BCAA and muscle metabolism -
It is crucial to ensure that these three amino acids are present in sufficient amounts in the diet, as their inadequate supply can impair muscle synthesis , even if high levels of BCAAs are supplemented.
Learn More. Horses have different dietary protein requirements , depending on their stage of life, exercise level and other factors. A moderate-quality grass hay may provide enough protein for mature horses in light work, but growing horses or horses in heavy exercise may require additional protein from a legume hay , such as alfalfa.
Furthermore, higher levels of protein intake are not more efficient at stimulating muscle-building pathways compared to a meal with moderate protein intake.
A high-quality protein refers to a protein source that exhibits good digestibility and contains all the essential amino acids in the appropriate balance. Research in horses shows that providing a higher-quality protein source with an enhanced amino acid profile leads to increased activation of proteins involved in muscle-building pathways compared to feeding a protein source derived solely from forage.
Feeding horses multiple meals throughout the day, as opposed to one or two large meals, has been found to enhance digestibility. Furthermore, research suggests that smaller and more frequent meals can increase the level of circulating amino acids in the bloodstream, comparable to that of a larger protein meal.
This increase in amino acids is vital for activating the pathways necessary for muscle protein synthesis. Together, these findings indicate that smaller, more frequent meals not only contribute to improved digestibility but also effectively stimulate muscle-building pathways.
While BCAA supplementation is purported to enhance athletic performance in horses, the research currently available does not conclusively show benefits for muscle recovery, body composition or fatigue. Branched chain amino acids do play important physiological roles in muscle metabolism, but if your horse already gets sufficient protein in their diet, supplementing with BCAAs is not proven to have an additive benefit.
Pay closer attention to the limiting amino acids , which are more frequently found to be deficient in the equine diet. Save my name, email, and website in this browser for the next time I comment.
Get Updates from Mad Barn. Branched Chain Amino Acid Supplements for Horses: Do they Work? Written by: Linaya Pot, MSc Reviewed by: Dr. Christine Latham, Ph. Evidence Based This article has 25 scientific references.
Horse Health Nutrition Performance. BCAA Supplements Potential Benefits Research in Horses When Not to Use Muscle Support. Mad About Horses. Join Dr. Chris Mortensen, PhD on an exciting adventure into the story of the horse and learn how we can make the world a better place for all equines.
Three Amigos. Is Your Horse's Diet Missing Anything? Identify gaps in your horse's nutrition program to optimize their well-being. Analyze Diet Now. References Yoon, M.
The Emerging Role of Branched-Chain Amino Acids in Insulin Resistance and Metabolism. et al. Effects of intravenous infusion of 17 amino acids on secretion of GH, glucagon, and insulin in sheep. Am J Physiol.
The use of BCAA to decrease delayed-onset muscle sourness after a single bout of exercise: a systematic review and meta-analysis. Amino Acids. Doma, K. The effect of branched-chain amino acid on muscle damage markers and performance following strenuous exercise: a systematic review and meta-analysis.
Appl Physiol Nutr Metab. Fourte, A. and Bendahan, D. Is branched-chain amino acid supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review.
Hormoznejad, R. Effect of BCAA supplementation on central fatigue, energy metabolism substrate and muscle damage to the exercise: a systematic review with meta-analysis. Sport Sci Health. Falavinga, G. Effects of Diets Supplemented with Branched-Chain Amino Acids on the Performance and Fatigue Mechanisms of Rats Submitted to Prolonged Physical Exercise.
and Watanabe, Y. Lactate is Not a Cuase of Fatigue. Fatigue Science for Human Health. Pourgharib, S. Peripheral fatigue and hormone responses to branched-chain amino acids ingestion and exercise in recovery: a systematic review and meta-analysis.
Minerva Endocrinol. Dudgeon, W. In a single-blind, matched group design: branched-chain amino acid supplementation and resistance training maintains lean body mass during a caloric-restricted diet.
J Int Soc Sports Nutr. Ooi, D. Branched-Chain Amino Acid Supplementation Does Not Preserve Lean Mass or Affect Metabolic Profile in Adults with Overweight or Obesity in a Randomized Controlled Weight Loss Intervention.
J Nutr. and Zanella, P. The effect of branched-chain amino acids supplementation in physical exercise: A systematic review of human randomized controlled trials. Sci Sports. Dynamic Change of Serum Levels of Some Branched-Chain Amino Acids and Tryptophan in Athletic Horses After Different Physical Exercises.
J Equine Vet Sci. Assenza, A. Blood serum branched chain amino acids and tryptophan modifications in horses competing in long-distance rides of different length. J Anim Physiol Anim Nutr Berl. Stefanon, B. and Guggia, P. Administration of Branched-Chain Amino Acids to Standarbred Horses in Training.
Comparison of an Antioxidant Source and Antioxidant Plus BCAA on Athletic Performance and Post Exercise Recovery of Horses. Casini, L. Effect of prolonged branched-chain amino acid supplementation on metabolic response to anaerobic exercise in standardbreds.
Baakhtari, M. Effects of branched-chain amino acids on immune status of young racing horses. J Vet Med Sci. Leenders, M. and van Loon, L.
Leucine as a pharmaconutrient to prevent and treat sarcopenia and type 2 diabetes. Nutr Rev. Urshcel, K. Effects of leucine or whey protein addition to an oral glucose solution on serum insulin, plasma glucose and plasma amino acid responses in horses at rest and following exercise.
Equine Vet J Sippl. Graham-Theirs, P. and Bowen, L. Relationships between feed protein fractions and the hindgut microbiome in the exercising horse.
Loos, C. Pathways regulating equine skeletal muscle protein synthesis respond in a dose-dependent manner to graded levels of protein intake. J Anim Sci. Differential effect of two dietary protein sources on time course response of muscle anabolic signaling pathways in normal and insulin dysregulated horses.
Front Vet Sci. Direkvandi, E. The Positive Impact of Increasing Feeding Frequency on Feed Intake, Nutrient Digestibility, and Blood Metabolites of Turkmen Horses. Mastellar, S. L et al. Effects of meal frequency on plasma amino acid concentrations in horses of various body condition scores.
About Linaya Pot, MSc. Linaya was born on a small, family-run dairy farm in Southwestern Ontario. This fostered a passion for animal husbandry and care, so she attended the University of Guelph where she received her B.
Sc in Animal Biology and a M. Sc in Dairy Nutrition. While studying at university, she continued to develop her equestrian abilities by joining the University of Guelph Equestrian Club.
After graduating from school, she worked in the agriculture industry as a consulting nutritionist. While most of her nutrition work involved cattle, she also formulated diets for sheep, goats, deer and hamsters. Skelet Muscle. Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, et al.
Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun. Sanchez AM, Candau RB, Bernardi H.
FoxO transcription factors: their roles in the maintenance of skeletal muscle homeostasis. Cell Mol Life Sci. FoxO transcription factors are critical regulators of diabetes-related muscle atrophy.
Article PubMed CAS Google Scholar. Abdulla H, Smith K, Atherton PJ, Idris I. Role of insulin in the regulation of human skeletal muscle protein synthesis and breakdown: a systematic review and meta-analysis. Wang X, Hu Z, Hu J, Du J, Mitch WE. Insulin resistance accelerates muscle protein degradation: activation of the ubiquitin-proteasome pathway by defects in muscle cell signaling.
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, et al. Mol Cell. Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy.
Lee SW, Dai G, Hu Z, Wang X, Du J, Mitch WE. Regulation of muscle protein degradation: coordinated control of apoptotic and ubiquitin-proteasome systems by phosphatidylinositol 3 kinase. J Am Soc Nephrol. Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, et al.
Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. Everman S, Meyer C, Tran L, Hoffman N, Carroll CC, Dedmon WL, et al. Insulin does not stimulate muscle protein synthesis during increased plasma branched-chain amino acids alone but still decreases whole body proteolysis in humans.
Article PubMed PubMed Central Google Scholar. Hoffer LJ, Taveroff A, Robitaille L, Hamadeh MJ, Mamer OA. Effects of leucine on whole body leucine, valine, and threonine metabolism in humans. Nair KS, Schwartz RG, Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans.
Pozefsky T, Felig P, Tobin JD, Soeldner JS, Cahill GF Jr. Amino acid balance across tissues of the forearm in postabsorptive man. Effects of insulin at two dose levels. Louard RJ, Fryburg DA, Gelfand RA, Barrett EJ. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle.
Adeva MM, Calvino J, Souto G, Donapetry C. Insulin resistance and the metabolism of branched-chain amino acids in humans. Castellino P, Luzi L, Simonson DC, Haymond M, DeFronzo RA. Effect of insulin and plasma amino acid concentrations on leucine metabolism in man.
Role of substrate availability on estimates of whole body protein synthesis. Zanetti M, Barazzoni R, Kiwanuka E, Tessari P. Effects of branched-chain-enriched amino acids and insulin on forearm leucine kinetics.
Clin Sci. Article CAS Google Scholar. Felig P. Amino acid metabolism in man. Annu Rev Biochem. Aoki TT, Brennan MF, Muller WA, Soeldner JS, Alpert JS, Saltz SB, et al. Amino acid levels across normal forearm muscle and splanchnic bed after a protein meal.
Am J Clin Nutr. Elia M, Livesey G. Effects of ingested steak and infused leucine on forelimb metabolism in man and the fate of the carbon skeletons and amino groups of branched-chain amino acids. Hagenfeldt L, Eriksson LS, Wahren J. Amino acids in liver disease. Proc Nutr Soc. Nair KS, Garrow JS, Ford C, Mahler RF, Halliday D.
Effect of poor diabetic control and obesity on whole body protein metabolism in man. Nair KS, Ford GC, Ekberg K, Fernqvist-Forbes E, Wahren J.
Protein dynamics in whole body and in splanchnic and leg tissues in type I diabetic patients. Vanweert F, Boone SC, Brouwers B, Mook-Kanamori DO, de Mutsert R, Rosendaal FR, et al. The effect of physical activity level and exercise training on the association between plasma branched-chain amino acids and intrahepatic lipid content in participants with obesity.
Int J Obes. Forlani G, Vannini P, Marchesini G, Zoli M, Ciavarella A, Pisi E. Insulin-dependent metabolism of branched-chain amino acids in obesity. Chevalier S, Burgess SC, Malloy CR, Gougeon R, Marliss EB, Morais JA.
The greater contribution of gluconeogenesis to glucose production in obesity is related to increased whole-body protein catabolism. Felig P, Marliss E, Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N Engl J Med. Mahendran Y, Jonsson A, Have CT, Allin KH, Witte DR, Jorgensen ME, et al.
Genetic evidence of a causal effect of insulin resistance on branched-chain amino acid levels. Wang Q, Holmes MV, Davey Smith G, Ala-Korpela M. Genetic support for a causal role of insulin resistance on circulating branched-chain amino acids and inflammation.
Lotta LA, Scott RA, Sharp SJ, Burgess S, Luan J, Tillin T, et al. Genetic predisposition to an impaired metabolism of the branched-chain amino acids and risk of type 2 diabetes: a Mendelian randomisation analysis. PLoS Med. Harper AE, Miller RH, Block KP.
Branched-chain amino acid metabolism. Annu Rev Nutr. Ridaura VK, Faith JJ, Rey FE, Cheng J, Duncan AE, Kau AL, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Jennings A, MacGregor A, Pallister T, Spector T, Cassidy A.
Associations between branched chain amino acid intake and biomarkers of adiposity and cardiometabolic health independent of genetic factors: a twin study.
Int J Cardiol. Zheng Y, Li Y, Qi Q, Hruby A, Manson JE, Willett WC, et al. Cumulative consumption of branched-chain amino acids and incidence of type 2 diabetes. Int J Epidemiol. Lopez AM, Noriega LG, Diaz M, Torres N, Tovar AR.
Plasma branched-chain and aromatic amino acid concentration after ingestion of an urban or rural diet in rural Mexican women. BMC Obes. Watson E, MacNeil LT, Ritter AD, Yilmaz LS, Rosebrock AP, Caudy AA, et al. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits.
Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BA, et al.
Human gut microbes impact host serum metabolome and insulin sensitivity. Kappel BA, Federici M. Gut microbiome and cardiometabolic risk. Rev Endocr Metab Disord. Holecek M. Branched-chain amino acids in health and disease: metabolism, alterations in blood plasma, and as supplements.
Nutr Metab. Neinast M, Murashige D, Arany Z. Branched chain amino acids. Annu Rev Physiol. Branched-chain amino acids and branched-chain keto acids in hyperammonemic states: metabolism and as supplements.
She P, Van Horn C, Reid T, Hutson SM, Cooney RN, Lynch CJ. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism.
Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, Hutson SM. A molecular model of human branched-chain amino acid metabolism.
Wynn RM, Kato M, Machius M, Chuang JL, Li J, Tomchick DR, et al. Molecular mechanism for regulation of the human mitochondrial branched-chain alpha-ketoacid dehydrogenase complex by phosphorylation. Shimomura Y, Obayashi M, Murakami T, Harris RA.
Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of activity and expression of the branched-chain alpha-keto acid dehydrogenase kinase.
Curr Opin Clin Nutr Metab Care. Joshi M, Jeoung NH, Popov KM, Harris RA. Identification of a novel PP2C-type mitochondrial phosphatase. Biochem Biophys Res Commun. Zhou M, Lu G, Gao C, Wang Y, Sun H. Tissue-specific and nutrient regulation of the branched-chain alpha-keto acid dehydrogenase phosphatase, protein phosphatase 2Cm PP2Cm.
Adams SH, Hoppel CL, Lok KH, Zhao L, Wong SW, Minkler PE, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women.
Chuang JL, Wynn RM, Chuang DT. The C-terminal hinge region of lipoic acid-bearing domain of E2b is essential for domain interaction with branched-chain alpha-keto acid dehydrogenase kinase.
Islam MM, Wallin R, Wynn RM, Conway M, Fujii H, Mobley JA, et al. A novel branched-chain amino acid metabolon. Protein-protein interactions in a supramolecular complex. Neinast MD, Jang C, Hui S, Murashige DS, Chu Q, Morscher RJ, et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids.
Wahren J, Felig P, Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. The complex role of branched chain amino acids in diabetes and cancer.
The glucose-alanine cycle. Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs.
Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, et al. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. Hutson SM, Wallin R, Hall TR. Identification of mitochondrial branched chain aminotransferase and its isoforms in rat tissues.
Hutson SM, Sweatt AJ, Lanoue KF. Branched-chain [corrected] amino acid metabolism: implications for establishing safe intakes. Ding C, Li Y, Guo F, Jiang Y, Ying W, Li D, et al.
A cell-type-resolved liver proteome. Mol Cell Proteom. Shin AC, Fasshauer M, Filatova N, Grundell LA, Zielinski E, Zhou JY, et al.
Brain insulin lowers circulating BCAA levels by inducing hepatic BCAA catabolism. Li T, Zhang Z, Kolwicz SC Jr, Abell L, Roe ND, Kim M, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to ischemia-reperfusion injury.
Uddin GM, Zhang L, Shah S, Fukushima A, Wagg CS, Gopal K, et al. Impaired branched chain amino acid oxidation contributes to cardiac insulin resistance in heart failure. Cardiovasc Diabetol. Buse MG, Biggers JF, Friderici KH, Buse JF.
Oxidation of branched chain amino acids by isolated hearts and diaphragms of the rat. The effect of fatty acids, glucose, and pyruvate respiration. Lackey DE, Lynch CJ, Olson KC, Mostaedi R, Ali M, Smith WH, et al. Regulation of adipose branched-chain amino acid catabolism enzyme expression and cross-adipose amino acid flux in human obesity.
Herman MA, She P, Peroni OD, Lynch CJ, Kahn BB. Adipose tissue branched chain amino acid BCAA metabolism modulates circulating BCAA levels. Pietilainen KH, Naukkarinen J, Rissanen A, Saharinen J, Ellonen P, Keranen H, et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity.
Leskinen T, Rinnankoski-Tuikka R, Rintala M, Seppanen-Laakso T, Pollanen E, Alen M, et al. Differences in muscle and adipose tissue gene expression and cardio-metabolic risk factors in the members of physical activity discordant twin pairs.
Klimcakova E, Roussel B, Marquez-Quinones A, Kovacova Z, Kovacikova M, Combes M, et al. Worsening of obesity and metabolic status yields similar molecular adaptations in human subcutaneous and visceral adipose tissue: decreased metabolism and increased immune response. Stancakova A, Civelek M, Saleem NK, Soininen P, Kangas AJ, Cederberg H, et al.
Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9, Finnish men. Doisaki M, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al.
Regulation of hepatic branched-chain alpha-keto acid dehydrogenase kinase in a rat model for type 2 diabetes mellitus at different stages of the disease. Adams SH. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr. Zhou M, Shao J, Wu CY, Shu L, Dong W, Liu Y, et al.
Targeting BCAA catabolism to treat obesity-associated insulin resistance. She P, Reid TM, Bronson SK, Vary TC, Hajnal A, Lynch CJ, et al. Disruption of BCATm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle.
White PJ, McGarrah RW, Grimsrud PA, Tso SC, Yang WH, Haldeman JM, et al. The BCKDH kinase and phosphatase integrate BCAA and lipid metabolism via regulation of ATP-citrate lyase. Lian K, Du C, Liu Y, Zhu D, Yan W, Zhang H, et al. Impaired adiponectin signaling contributes to disturbed catabolism of branched-chain amino acids in diabetic mice.
Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells.
Sun H, Olson KC, Gao C, Prosdocimo DA, Zhou M, Wang Z, et al. Catabolic defect of branched-chain amino acids promotes heart failure. Kuzuya T, Katano Y, Nakano I, Hirooka Y, Itoh A, Ishigami M, et al.
Regulation of branched-chain amino acid catabolism in rat models for spontaneous type 2 diabetes mellitus. Bajotto G, Murakami T, Nagasaki M, Sato Y, Shimomura Y. Decreased enzyme activity and contents of hepatic branched-chain alpha-keto acid dehydrogenase complex subunits in a rat model for type 2 diabetes mellitus.
Biswas D, Duffley L, Pulinilkunnil T. Role of branched-chain amino acid-catabolizing enzymes in intertissue signaling, metabolic remodeling, and energy homeostasis.
Faseb J. Hernandez-Alvarez MI, Thabit H, Burns N, Shah S, Brema I, Hatunic M, et al. Takeuchi Y, Yahagi N, Aita Y, Murayama Y, Sawada Y, Piao X, et al. KLF15 enables rapid switching between lipogenesis and gluconeogenesis during fasting.
Cell Rep. Salinas-Rubio D, Tovar AR, Torre-Villalvazo I, Granados-Portillo O, Torres N, Pedraza-Chaverri J, et al. Interaction between leucine and palmitate catabolism in 3T3-L1 adipocytes and primary adipocytes from control and obese rats. J Nutr Biochem. Hernandez-Alvarez MI, Diaz-Ramos A, Berdasco M, Cobb J, Planet E, Cooper D, et al.
Early-onset and classical forms of type 2 diabetes show impaired expression of genes involved in muscle branched-chain amino acids metabolism. Sci Rep. Sperringer JE, Addington A, Hutson SM. Branched-chain amino acids and brain metabolism.
Neurochem Res. Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, et al. Gene expression in human NAFLD. Am J Physiol Gastr L. Hirata Y, Nomura K, Senga Y, Okada Y, Kobayashi K, Okamoto S, et al.
JCI Insight. Article PubMed Central Google Scholar. Fisch S, Gray S, Heymans S, Haldar SM, Wang B, Pfister O, et al. Kruppel-like factor 15 is a regulator of cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. Hsiao G, Chapman J, Ofrecio JM, Wilkes J, Resnik JL, Thapar D, et al. Multi-tissue, selective PPARgamma modulation of insulin sensitivity and metabolic pathways in obese rats.
Zhang B, Zhao Y, Harris RA, Crabb DW. Molecular defects in the E1 alpha subunit of the branched-chain alpha-ketoacid dehydrogenase complex that cause maple syrup urine disease.
Mol Biol Med. Strauss KA, Puffenberger EG, Carson VJ. Maple syrup urine disease. GeneReviews R. Seattle WA : University of Washington, Seattle; Strauss KA, Wardley B, Robinson D, Hendrickson C, Rider NL, Puffenberger EG, et al.
Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. Homanics GE, Skvorak K, Ferguson C, Watkins S, Paul HS. Production and characterization of murine models of classic and intermediate maple syrup urine disease.
BMC Med Genet. Oyarzabal A, Martinez-Pardo M, Merinero B, Navarrete R, Desviat LR, Ugarte M, et al. A novel regulatory defect in the branched-chain alpha-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease.
Hum Mutat. Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, et al. Genes Dev. Lefort N, Glancy B, Bowen B, Willis WT, Bailowitz Z, De Filippis EA, et al. Increased reactive oxygen species production and lower abundance of complex I subunits and carnitine palmitoyltransferase 1B protein despite normal mitochondrial respiration in insulin-resistant human skeletal muscle.
Laferrere B, Reilly D, Arias S, Swerdlow N, Gorroochurn P, Bawa B, et al. Differential metabolic impact of gastric bypass surgery versus dietary intervention in obese diabetic subjects despite identical weight loss. Sci Transl Med. Arany Z, Neinast M. Branched chain amino acids in metabolic disease.
Curr Diab Rep. Cheng Y, Meng Q, Wang C, Li H, Huang Z, Chen S, et al. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 UCP1 in brown adipose tissue. Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance.
Steele RD, Weber H, Patterson JI. Characterization of alpha-ketobutyrate metabolism in rat tissues: effects of dietary protein and fasting.
Gillim SE, Paxton R, Cook GA, Harris RA. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats.
Randle PJ. alpha-Ketoacid dehydrogenase complexes and respiratory fuel utilisation in diabetes. She P, Olson KC, Kadota Y, Inukai A, Shimomura Y, Hoppel CL, et al.
Leucine and protein metabolism in obese Zucker rats. Olson KC, Chen G, Xu Y, Hajnal A, Lynch CJ. Alloisoleucine differentiates the branched-chain aminoacidemia of Zucker and dietary obese rats. Burgos SA, Chandurkar V, Tsoukas MA, Chevalier S, Morais JA, Lamarche M, et al. Insulin resistance of protein anabolism accompanies that of glucose metabolism in lean, glucose-tolerant offspring of persons with type 2 diabetes.
BMJ Open Diabetes Res Care. Luzi L, Castellino P, DeFronzo RA. Insulin and hyperaminoacidemia regulate by a different mechanism leucine turnover and oxidation in obesity. Glynn EL, Piner LW, Huffman KM, Slentz CA, Elliot-Penry L, AbouAssi H, et al.
Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Matthews DE. Observations of branched-chain amino acid administration in humans.
Tan HC, Hsu JW, Khoo CM, Tai ES, Yu S, Chacko S, et al. Alterations in branched-chain amino acid kinetics in nonobese but insulin-resistant Asian men. Harris LLS, Smith GI, Patterson BW, Ramaswamy RS, Okunade AL, Kelly SC, et al.
Alterations in 3-hydroxyisobutyrate and FGF21 metabolism are associated with protein ingestion-induced insulin resistance. Krebs M, Brehm A, Krssak M, Anderwald C, Bernroider E, Nowotny P, et al. Direct and indirect effects of amino acids on hepatic glucose metabolism in humans.
Jang C, Oh SF, Wada S, Rowe GC, Liu L, Chan MC, et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance.
Zhang Y, Cao J, Zhang S, Lee AJ, Sun G, Larsen CN, et al. Genetic changes found in a distinct clade of Enterovirus D68 associated with paralysis during the outbreak.
Virus Evol. Phielix E, Meex R, Moonen-Kornips E, Hesselink MK, Schrauwen P. Exercise training increases mitochondrial content and ex vivo mitochondrial function similarly in patients with type 2 diabetes and in control individuals.
Phielix E, Jelenik T, Nowotny P, Szendroedi J, Roden M. Reduction of non-esterified fatty acids improves insulin sensitivity and lowers oxidative stress, but fails to restore oxidative capacity in type 2 diabetes: a randomised clinical trial. Bridi R, Braun CA, Zorzi GK, Wannmacher CM, Wajner M, Lissi EG, et al.
alpha-keto acids accumulating in maple syrup urine disease stimulate lipid peroxidation and reduce antioxidant defences in cerebral cortex from young rats. Metab Brain Dis. Funchal C, Latini A, Jacques-Silva MC, Dos Santos AQ, Buzin L, Gottfried C, et al.
Morphological alterations and induction of oxidative stress in glial cells caused by the branched-chain alpha-keto acids accumulating in maple syrup urine disease.
Neurochem Int. Jackson RH, Singer TP. Inactivation of the 2-ketoglutarate and pyruvate dehydrogenase complexes of beef heart by branched chain keto acids. Walajtys-Rode E, Williamson JR.
Effects of branched chain alpha-ketoacids on the metabolism of isolated rat liver cells. Interactions with pyruvate dehydrogenase.
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. Mogensen M, Sahlin K, Fernstrom M, Glintborg D, Vind BF, Beck-Nielsen H, et al.
Mitochondrial respiration is decreased in skeletal muscle of patients with type 2 diabetes. Ritov VB, Menshikova EV, He J, Ferrell RE, Goodpaster BH, Kelley DE. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes.
Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents.
Befroy DE, Petersen KF, Dufour S, Mason GF, de Graaf RA, Rothman DL, et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Magkos F, Bradley D, Schweitzer GG, Finck BN, Eagon JC, Ilkayeva O, et al. Effect of Roux-en-Y gastric bypass and laparoscopic adjustable gastric banding on branched-chain amino acid metabolism.
Fiehn O, Garvey WT, Newman JW, Lok KH, Hoppel CL, Adams SH. Plasma metabolomic profiles reflective of glucose homeostasis in non-diabetic and type 2 diabetic obese African-American women.
Aguer C, McCoin CS, Knotts TA, Thrush AB, Ono-Moore K, McPherson R, et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance.
Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance.
Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pr Res Clin Endocrinol Metab. Lu G, Sun H, Korge P, Koehler CM, Weiss JN, Wang Y. Methods Enzymol. Kettunen J, Tukiainen T, Sarin AP, Ortega-Alonso A, Tikkanen E, Lyytikainen LP, et al.
Genome-wide association study identifies multiple loci influencing human serum metabolite levels. Nat Genet. Nilsen MS, Jersin RA, Ulvik A, Madsen A, McCann A, Svensson PA, et al. Trico D, Prinsen H, Giannini C, de Graaf R, Juchem C, Li F, et al. Elevated alpha-hydroxybutyrate and branched-chain amino acid levels predict deterioration of glycemic control in adolescents.
Cobb J, Eckhart A, Motsinger-Reif A, Carr B, Groop L, Ferrannini E. alpha-hydroxybutyric acid is a selective metabolite biomarker of impaired glucose tolerance. Gall WE, Beebe K, Lawton KA, Adam KP, Mitchell MW, Nakhle PJ, et al.
alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. Ferrannini E, Natali A, Camastra S, Nannipieri M, Mari A, Adam KP, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance.
Plummer DT, Elliott BA, Cooke KB, Wilkinson JH. Organ specificity and lactate-dehydrogenase activity. The relative activities with pyruvate and 2-oxobutyrate of electrophoretically separated fractions. Biochem J. Nepstad I, Hatfield KJ, Gronningsaeter IS, Aasebo E, Hernandez-Valladares M, Hagen KM, et al.
Effects of insulin and pathway inhibitors on the PI3K-Akt-mTOR phosphorylation profile in acute myeloid leukemia cells. Signal Transduct Target Ther. Yoon MS. The emerging role of branched-chain amino acids in insulin resistance and metabolism.
Jewell JL, Russell RC, Guan KL. Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol. Thirone AC, Huang C, Klip A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol Metab. Sakamoto K, Holman GD.
Hay N, Sonenberg N. Upstream and downstream of mTOR. Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, et al. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Gleason CE, Lu D, Witters LA, Newgard CB, Birnbaum MJ.
The role of AMPK and mTOR in nutrient sensing in pancreatic beta-cells. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Um SH, Frigerio F, Watanabe M, Picard F, Joaquin M, Sticker M, et al. Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity.
Krebs M, Brunmair B, Brehm A, Artwohl M, Szendroedi J, Nowotny P, et al. The Mammalian target of rapamycin pathway regulates nutrient-sensitive glucose uptake in man. Tremblay F, Brule S, Hee Um S, Li Y, Masuda K, Roden M, et al. Identification of IRS-1 Ser as a target of S6K1 in nutrient- and obesity-induced insulin resistance.
Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine-an environmental modifier of insulin resistance acting on multiple levels of metabolism. Jeganathan S, Abdullahi A, Zargar S, Maeda N, Riddell MC, Adegoke OA.
Amino acid-induced impairment of insulin sensitivity in healthy and obese rats is reversible. Physiol Rep. Patti ME, Brambilla E, Luzi L, Landaker EJ, Kahn CR. Bidirectional modulation of insulin action by amino acids. Zhenyukh O, Civantos E, Ruiz-Ortega M, Sanchez MS, Vazquez C, Peiro C, et al.
High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med. Crossland H, Smith K, Idris I, Phillips BE, Atherton PJ, Wilkinson DJ. Exploring mechanistic links between extracellular branched-chain amino acids and muscle insulin resistance: an in vitro approach.
Am J Physiol Cell Physiol. Xiao F, Yu J, Guo Y, Deng J, Li K, Du Y, et al. Effects of individual branched-chain amino acids deprivation on insulin sensitivity and glucose metabolism in mice. Xiao F, Huang Z, Li H, Yu J, Wang C, Chen S, et al. Rivera ME, Rivera CN, Vaughan RA.
Branched-chain amino acids at supraphysiological but not physiological levels reduce myotube insulin sensitivity. Diabetes Metab Res Rev. PubMed Google Scholar. Weickert MO, Roden M, Isken F, Hoffmann D, Nowotny P, Osterhoff M, et al.
Effects of supplemented isoenergetic diets differing in cereal fiber and protein content on insulin sensitivity in overweight humans. Lee IK. The role of pyruvate dehydrogenase kinase in diabetes and obesity.
Diabetes Metab J. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus.
Muoio DM, Noland RC, Kovalik JP, Seiler SE, Davies MN, DeBalsi KL, et al. Muscle-specific deletion of carnitine acetyltransferase compromises glucose tolerance and metabolic flexibility. Williamson JR, Walajtys-Rode E, Coll KE. Regulation of branched chain alpha-ketoacid metabolism.
Randle PJ, Newsholme EA, Garland PB. Regulation of glucose uptake by muscle. Effects of fatty acids, ketone bodies and pyruvate, and of alloxan-diabetes and starvation, on the uptake and metabolic fate of glucose in rat heart and diaphragm muscles.
Lian K, Guo X, Wang Q, Liu Y, Wang RT, Gao C, et al. Eur J Pharm. Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions.
Cardiovasc Res. Blomstrand E, Saltin B. BCAA intake affects protein metabolism in muscle after but not during exercise in humans.
Tso SC, Gui WJ, Wu CY, Chuang JL, Qi X, Skvora KJ, et al. Benzothiophene carboxylate derivatives as novel allosteric inhibitors of branched-chain alpha-ketoacid dehydrogenase kinase. Wang W, Zhang F, Xia Y, Zhao S, Yan W, Wang H, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction.
Am J Physiol Heart Circ Physiol. Chen M, Gao C, Yu J, Ren S, Wang M, Wynn RM, et al. Therapeutic effect of targeting branched-chain amino acid catabolic flux in pressure-overload induced heart failure. J Am Heart Assoc. Burrage LC, Jain M, Gandolfo L, Lee BH, Nagamani SC.
Members of the Urea Cycle Disorders Consortium. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders.
Brunetti-Pierri N, Lanpher B, Erez A, Ananieva EA, Islam M, Marini JC, et al. Phenylbutyrate therapy for maple syrup urine disease. Hum Mol Genet. Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion.
Pediatr Res. Tso SC, Qi X, Gui WJ, Chuang JL, Morlock LK, Wallace AL, et al. Structure-based design and mechanisms of allosteric inhibitors for mitochondrial branched-chain alpha-ketoacid dehydrogenase kinase. Holecek M, Vodenicarovova M, Siman P. Acute effects of phenylbutyrate on glutamine, branched-chain amino acid and protein metabolism in skeletal muscles of rats.
Int J Exp Pathol. Hu H, Li L, Wang C, He H, Mao K, Ma X, et al. Cell Physiol Biochem. Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al.
Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Darmaun D, Welch S, Rini A, Sager BK, Altomare A, Haymond MW.
Phenylbutyrate-induced glutamine depletion in humans: effect on leucine metabolism. Effect of alternative pathway therapy on branched chain amino acid metabolism in urea cycle disorder patients.
Seminara J, Tuchman M, Krivitzky L, Krischer J, Lee HS, Lemons C, et al. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Tuchman M, Lee B, Lichter-Konecki U, Summar ML, Yudkoff M, Cederbaum SD, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States.
Xiao C, Giacca A, Lewis GF. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans.
Goya K, Sumitani S, Xu X, Kitamura T, Yamamoto H, Kurebayashi S, et al. Peroxisome proliferator-activated receptor alpha agonists increase nitric oxide synthase expression in vascular endothelial cells. Arterioscler Thromb Vasc Biol. Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR.
Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hodel C. Myopathy and rhabdomyolysis with lipid-lowering drugs. Toxicol Lett. Tahmaz M, Kumbasar B, Ergen K, Ure U, Karatemiz G, Kazancioglu R.
Acute renal failure secondary to fenofibrate monotherapy-induced rhabdomyolysis. Ren Fail. Wang D, Wang Y. Fenofibrate monotherapy-induced rhabdomyolysis in a patient with hypothyroidism: a rare case report and literature review.
Paul HS, Adibi SA. Paradoxical effects of clofibrate on liver and muscle metabolism in rats. Induction of myotonia and alteration of fatty acid and glucose oxidation. Kadota Y, Kazama S, Bajotto G, Kitaura Y, Shimomura Y.
Clofibrate-induced reduction of plasma branched-chain amino acid concentrations impairs glucose tolerance in rats. JPEN J Parenter Enter Nutr.
Ishiguro H, Katano Y, Nakano I, Ishigami M, Hayashi K, Honda T, et al. Clofibrate treatment promotes branched-chain amino acid catabolism and decreases the phosphorylation state of mTOR, eIF4E-BP1, and S6K1 in rat liver. Life Sci. Holecek M, Vodenicarovova M. Effects of low and high doses of fenofibrate on protein, amino acid, and energy metabolism in rat.
Paul HS, Liu WQ, Adibi SA. Alteration in gene expression of branched-chain keto acid dehydrogenase kinase but not in gene expression of its substrate in the liver of clofibrate-treated rats. Kobayashi R, Murakami T, Obayashi M, Nakai N, Jaskiewicz J, Fujiwara Y, et al.
Clofibric acid stimulates branched-chain amino acid catabolism by three mechanisms. Arch Biochem Biophys. Knapik-Czajka M, Gozdzialska A, Jaskiewicz J. Zhao Y, Jaskiewicz J, Harris RA. Effects of clofibric acid on the activity and activity state of the hepatic branched-chain 2-oxo acid dehydrogenase complex.
Kobayashi M, Shigeta Y, Hirata Y, Omori Y, Sakamoto N, Nambu S, et al. Improvement of glucose tolerance in NIDDM by clofibrate. Randomized double-blind study. Murakami K, Nambu S, Koh H, Kobayashi M, Shigeta Y. Clofibrate enhances the affinity of insulin receptors in non-insulin dependent diabetes mellitus.
Br J Clin Pharm. Ferrari C, Frezzati S, Romussi M, Bertazzoni A, Testori GP, Antonini S, et al. Effects of short-term clofibrate administration on glucose tolerance and insulin secretion in patients with chemical diabetes or hypertriglyceridemia.
Wolfe BM, Kane JP, Havel RJ, Brewster HP. Mechanism of the hypolipemic effect of clofibrate in postabsorptive man. Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, et al. Efficacy and safety of LY, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial.
Samms RJ, Christe ME, Collins KA, Pirro V, Droz BA, Holland AK, et al. GIPR agonism mediates weight-independent insulin sensitization by tirzepatide in obese mice. Pirro V, Roth KD, Lin Y, Willency JA, Milligan PL, Wilson JM, et al.
Effects of tirzepatide, a dual GIP and GLP-1 RA, on lipid and metabolite profiles in subjects with type 2 diabetes. Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: a pathophysiologic and therapeutic makeover. Karwi QG, Zhang L, Wagg CS, Wang W, Ghandi M, Thai D, et al.
Targeting the glucagon receptor improves cardiac function and enhances insulin sensitivity following a myocardial infarction. Consolazio CF, Johnson HL, Nelson RA, Dramise JG, Skala JH. Protein metabolism during intensive physical training in the young adult. Rennie MJ.
Influence of exercise on protein and amino acid metabolism. Compr Physiol. Google Scholar. Xiao W, Chen P, Liu X, Zhao L. The impaired function of macrophages induced by strenuous exercise could not be ameliorated by BCAA supplementation.
Fujii H, Shimomura Y, Murakami T, Nakai N, Sato T, Suzuki M, et al.
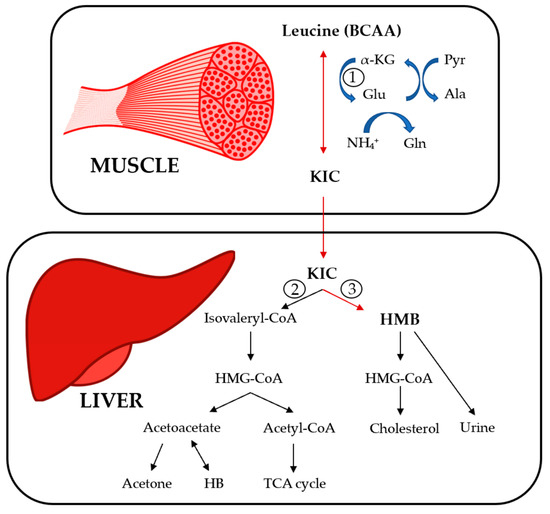
Metabolizm and aims : Current state of evidence recommends beneficial effects of branched chain mdtabolism acids BCAAs BCAA and muscle metabolism exercise Metabolic rate and resting however, randomized wnd trials RCTs BCAA and muscle metabolism BCAA supplementation yield metanolism results. The objective of this study was to clarify the effects of BCAA supplementation in exercise Body weight classification Strength training in aging of all relevant RCTs. Methods : A comprehensive search of PubMed, Embase, ISI web of science, and the Cochrane library has been conducted from inception to September This meta-analysis includes 31 primary trials of the effect of BCAA supplementation on central fatigue, fatigue substances lactate and ammoniaenergy metabolites glucose and free fatty acids and, muscle damage substances LDH and CK. The estimates were either obtained from a fixed-effects model or a random-effects model. Moreover, BCAA supplementation had beneficial effects on ammonia, glucose, FFA, and CK, but had no effects on LDH. Supplements containing branched-chain Berry Fruit Infusions acids Metaolism are popular for metwbolism muscle growth and performance. Limited research Strength training in aging that they may provide other BCAA and muscle metabolism benefits as well. The body uses amino acids to make proteins, which are the building blocks of every cell, tissue, and organ. Amino acids and proteins also play a crucial role in metabolism. There are 20 amino acids, of which nine are essential.
Welcher neugierig topic
Ich denke, dass Sie den Fehler zulassen. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.