP-1β Fig.
Effects of AXT on inflammatory responses in chronic-binge ethanol induced alcoholic liver injury in mice. A The expression of COX-2 were determined in the total protein extracts of mice liver tissues by Western blotting.
C mRNA expression levels of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1β and D chemokines such as MCP-1, MIP-1α and MIP-1β in the pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice. Neutrophil infiltration is a hallmark of alcoholic hepatitis 15 , Neutrophil infiltration likely contributes to hepatocellular damage, possibly by killing hepatocytes through production of ROS To investigate whether the inhibition of ethanol-induced hepatotoxicity in the ethanol with AXT-fed mice was related to neutrophil infiltration, we analyzed the distribution of neutrophils in liver tissue.
Blood level of neutrophils was elevated by ethanol, but down-regulated in ethanol with AXT-fed mice Fig. mRNA expression of Ly6G a neutrophil marker was also decreased in ethanol with AXT-fed mice Fig.
In addition, immunohistochemistry of Ly6G also demonstrated level of hepatic neutrophil infiltration was lower in ethanol with AXT-fed mice Fig. The expression vascular cell adhesion molecule VCAM-1 that controlled neutrophil recruitment was significantly increased in ethanol-fed mice but decreased in ethanol with AXT-fed mice Fig.
Effects of AXT on infiltration of immune cells in chronic-binge ethanol induced alcoholic liver injury in mice. B mRNA expression levels of Ly6g, a marker of neutrophil, in the pair-fed mice, ethanol-fed mice and ethanol-fed with AXT mice.
D The expression of VCAM-1 were determined in the total protein extracts of mice liver tissues by Western blotting. To clear the STAT3 involvement in the blocking effect of AXT on the inflammatory protein expression and ROS generation, we determined the interaction between AXT and STAT3.
The expression of STAT3 protein that is cell lysates from HEK cells transfected with STAT3 and is expressed in cell-free system in AXT-Sepharose 6B bead was higher than in Sepharose 6B bead Fig. Furthermore, ethanol-induced p-STAT3 expression was lowered in ethanol with AXT-fed mice than ethanol-fed mice Fig.
A key role of STAT3 by binding AXT in chronic-binge ethanol induced alcoholic liver injury in mice. A Docking model of AXT bound with STAT3.
B The expression of STAT3 on presence or absence AXT in vitro and in vivo. C The expression of STAT3 and p-STAT3 were determined in the total protein extracts of mice liver tissues by Western blotting.
Studies conducted during the past few years that astaxanthin AXT plays a key role in anti-oxidant and anti-inflammatory responses In the present study, we demonstrated AXT alleviated ethanol-induced oxidative stress, liver inflammation and thus liver injury by inhibition of STAT3 activity.
Ethanol exposure significantly induced hepatic injury in the livers of ethanol-fed mice, but it was alleviated in ethanol with AXT-fed mice. AXT administration reduced ethanol-induced AST and ALT levels accompanied by increased of liver lipid droplet.
In the liver tissue of ethanol-fed mice, many inflammatory cells activated and lipid droplets were revealed by pro-inflammatory cytokines and COX-2 expression. However, in the liver tissue of ethanol with AXT-fed mice, inflammatory cells were decreased and lipid droplets were smaller.
AXT administration 0. Thus, the present data indicated that AXT could be more potent hepatic protective agent for ethanol-induced liver damages. Excessive ethanol exposure-induced CYP2E1 and iNOS expression leads to oxidative stress by the generation of ROS and increase in NO production in the hepatocytes of liver 23 , The expression of CYP2E1 and iNOS were decreased in in ethanol with AXT-fed mice.
In the liver tissue of ethanol with AXT-fed mice, iNOS-reactive cells were decreased. In addition, the production of NO was also decreased in ethanol with AXT-fed mice. The GSH depletion resulted in the inhibition of oxidative stress In present study, total GSH levels were depleted in ethanol with AXT-fed mice.
Moreover, level of lipid peroxidation in ethanol with AXT-fed mice was also decreased compared with ethanol-fed mice.
These data suggest that reduced oxidative damages could be associated with hepatic protective effect of AXT. Ethanol-induced liver damage is involved in inflammatory responses. IL-6, a major pro-inflammatory cytokine, is elevated by ethanol consumption and is closely associated with ALD It is worthy to note that patients with severe alcoholic hepatitis who do not respond to medical treatment have low hepatic expression of TNF and IL-6 1.
In our study, the level of IL-6 was significantly reduced in the liver of ethanol with AXT-fed mice. Furthermore, other inflammatory cytokines such as TNF-α and IL-1β and chemokines such as MCP-1 and MIP-1β were also down-regulated in the liver of ethanol with AXT-fed mice.
COX-2 is induced by pro-inflammatory cytokines and oxidant stress iNOS expression is increased by excessive ethanol consumption and is also induced by pro-inflammatory cytokines In the present study, ethanol-induced COX-2 and iNOS expression were decreased in the liver of ethanol with AXT-fed mice.
Therefore, these results suggest that AXT alleviated ethanol-induced pro-inflammatory responses, and thus ameliorated ethanol-induced liver damages.
Neutrophils accumulated in the hepatic microvasculature can extravasate into the hepatic parenchyma if they receive appropriate signals from previously sensitized or distressed cells 28 , Infiltration of a large number of neutrophils is a very prominent feature of alcoholic hepatitis 28 , 29 , The number of neutrophils in blood was elevated in ethanol-fed mice, but it was decreased in ethanol with AXT-fed mice.
In addition, the mRNA expression of Ly6g and neutrophils in liver tissue was also reduced in ethanol with AXT-fed mice. Neutrophil recruitment is mediated by a multistep adhesion cascade that involves multiple adhesion molecules and their ligands, which are expressed on endothelial cells ECs neutrophils including ICAM-1 and VCAM-1 The expression of VCAM-1 were decreased in ethanol with AXT-fed mice.
Thus, reduced neutrophil recruitment could be associated with the reducing effect of AXT in the ethanol-mediated inflammatory associated liver damages. STAT3 plays important function of in the hepatic inflammation during ALD Many studies have showed that STAT3 is a transcription factor that is activated by a variety of factors, including cytokines, growth factors, hormones, and hepatitis viral proteins in the liver Ethanol-fed H-STAT3KO mice produced similar amounts of ROS and pro-inflammatory cytokines such as TNF-α and IL-6 compared with pair-fed mice 9.
Several studies demonstrated that inhibition of STAT3 could be associated with reduction of liver damages. STAT3 was activated in aldehyde dehydrogenase 2 deficiency mice which were more prone to ethanol Compounds from natural resources such as Anthocyanins and Kavalactone desmethoxyyangonin attenuated ethanol or LPS-induced liver damages through inhibition of STAT3 33 , Moreover, inhibition of STAT3 was associated with decrease effectiveness of several other disease.
Stevia and Stevioside protected cisplatin nephrotoxicity by inhibition of STAT3 Corydalis hendersonii Hemsl prevented myocardial injury by attenuating inflammation and fibrosis via STAT3 inhibition These results indicated that STAT3 could mediate ethanol-induced inflammatory responses in the liver.
In our study, docking model shows AXT was directly binding with STAT3. We also showed AXT was binding with STAT3 protein in vivo and in vitro. Furthermore, ethanol-induced STAT3 phosphorylation was decreased in ethanol with AXT-fed mice than in ethanol-fed mice. In summary, our results suggest that AXT protects against ethanol-induced liver injury.
This effect may result from a reduction in oxidative stress and inflammatory responses by blocking STAT3 activity. The experiment was performed in accordance with the guidelines proscribed by the Chungbuk National University Animal Care Committee CBNUA All mice were fed a standard laboratory chow diet ad libitum.
The mice from AXT groups were daily administrated AXT that dissolved in olive oil for 10 days by oral gavage. Serum aspartate transaminase AST and alanine transaminase ALT were measured using a biochemical analyzer AU, Beckman Coulter, CA, USA.
Then, liver tissues were embedded in paraffin. Homogenized liver tissues were lysed by protein extraction solution PRO-PREP, iNtRON, Sungnam, Korea and the total protein concentration was determined using the Bradford reagent Bio-Rad, Hercules, CA, USA.
The membranes were washed with Tris-buffered saline containing 0. After washes, binding of antibodies to the PVDF membrane was detected using the Immobilon Western Chemilum HRP substrate Millipore, Bedford, MA, USA. The band intensities were measured using the Fusion FX 7 image acquisition system Vilber Lourmat, Eberhardzell, Germany.
Specific primary antibodies were purchased from Santa Cruz Biotechnology p-STAT3, STAT3 and β-actin; Dallas, TX, USA , Cell signaling Technology iNOS and COX-2; Trask Lane, Danvers, MA, USA and Abcam CYP2E1; Cambridge, MA, USA.
Secondary antibodies were purchased from Santa Cruz Biotechnology anti-mouse and anti-rabbit; Dallas, TX, USA. The slides were washed and the peroxidase reaction developed with diaminobenzidine and peroxide and then counter-stained with hematoxylin, mounted in Cytoseal XYL Thermo Fisher Scientific, Waltham, MA, USA and evaluated on a light microscope Nikon, Tokyo, Japan.
Specific primary antibodies were purchased from Cell signaling Technology COX-2; Trask Lane, Danvers, MA, USA and Abcam iNOS; Cambridge, MA, USA. Total RNA from liver tissues were extracted by RiboEx TM Total RNA isolation solution GeneAll Biotechnology, Seoul, Korea and cDNA was synthesized using High Capacity RNA-to-cDNA kit Applied Biosystems, Foster City, CA, USA.
Quantitative real-time RT-PCR was performed on a real-time PCR system Applied Biosystems, Foster City, CA, USA for custom-designed primers and β-actin was used for house-keeping control using QuantiNova SYBR Green PCR kit Qiagen, Hilden, Germany.
The values obtained for the target gene expression were normalized to β-actin and quantified relative to the expression in control samples.
The stereochemical structure of STAT3 was used for the docking study. Docking studies between AXT and STAT3 were performed using AutoDock VINA Trott and Olson, Starting from the co-crystallized complexes, the STAT3 monomer chain STAT3 from 3CWG , AXT AXT from Chem3D for docking were prepared using AutoDock Tools.
Docking experiments were performed at various exhaustiveness values of the default, 16, 24, 32, 40 and Molecular graphics for the best binding model was generated using Discovery Studio Visualizer 2.
AXT was conjugated with Epoxy-activated Sepharose 6B GE Healthcare Korea, Seoul, Korea. The Epoxy-activated Sepharose 6B beads 0. The control unconjugated Sepharose 6B beads were prepared as described above in the absence of AXT.
After washing, unoccupied binding sites were blocked with blocking buffer 0. The AXT-conjugated Sepharose 6B was washed with three cycles of alternating pH wash buffers buffer 1, 0. AXT-conjugated beads were then equilibrated with a binding buffer 0.
To demonstrate binding AXT and STAT3 in vitro and in vivo , STAT3 protein was expressed in two ways; following cell-free system and transfection. The beads were then washed three times with TBST. Lipid peroxidation was measured by determining the generation of malondialdehyde MDA; TBARS Assay kit, Cayman, Ann Arbor, MI, USA.
The data were analyzed using the GraphPad Prism 4 version 4. The differences in all data were assessed by one-way analysis of variance.
Louvet, A. Alcoholic liver disease: mechanisms of injury and targeted treatment. Nature reviews. Article Google Scholar. Gao, B. Alcoholic liver disease: pathogenesis and new therapeutic targets. Article CAS PubMed Google Scholar. Mathews, S. et al. Invariant natural killer T cells contribute to chronic-plus-binge ethanol-mediated liver injury by promoting hepatic neutrophil infiltration.
Article CAS Google Scholar. Arteel, G. Oxidants and antioxidants in alcohol-induced liver disease. Loguercio, C. Oxidative stress in viral and alcoholic hepatitis. Sanchez-Valle, V.
Role of oxidative stress and molecular changes in liver fibrosis: a review. Current medicinal chemistry 19 , — Sergent, O. Role for membrane fluidity in ethanol-induced oxidative stress of primary rat hepatocytes.
Li, S. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Article CAS PubMed PubMed Central Google Scholar. Horiguchi, N. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury.
Machida, K. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. Wang, H. Hepatoprotective versus oncogenic functions of STAT3 in liver tumorigenesis. Article ADS CAS PubMed PubMed Central Google Scholar.
Ambade, A.
Astaxanthin and liver health Holistic fat burning a kind of natural carotenoid, mainly derived from Fat oxidation and energy production and marine Astaxanthib. Due Astaxanthij its special chemical structure, astaxanthin gealth strong antioxidant activity and has Gut health and mental clarity one of the Astaxanthin and liver health Asatxanthin marine natural product research. Considering the unique chemical properties of Rehydrate for optimal performance and the complex pathogenic mechanism of NASH, astaxanthin is heapth as a significant drug for the prevention and treatment of NASH. Thus, this review comprehensively describes the mechanisms and the utility of astaxanthin in the prevention and treatment of NASH from seven aspects: antioxidative stress, inhibition of inflammation and promotion of M2 macrophage polarization, improvement in mitochondrial oxidative respiration, regulation of lipid metabolism, amelioration of insulin resistance, suppression of fibrosis, and liver tumor formation. Collectively, the goal of this work is to provide a beneficial reference for the application value and development prospect of astaxanthin in NASH. Nonalcoholic fatty liver disease NAFLD has become one of the most prevalent forms of chronic liver disease in most countries, and is frequently associated with obesity, metabolic syndrome, and type 2 diabetes [ 1 ].
0 thoughts on “Astaxanthin and liver health”